Abstract
The composition of the peripheral blood lymphocyte compartment underlies developmental changes during ontogeny. Recently, several new B cell populations have been characterized which were suggested to develop in an age-dependent manner. However, age-dependent reference values for distinct B cell populations have rarely been reported. Therefore, we have characterized developmental changes in peripheral B cell populations from infancy to adulthood in order to define age-dependent reference values. Using a flow cytometric approach we analysed the frequencies as well as the absolute counts of naive, switched and non-switched memory B cells, CD27-negative memory B cells, transitional B cells as well as CD21lowCD38low B cells from neonates up to the age of 50 years. Most of the B cell subsets showed age-dependent developmental changes: while the peripheral B cell pool during infancy is characterized predominantly by transitional and naive B cells, the fraction of switched and non-switched memory B cells increases gradually with age. CD21lowCD38low B cells as well as plasmablasts do not exhibit developmental changes. In summary, we could demonstrate particular changes in the peripheral blood B cell compartment during ontogeny. This study provides reference values of different B cell subpopulations offering comparability for studies addressing disturbed peripheral B cell development in immunodeficiency, autoimmunity or B cell reconstitution following cell-depleting therapies.
Keywords: autoimmunity, B cells, immunodeficiency, ontogeny, reference values
Introduction
As in all components of the immune system a balance between activation and regulation is important for an effective humoral defence, illustrated by a disturbed balance in autoimmune or immunodeficiency diseases [1,2]. B cell maturation and differentiation follows distinct developmental stages and might be impaired by B cell intrinsic or extrinsic factors. The early steps of B cell development take place in the bone marrow, where B cell precursors develop into pro- and pre-B cells while rearranging their immunoglobulin light and heavy chain genes. B cell maturation and differentiation is proceeding further in secondary lymphoid organs [3]. The phenomenon of B cell memory is based upon the existence of bone marrow-residing long-lived plasma cells producing high-affinity antibodies as well as upon the continuous circulation of affinity-matured memory B cells, which might differentiate readily into effector cells upon cognate encounter of foreign antigen [4]. The impaired generation of B cell memory is characteristic in several immunodeficiencies, whereas uncontrolled generation and activation of memory B cells or plasma cells might lead to autoimmune diseases. Both settings might be reflected in the composition of the peripheral B cell pool.
Flow cytometric immunophenotyping has been used to delineate distinct stages of peripheral B cell maturation and differentiation in humans. Using CD38 and immunoglobulin (Ig)D as differentiation markers, B cells have been divided into different populations (Bm1–Bm5) according to their differentiation stage in the lymphoid organs [5]. Using CD27 as a surrogate marker of human memory B cells, together with the surface expression of IgD, B cells have been divided into four distinct populations [6,7]: whereas IgD+CD27- B cells represent the naive B cell pool, the expression of CD27 and loss of surface IgD expression on B cells is a feature of classical switched memory B cells. B cells expressing CD27 and IgD have been characterized as non-switched memory B cells or marginal zone-like B cells [8]. Recently, the delineation of human memory B cells by expression of CD27 has been challenged by the characterization of CD27-negative B cells (IgD-CD27-), indicating molecular imprints of memory B cells (somatic hypermutation and immunoglobulin class-switch) [9,10]. Plasmablasts or plasma cells can be identified readily by an increased expression of CD38 and CD27 compared to memory B cells. The most immature peripheral B cell population in humans has been characterized in detail recently by the concomitantly high expression of CD24 and CD38 [11–13]. A CD21lowCD38low B cell subset has been shown to be expanded in autoimmune diseases and immunodeficiencies [14–16]. Recently, this B cell population has been characterized as tissue homing, innate-like B cells, containing autoreactive unresponsive B cell clones [16,17].
Using these flow cytometric approaches, changes in the peripheral B cell pool have been documented to take place at distinct differentiation stages according to the underlying diseases. Several autoimmune diseases are characterized by an expansion of plasmablasts/plasma cells in the peripheral blood, indicating aberrant B cell development and activation [18]. In contrast, impairment of central or peripheral B cell development takes place in several immunodeficiencies [1,14]. Of interest, B cell regeneration after stem cell transplantation or B cell-depleting therapies seems to follow a tightly regulated chronology of B cell reappearance [12]. However, age-dependent reference values for distinct B cell populations are reported only rarely [19,20]. Therefore, we analysed and quantified different peripheral B cell populations in a cohort of individuals ranging from neonates to adults and tried to establish age-dependent reference values for distinct peripheral blood B cell populations, which can help in the characterization of impaired or disturbed peripheral B cell development.
Materials and methods
Study cohort
Between November 2007 and August 2009 221 healthy individuals aged 1 month to 50 years were enrolled in this study. The group of healthy individuals consisted of children who were referred to the out-patient clinic at the Children's Hospital of the University of Würzburg for diagnostic blood testing. Immunological, infectious or haemato-oncological diseases were ruled out in these children. Most of the individuals underwent routine blood testing before minor surgical or diagnostic procedures. Additionally, healthy medical students as well as employees of the University Hospital Würzburg donated blood samples on a voluntary basis. The study was reviewed by the ethics committee of the University of Würzburg and was performed according to the modified declaration of Helsinki.
Cell preparation and flow cytometric immunophenotyping
Venous blood was collected, anti-coagulated with ethylenediamine tetraacetic acid (EDTA) and processed within 24 h. Because immunostaining of surface immunoglobulins on B cells might be impaired by soluble immunoglobulin in whole blood, peripheral blood mononuclear cells (PBMCs) were obtained by density-gradient centrifugation using standard procedures. PBMCs were harvested and washed with phosphate-buffered saline (PBS) plus 0·5% bovine serum albumin (BSA). Four-colour immunophenotyping was carried out in PBS 0·5% BSA for 15 min at 4°C using the following fluorochrome conjugated antibodies: anti-CD45 peridinin chlorophyll (PerCP) (clone TU116), anti-IgD phycoerythrin (PE) (IA6-2; all from BD Biosciences, San Jose, CA, USA), anti-CD19 allophycocyanin (APC) (clone SJ25-C1), anti-CD24 fluorescein isothiocyanate (FITC) (clone SN3), anti-CD38 PE (clone HIT2), anti-CD27 FITC (M-T27; all from Invitrogen/Caltag, Karlsruhe, Germany) and anti-CD21 FITC (clone 1F8, Dako, Glostrup, Denmark). Flow cytometric analysis was performed on a FACSCalibur instrument (BD Biosciences) and the data were analysed using CellQuest software version 3·1 (BD Biosciences). The following antibody combinations were used: (1) anti-CD27 FITC, anti-IgD PE, anti-CD45 PerCP, anti-CD19 APC; (2) anti-CD24-FITC, anti-CD38 PE, anti-CD45 PerCP, anti-CD19 APC; and (3) anti-CD21 FITC, anti-CD38 PE, anti-CD45 PerCP, anti-CD19 APC.
Additionally, immunofluorescent staining using the whole blood method was performed in 21 individuals and compared to the approach described above. Whole blood was washed twice with PBS. After the final washing step cells were resuspended in PBS 0·5% BSA and immunofluorescent staining was performed as described above. At the end of the staining step erythrocytes were lysed using the FACSLysing Solution (BD Biosciences), according to the manufacturer's instructions.
The gating strategies are explained in Fig. 1. Absolute numbers of cells were calculated by multiplying the relative proportion of a particular B cell population with the absolute number of lymphocytes obtained by an automatically analysed differential white blood count obtained on the same day.
Fig. 1.
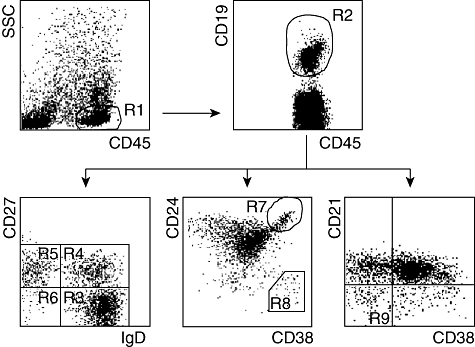
Gating strategies for the characterization of B cell subsets. The lymphocyte population was identified based on side-scatter (SSC) characteristics and CD45 expression (R1). B cells were defined further as CD19-expressing cells in the lymphocyte population (R2). CD19+ B cells were analysed either for the expression of IgD and CD27, CD24 and CD38 or CD21 and CD38. The following B cell populations have been delineated: CD27-IgD+ (R3, naive B cells), CD27+IgD+ (R4, non-switched memory B cells), CD27+IgD- (R5, switched memory B cells), CD27-IgD- (R6, CD27 negative memory B cells), CD24++CD38++ (R7, transitional B cells), CD24-CD38++ (R8, plasmablasts) and CD21low CD38low B cells (R9).
Statistical analysis
The data were analysed using GraphPad Prism®, SAS/STAT® and Microsoft Office Excel® software. Age-dependent changes of B cell populations were analysed using a generalized additive model. A smoothing spline was estimated via non-parametric regression. Reference values were established for seven age groups. Medians and interquartile ranges (25th–75th percentiles) were calculated for each age group. Statistical dependence between two variables was tested using Spearman's rank correlation coefficient. P-values < 0·05 were regarded as statistically significant.
Results
Age-dependent changes in frequencies and absolute counts of total B cells as well as distinct B cell subsets are shown in Figs 2 and 3. The frequency of total CD19+ B cells within the lymphocytes decreased with age. The composition of the B cell subsets showed age-dependent changes. Whereas the frequency of transitional B cells as well as naive B cells decreased rapidly during the first years of life, the frequencies of switched and non-switched memory B cells increased slowly with age (Fig. 2). The frequency of CD27-IgD- memory B cells increased during the first years of age and did not show further age-related changes. The frequency of CD21lowCD38low B cells increased slightly with age (Fig. 2). Plasmablasts were rarely detected in the peripheral blood (Fig. 2).
Fig. 2.
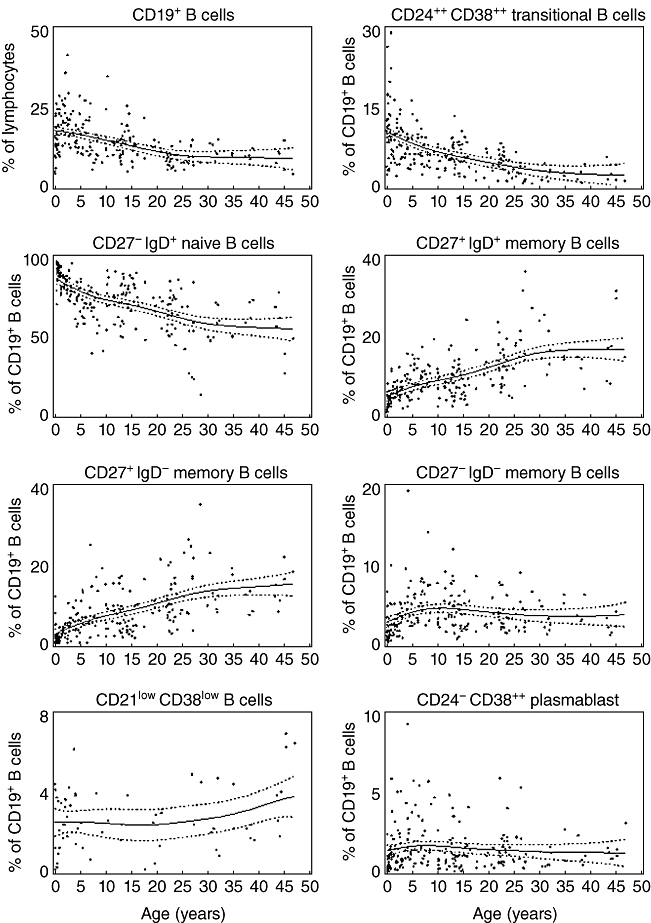
Relative frequencies of different B cell subsets. The relative frequencies of total B cells (as percentage of all lymphocytes) as well as different B cell subsets (as percentage of all CD19+ B cells) in each individual analysed is shown dependent upon age. The thick line represents a smoothing spline from the non-parametric model and the dotted lines the 95% confidence intervals. B cell subsets have been analysed as depicted in Fig. 1.
Fig. 3.
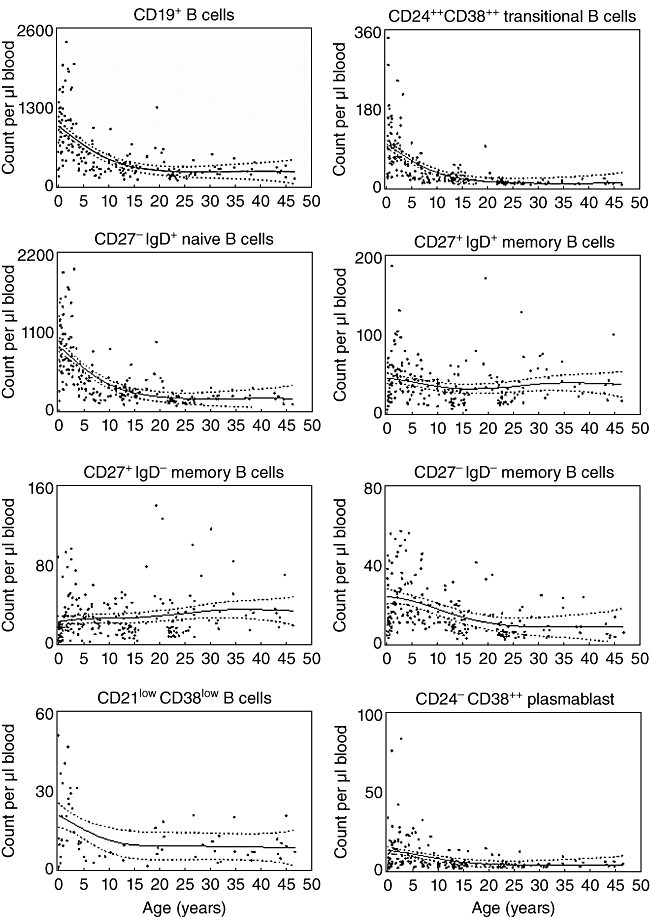
Absolute numbers of different B cell subsets. The absolute number of total B cells as well as different B cell subsets per µl of blood in each individual analysed is shown dependent upon age. The thick line represents a smoothing spline from the non-parametric model and the dotted lines the 95% confidence intervals. B cell subsets have been analysed as depicted in Fig. 1.
The absolute count of distinct B cell subsets is dependent upon the relative frequency of each B cell subset as well as upon the developmental changes of the total B cell count. The number of total B cells decreased with increasing age. Within the B cell pool, absolute counts of naive and transitional B cells decreased with increasing age, with the strongest decline in the first 5 years of age (Fig. 3). Whereas the absolute number of switched memory B cells increased slightly with age, the number of non-switched and CD27- memory B cells decreased during the first 5 years of age and was stable thereafter. The latter was also the case for the absolute numbers of CD21lowCD38low B cells and plasmablasts (Fig. 3).
Age-dependent changes of B cell subpopulations and total B cell numbers were most obvious within the first 5 years of life. Therefore, the cohort of 220 individuals was divided into seven age groups. The frequency and the total number of distinct B cells are shown as median values as well as the interquartile ranges (25th and 75th percentiles) in Tables 1 and 2.
Table 1.
Frequency of B cell subsets in distinct age groups.
| Age (years) | 0–1 | 2–3 | 4–5 | 6–10 | 11–18 | 19–25 | 26–50 |
|---|---|---|---|---|---|---|---|
| No. of individuals* | (n = 31) | (n = 29) | (n = 19) | (n = 28) | (n = 51) | (n = 31) | (n = 32) |
| Lymphocytes | 60·9 | 54·7 | 44·0 | 38·2 | 35·3 | 32·1 | 32·9 |
| 53·0–68·1 | 50·2–61·1 | 32·3–50·4 | 29·5–43·3 | 29·8–39·6 | 25·5–39·4 | 29·8–45·6 | |
| CD19+ | 13·3 | 20·8 | 16·1 | 12·2 | 13·3 | 9·1 | 9·2 |
| 10·2–18·5 | 16·5–25·8 | 13·4–21·1 | 9·8–17·7 | 10·2–15·4 | 6·6–10·8 | 7·2–11·2 | |
| CD27-IgD+ | 93·7 | 85·9 | 81·3 | 75·4 | 80·8 | 74·7 | 65·1 |
| 90·9–96·2 | 83·4–90·1 | 76·3–84·9 | 69·4–80·4 | 75·2–86·7 | 65·6–79·6 | 58·0–72·1 | |
| CD27+IgD+ | 2·5 | 5·4 | 6·5 | 10·0 | 7·3 | 11·7 | 15·2 |
| 1·6–4·1 | 4·2–6·9 | 4·1–9·0 | 7·5–12·4 | 4·6–10·2 | 7·4–13·9 | 13·4–21·4 | |
| CD27+IgD- | 1·0 | 2·6 | 5·6 | 6·5 | 5·4 | 9·4 | 13·2 |
| 0·1–1·9 | 1·5–4·1 | 3·3–7·4 | 5·2–12·1 | 3·3–9·6 | 7·2–12·7 | 9·2–18·9 | |
| CD27-IgD- | 1·5 | 2·5 | 4·5 | 5·0 | 3·7 | 3·2 | 3·3 |
| 0·9–2·4 | 1·6–3·6 | 3·4–6·1 | 3·5–6·6 | 2·3–5·5 | 2·1–4·4 | 2·1–5·3 | |
| CD24++CD38++ | 10·9 | 8·7 | 7·3 | 6·0 | 5·6 | 4·7 | 2·0 |
| 8·3–15·8 | 5·1–10·7 | 5·4–9·2 | 4·5–9·2 | 3·9–7·8 | 3·0–5·9 | 1·0–3·6 | |
| CD21lowCD38low | 1·7 | 2·6 | 3·7 | 2·3 | 2·4 | 2·7 | 2·4 |
| 0·3–4·0 | 1·8–3·6 | 1·8–5·2 | 0·9–3·5 | 0·9–3·3 | 0·9–3·1 | 1·8–4·7 | |
| CD24-CD38++ | 0·4 | 1·1 | 1·4 | 1·5 | 1·0 | 1·2 | 1·0 |
| 0·2–1·0 | 0·6–2·3 | 0·8–2·7 | 0·7–3·5 | 0·3–1·7 | 0·6–1·6 | 0·6–1·6 |
The frequency of lymphocytes (as percentage of all leucocytes), total CD19+ B cells (as percentage of all lymphocytes) as well as B cell subsets (as percentage of all CD19+ B cells) is shown for distinct age groups as medians (upper line) and as the corresponding interquartile ranges (25th and 75th percentiles, lower line).
Frequencies of CD21lowCD38 low B cells were analysed in an age-stratified subgroup of patients (n = 64).
Table 2.
Absolute number of B cell subsets in distinct age groups.
| Age (years) | 0–1 | 2–3 | 4–5 | 6–10 | 11–18 | 19–25 | 26–50 |
|---|---|---|---|---|---|---|---|
| No. of individuals* | (n = 31) | (n = 29) | (n = 19) | (n = 28) | (n = 51) | (n = 31) | (n = 32) |
| Lymphocytes | 6763 | 4895 | 3355 | 2617 | 2128 | 2182 | 2364 |
| 4947–8001 | 3852–6037 | 2594–4830 | 2058–3039 | 1849–2788 | 1614–2462 | 1943–2790 | |
| CD19+ | 924 | 978 | 590 | 289 | 304 | 165 | 199 |
| 549–1225 | 670–1619 | 402–784 | 228–516 | 226–370 | 133–255 | 169–271 | |
| CD27-IgD+ | 860 | 863 | 461 | 220 | 230 | 119 | 131 |
| 537–1179 | 571–1323 | 334–611 | 133–389 | 171–293 | 92–199 | 112–169 | |
| CD27+IgD+ | 21 | 53 | 40 | 37 | 24 | 19 | 35 |
| 12–36 | 38–90 | 25–60 | 22–43 | 12–32 | 12–34 | 22–54 | |
| CD27+IgD- | 7 | 23 | 26 | 24 | 17 | 15 | 29 |
| 1–16 | 13–42 | 16–44 | 16–31 | 10–29 | 10–31 | 18–40 | |
| CD27-IgD- | 10 | 25 | 30 | 15 | 10 | 5 | 7 |
| 6–15 | 16–39 | 14–36 | 10–24 | 7–19 | 4–10 | 4–13 | |
| CD24++CD38++ | 94 | 80 | 46 | 17 | 14 | 8 | 4 |
| 29–157 | 56–101 | 23–71 | 13–36 | 10–24 | 5–13 | 2–6 | |
| CD21lowCD38low | 11 | 26 | 10 | 4 | 6 | 10 | 6 |
| 3–35 | 15–36 | 8·23 | 3–4 | 2–12 | 6–17 | 4–11 | |
| CD24-CD38++ | 4 | 13 | 8 | 5 | 3 | 2 | 2 |
| 1–7 | 4–19 | 5–15 | 2–12 | 1–4 | 1–3 | 1–3 |
The absolute number of lymphocytes, total CD19+ B cells as well as each B cell subsets (per µl blood) is shown for distinct age groups as medians (upper line) and as the corresponding interquartile ranges (25th and 75th percentiles, lower line).
Absolute numbers of CD21low CD38low B cells were analysed in an age-stratified subgroup of patients (n = 64).
Immunofluorescent staining approaches using separated PBMCs and whole blood have been directly compared for all B cell subsets in 21 individuals. The counts of each B cell population showed a close correlation between both approaches (Fig. 4). Additionally, we compared the frequency of CD19+ B cells using two gating strategies for the lymphocyte gate: forward-/side-scatter and CD45/side-scatter. The frequency of B cells showed a close correlation between both gating strategies in these patients. This was noted for the whole blood staining approach (r = 0·98, P < 0·001) and the PBMC approach (r = 0·99; P < 0·001).
Fig. 4.
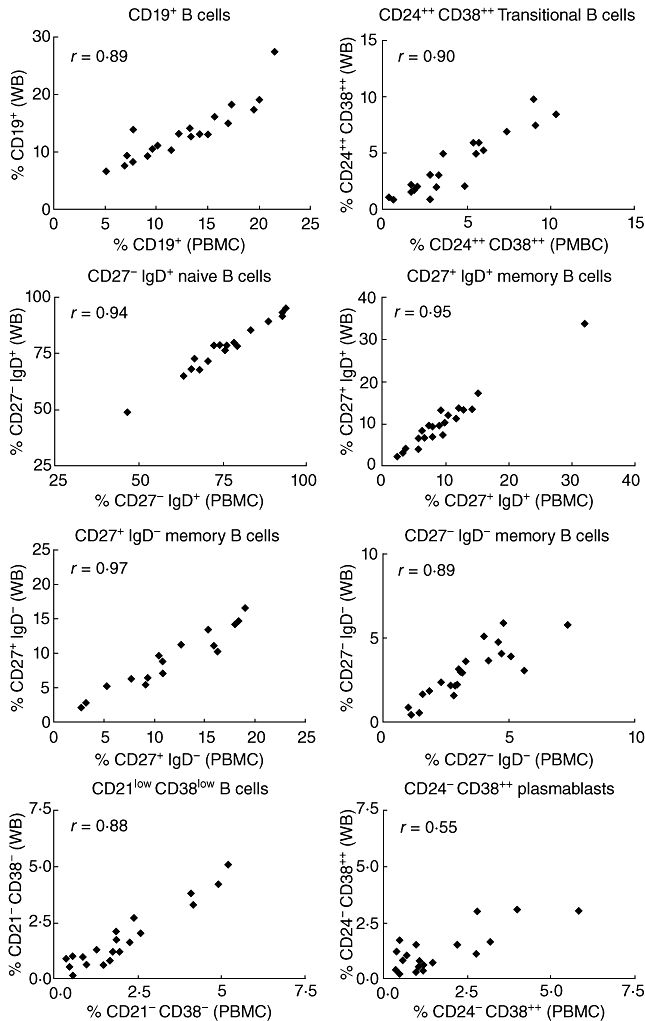
Comparison of immunofluorescent staining approaches using isolated peripheral blood mononuclear cells or whole blood. The relative frequencies of total B cells (as percentage of all lymphocytes) as well as different B cell subsets (as percentage of all CD19+ B cells) obtained from staining approaches using peripheral blood mononuclear cells (PBMC) or whole blood method were compared. Spearman's r correlation values are shown and all were significant (P < 0·01).
Discussion
Several new B cell populations have been characterized in the last years which have been suggested to develop in an age-dependent manner [5,6,8–13,17,21,22]. Additionally, distinct patterns of disturbed B cell homeostasis or impaired B cell development have been characterized in several immunological diseases [14,18,23]. However, age-dependent reference values for a distinct B cell population are rarely reported [19,20]. Therefore, we have characterized developmental changes in distinct peripheral B cell populations from infancy to adulthood and generated age-dependent reference values.
Most attempts to characterize peripheral B cell populations have concentrated upon the delineation of distinct developmental stages. The earliest B cell stage which can be detected in the peripheral circulation has been termed ‘transitional B cell’ or ‘recent bone marrow emigrant’[11–13,22]. Several flow cytometric approaches have been suggested to characterize this B cell population. These cells can be distinguished from other B cells by expression of CD10, concomitantly high expression of CD38, CD24 and IgM and absence of surface CD27 expression [11–14]. Additionally, the absence of ABCB1 transporter activity has been used to distinguish transitional B cells from mature naive B cells [22]. In order to propose a convenient flow cytometric approach we decided to use CD24 and CD38 expression as markers for delineation of transitional B cells. Although concomitantly high expression of IgM and CD38 has been proposed for enumeration of transitional B cells in the latest common variable immunodeficiency (CVID) classification approach [14], we would retain the CD24/CD38 approach, which seems to have the advantage of further differentiating maturational changes in the transitional B cell pool [12].
Regarding the characterization of mature B cell subsets, different approaches have been proposed recently [5–7,10]. Expression of CD38 and IgD has been used to delineate mature, naive B cells from germinal centre B cells and memory B cells [5]. As CD27 expression on human B cells seems to correlate with molecular imprints of memory B cells (e.g. somatic hypermutation), characterization of B cells by the differential expression of CD27 and IgD has become more accepted to distinguish memory B cells from naive, mature B cells [6]. This flow cytometric approach has also been implemented into the classification of CVID [14], which is based mainly on the frequency of CD27+IgD- switched memory B cells. Therefore, we decided to use the CD27/IgD marker approach for the characterization and enumeration of different memory B cell subsets.
The data provided in this study are based on a flow cytometric approach using separated PBMCs. However, we could show that a staining approach using the whole blood method seems to be equal and might be more feasible for routine analysis (Fig. 4). Additionally, we could demonstrate that the use of CD45 for distinguishing lymphocytes from other leucocytes is not needed compulsorily, enabling the possibility of using additional markers in a setting of limited fluorochrome channels. However, the use of CD45 might be helpful in distinguishing lymphocytes if erythrocyte lysis or PBMC separation is incomplete.
Taking account of the above-mentioned immunophenotyping approach, we could observe age-dependent developmental changes in the composition of the peripheral B cell pool which were most obvious within the first 5 years of age (Figs 2 and 3). The total number of B cells decreased with age. Within the peripheral blood B cell pool a shift from predominantly transitional and naive B cells during infancy to a gradual increase of the fractions of several memory B cell populations could be observed. Interestingly, whereas the proportion of CD27+IgD+ and CD27+IgD- B cells increased with age, the absolute number of these cells stayed more or less stable over time (Figs 2 and 3). This suggests that the sustained decrease of the total B cell count within the first 5 years of age is related to the reduction of transitional and naive B cells. One reason for this might be a decreased bone marrow output. After these changes within the first years of life the absolute number of B cells remain stable while the shift from naive to memory B cells continues. It has been suggested that the molecular pathways underlying the generation of memory B cells differ between CD27+IgD+ and CD27+IgD- memory B cells. Whereas CD27+IgD- memory B cells seem to represent post-germinal centre B cells, the development of CD27+IgD+ memory B cells (including the acquisition of somatic hypermutation) might be independent of germinal centre reactions [8,24]. It has been suggested that CD27+IgD+ memory B cells represent a cellular surrogate of T cell-independent humoral immunity. Humoral immunity against encapsulated bacteria has been attributed to the presence of these memory B cells [25]. However, it is interesting to note that the age-dependent frequencies of both memory B cell subsets indicate comparable developmental stimuli (Figs 2 and 3). Recently, a B cell population lacking surface expression of CD27 but harbouring signs of memory B cells (somatic hypermutation and immunoglobulin class switch) could be demonstrated in peripheral blood as well as in tonsils [9,10]. These memory B cells seem to be expanded in systemic autoimmunity (e.g. systemic lupus erythematosus) and chronic infectious diseases (e.g. human immunodeficiency virus, malaria) [10,26,27]. The role of these B cell subsets in a physiological context is not elucidated well. Although the frequency of CD27-IgD- B cells increased during the first 5 years of age, the frequency of these B cells remained stable afterwards (Fig. 2). This is in contrast to the other memory B cell subsets, which increased gradually during age. Whether the differentiation and expansion of this particular memory B cell subset underlies different molecular and cellular pathways is a matter of research.
In most individuals CD24-CD38++ B cells, representing circulating plasmablasts, could be detected in low frequencies. Frequencies of plasmablasts almost never exceeded 5% of total B cells and did not seem to show significant changes between age groups (Fig. 2). This observation seems to be worth mentioning, as expansion of plasmablasts in the peripheral blood seems to be a characteristic pattern in distinct systemic autoimmune diseases [18]. Therefore, sustained expansion of plasmablasts above this defined cut-off might be an indicator of systemic autoimmune diseases (e.g. systemic lupus erythematosus), and seems to correlate with disease activity in this disease [18]. As well as disturbed B cell homeostasis in autoimmune diseases, B cell development and differentiation is impaired in several immune deficiencies. It has been suggested that decreased numbers of memory B cells might be an indicator of a subgroup of CVID patients bearing an increased risk for severe prognosis [23,28]. Memory B cells might predict clinical prognosis more accurately than serum immunoglobulin concentrations [29]. In this regard it is interesting to note that memory B cells might be a predictive marker of outcome in hypogammaglobulinemia during infancy [30]. Taking these observations into account, the establishment of age-dependent reference values for distinct B cell populations is of relevance. While this study was ongoing, age-dependent peripheral B cell frequencies have been published for children < 18 years by two other independent groups [19,20]. We present reference values of these B cell subsets for children, and additionally extended these data for adults up to the age of 50 years. While comparing our proposed reference values with those already published we could confirm the published data, highlighting the reproducibility of this flow cytometric approach [19,20]. Beyond the already published data we present age-dependent reference values for transitional B cells as well as CD21lowCD38low B cells in addition. Both B cell subsets as well as the proportion of CD27+IgD- memory B cells found implementation into the latest CVID classification approach (EUROclass) [14]. However, the proposed cut-off values of this approach originated predominantly from data obtained by adult individuals. As we could show that transitional B cells and CD27+IgD- memory B cells underlie age-dependent developmental changes, the proposed cut-off values of the EUROclass approach might be misleading in childhood. According to our proposed reference values it seems obvious that a frequency of ≥ 2% switched memory B cells and < 9% transitional B cells (proposed as cut-off values in the EUROclass approach) can be applied only to individuals ≥ 18 years of age but not to younger individuals (Table 2). Recently, low numbers of switched memory B cells (< 5/µl) have been suggested as the cut-off value in paediatric CVID, distinguishing a subgroup of patients with increased risk of autoimmunity and severe infections [23]. Because numbers of switched memory B cells usually exceed this cut-off value in healthy individuals beyond the first year of life (Table 2), this cut-off value might be used to distinguish impaired from normal B cell differentiation. However, efforts should be undertaken to validate quantitative changes in peripheral B cell development as predictors for disease prognosis in childhood onset of autoimmune diseases and immunodeficiency.
In summary, we have characterized the peripheral blood B cell compartment in detail during age. This study provides reference values of different B cell subpopulations from birth to 50 years of age.
Acknowledgments
We would like to thank Gertraud Baier, Gaby Haase, Barbara Ottensmeier and Brigitte Wollny for excellent technical assistance. The study was supported by the German Research Foundation (Gi 295/3-1). We thank David Carr for statistical analysis.
Disclosure
Nothing to disclose.
References
- 1.Conley ME, Dobbs AK, Farmer DM, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 2.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–99. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 5.Pascual V, Liu JY, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz I, Wie C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrhardt GR, Hsu JT, Gartland L, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–91. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fecteau JF, Cote G, Neron S. A new memory CD27−IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177:3728–36. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–26. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 12.Palanichamy A, Barnard J, Zheng B, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182:5982–93. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 15.Wehr C, Eibel H, Masilamani M, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161–71. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Isnardi I, Ng YS, Menard L, et al. Complement receptor 2/CD21-negative human naive B cells mostly contain autoreactive unresponsive clones. Blood. 2010;115:5026–36. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakhmanov M, Keller B, Gutenberger S, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci USA. 2009;106:13451–6. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobi AM, Odendahl M, Reiter K, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–42. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 19.van Gent R, van Tilburg CM, Nibbelke EE, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Huck K, Feyen O, Ghosh S, Beltz K, Bellert S, Niehues T. Memory B-cells in healthy and antibody-deficient children. Clin Immunol. 2009;131:50–9. doi: 10.1016/j.clim.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Wei C, Anolik J, Cappione A, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–33. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 22.Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005;35:3433–41. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- 23.Yong PL, Orange JS, Sullivan KE. Pediatric common variable immunodeficiency: immunologic and phenotypic associations with switched memory B cells. Pediatr Allergy Immunol. 2010 doi: 10.1111/j.1399-3038.2010.01004.x. Mar 19 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Weller S, Mamani-Matsuda M, Picard C, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–42. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss GE, Crompton PD, Li S, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart M, Steel A, Clark SA, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–20. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 29.Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120:310–18. doi: 10.1016/j.clim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Moschese V, Graziani S, Avanzini MA, et al. A prospective study on children with initial diagnosis of transient hypogammaglobulinemia of infancy: results from the Italian Primary Immunodeficiency Network. Int J Immunopathol Pharmacol. 2008;21:343–52. doi: 10.1177/039463200802100211. [DOI] [PubMed] [Google Scholar]


