Abstract
Background
Smoking withdrawal is associated with significant deficits in the ability to initiate and maintain attention for extended periods of time (i.e. sustained attention; SA). However, the effects of smoking abstinence on the temporal dynamics of neurocognition during SA have not been evaluated.
Methods
Twenty adult smokers underwent functional magnetic resonance imaging scans following smoking as usual and after 24-hr abstinence. During scanning they completed a SA task with two levels of task difficulty, designed to measure both sustained (i.e. over the duration of the task) and transient (i.e. event-related) activation.
Results
Smoking abstinence significantly decreased task accuracy regardless of task difficulty. Compared to the smoking as usual, abstinence resulted in decreased sustained activation in right inferior and middle frontal gyri but increased transient activation across disperse cortical areas including precuneus and right superior frontal gyrus. Greater task difficulty was associated with even greater transient activation during abstinence in mostly right hemisphere regions including right inferior frontal gyrus.
Conclusions
Smoking withdrawal shifts the temporal and spatial dynamics of neurocognition from sustained, right prefrontal activation reflecting proactive cognitive control (Braver et al., 2009) to more disperse and transient activation reflecting reactive control.
Keywords: attention, fMRI, mixed-design, nicotine dependence, prefrontal cortex, smoking
Introduction
Cessation of smoking robustly disrupts sustained attention (SA), or the ability to initiate and maintain focus for extended periods of time (Foulds et al., 1996; Gilbert et al., 1997; Heishman, 1998; Wesnes and Warburton, 1983). SA deficits emerge shortly after cessation (Hendricks et al., 2006) and persist for weeks among some smokers (Gilbert et al., 2004). Though previous neuroimaging research has investigated the neurobiology of SA in non-addicted individuals (Coull et al., 1996; Lawrence et al., 2003; Sturm and Willmes, 2001) and the effects of nicotine on minimally deprived smokers (Hong et al., 2009; Lawrence et al., 2002), the neurobiological basis of SA deficits following smoking abstinence has not been evaluated.
Functional neuroimaging and human lesion studies have identified a network of brain regions that subserve SA including right prefrontal (PFC) and parietal cortices, thalamus and brainstem (Coull et al., 1996; Lawrence et al., 2003; Shallice et al., 2008; Sturm and Willmes, 2001). The right PFC is specifically thought to be involved in the top-down control of SA (Sturm and Willmes, 2001); whereas brainstem centers including the locus coeruleus subserve bottom-up regulation of arousal (Sara, 2009).
To date, two neuroimaging studies have investigated the neural basis of smoking and nicotine effects on SA. Lawrence et al. (Lawrence et al., 2002) reported that in minimally deprived smokers, transdermal nicotine as compared to placebo increased activation during SA in bilateral parietal cortex, left thalamus and caudate; and decreased activation in insula. Hong et al. (Hong et al., 2009) similarly examined the effects of nicotine in smokers with and without schizophrenia. Across diagnosis groups and task demand levels, nicotine increased SA activation in frontal and parietal cortices, anterior and posterior cingulate, striatum and thalamus. Thus, the available data suggest that nicotine increases activation in regions known to subserve SA.
In addition to studies of sustained attentional processes, neuroimaging studies have also investigated the effects of nicotine and smoking on other, more transient attentional processes using event related designs. In these studies, acute nicotine administration to nonsmokers and/or minimally deprived smokers decreased event-related activation during reorienting of attention (Thiel and Fink, 2008; Vossel et al., 2008); and selective and divided attention (Hahn et al., 2009). Moreover, nicotine enhanced deactivation in posterior and temporal regions in both smokers and nonsmokers during a reflexive shifting of attention (Ettinger et al., 2009). Finally, smoking decreased ACC activation in minimally deprived smokers during a task probing attentional control processes (Azizian et al., 2010). In sum, the extant literature suggests that nicotinic enhancement of attention (Levin et al., 2006) may be related to decreases in transient brain activation.
The current study extends previous neuroimaging studies by investigating the effects of smoking abstinence (24-hrs) using a well-validated sustained attention task—the rapid visual information processing (RVIP) task. In order to investigate whether smoking abstinence might differentially modulate the temporal dynamics of SA, we utilized a mixed block/event-related fMRI paradigm that dissociates sustained (i.e. over the duration of the task) and transient (i.e. trial-related) brain activation. Similar designs have resulted in the identification of dissociable cognitive control networks associated with sustained versus transient cognitive operations (de Frias et al., 2009; Fales et al., 2008; Jimura and Braver, 2009). In line with previous studies of the effects of nicotine administration, we hypothesized that smoking abstinence would decrease sustained activation in regions associated with the top-down control of SA, including the right PFC; but would increase sustained and/or transient activation in other regions recruited in an effort to maintain performance.
Materials and methods
Participants
Twenty-three adult smokers recruited from the community completed all aspects of the study. To be enrolled, participants had to report smoking ≥ 10 cigarettes per day for at least two years, have an afternoon expired-air carbon monoxide (CO) level greater than 10 ppm (in order to establish smoking status), be right-handed, free of serious health problems (e.g., hypertension), not currently undergoing treatment for a psychiatric illness, free of medications altering CNS functioning, test negative for illicit drug use by urinalysis, not have any conditions making MRI research unsafe, and among females, have a negative serum pregnancy test. Data from two subjects were unusable due to blurry vision and excessive head motion, respectively. In addition, one subject’s data was only included in sustained analyses due to button box malfunction. Thus, the final sample consisted of 20 smokers for sustained analyses and 19 smokers for transient analyses.
Procedures
All participants first completed a 1.5-hr screening session in which they read and signed an informed consent form approved by the Institutional Review Board, completed questionnaires regarding smoking history and suitability for fMRI research and provided a breath and saliva sample.
Participants who passed screening then completed one to five, 1-hr training sessions for the purpose of training participants on the RVIP task to a stable level of performance. This was typically achieved over the course of 1 to 3 visits to the lab, taking place over a 1 to 2 week period. In order to pass the training phase, participants had to achieve at least 50% accuracy on the RVIP on two practice tasks within 11 attempts (mean number of attempts = 6.0). During the training phase, participants were also placed in a mock scanner in order to habituate them to the scanning environment.
Following the training phase, participants completed two fMRI sessions that were identical except that prior to one session they were allowed to smoke their usual amount of cigarettes up until entering the scanning facility (satiated condition) while in the other session they were required to be 24-hrs abstinent from smoking prior to, and following, scanning (abstinent condition). Order of condition was randomly assigned and counterbalanced. The mean number of days between scans was 6.15 (SD = 2.87; min = 2 days, max = 12 days).
Expired air CO concentrations were measured using a handheld CO monitor (Vitalograph Inc., Lenexa, KS) and was calculated by subtracting the background (ambient) CO from the peak CO reading. Criterion CO in the abstinence condition was ≤ 6 ppm. Saliva samples were stored in an on site −80° freezer. Assays for cotinine (collected at screening and used to characterize the sample) and nicotine (collected at scanning sessions and 24-hr quit check and used to characterize manipulation effects on nicotine levels) were performed using gas chromatography modified for use of a capillary column (Jacob et al., 1981).
fMRI Task
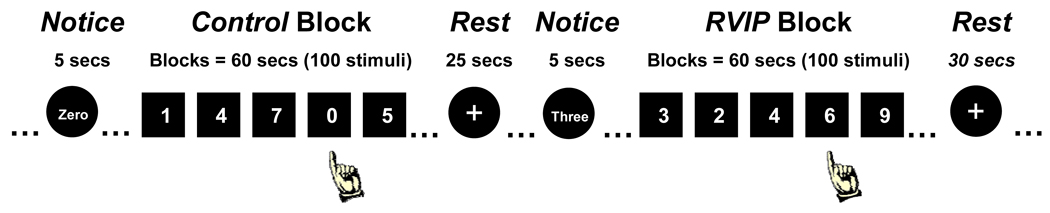
The task used in the present study (see Figure 1) was similar to that used in other fMRI studies of sustained visual attention (Coull et al., 1996; Lawrence et al., 2003; Lawrence et al., 2002). Each block of the task consisted of 60 s of single digits presented centrally at a rate of 100 digits/min (600 ms SOA). A total of 8 blocks were presented in a boxcar design—half of which were RVIP blocks and half of which were Control blocks. During RVIP blocks, the participants’ task was to press a button after each sequence of three odd (e.g., 5-1-3) or three even (e.g., 8-2-4) digits. During Control blocks, the task was to press a button each time the digit ‘0’ appeared. In both block types, targets were pseudorandomly distributed so that a total of 4–5 targets appeared in each block. Between each block a 30 s rest period occurred in which participants looked at a crosshair (Rest condition). Prior to each block, participants were notified of the target type for the following block. Total time for functional scanning during the RVIP was approximately 12.5 minutes and it was completed twice during each scanning session. The task resulted in performance measures including proportion of correctly identified targets and reaction time (RT) in response to correctly identified targets during both RVIP and Control blocks.
Figure 1.
The sustained attention task was comprised of Control and RVIP blocks. In control blocks, targets were the number ‘0’; in RVIP blocks, targets were the 3rd odd or even number presented in a row. Between each block were rest (25 s) and notice (5 s) periods.
Imaging Methods
A 4.0-T GE LX NVi scanner with 41 mT/m gradients was used for image acquisition. Blood-oxygen-level dependent (BOLD) functional images were collected for 34 contiguous axial slices (4 mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures. An inverse spiral pulse sequence sensitive to BOLD contrast was used, with TR = 1.5 s, TE = 6 ms, FOV = 24 cm, matrix = 64 × 64, flip angle = 60°, and in-plane resolution = 3.75 mm2. After completion of the functional data collection, a T1-weighted 3D fast spoiled gradient recalled (FSPGR) structural image was collected for 68 slices (1.9 mm thick) with TR = 12.3 s, TE = 5.4 ms, FOV = 24 cm, matrix = 256 × 256, flip angle = 20°, and in-plane resolution = 0.9375 mm2.
Preprocessing was conducted using statistical parametric mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London) to remove noise and artifacts. The first four volumes of each run were discarded to allow for T1 stabilization. All functional images underwent correction for acquisition timing and for head motion using rigid-body rotation and translation (Friston et al., 1994). Each participant’s data was then subsequently warped into a standard stereotaxic space (Montreal Neurological Institute) with an isotropic 2 mm voxel size and smoothed with an 8 mm FWHM Gaussian filter.
The goal of neuroimaging analysis was to identify voxels revealing significant effects of smoking condition either alone (Condition: abstinent, satiated) or in interaction with task difficulty level (Task: RVIP, control) on sustained and transient activation. Each participant’s data from each session was entered into two separate first-level whole brain analysis using the General Linear Model (Friston et al., 1994), one to examine sustained activation and the other to examine transient activity. For the sustained activation first level model, each task block (RVIP or control) was modeled as a boxcar function convolved with a canonical hemodynamic response function (HRF) that began at the onset of the first stimulus in the block and ended with the last (duration = 60 sec). Motion parameters were also included as nuisance covariates and a high-pass filter (128 sec) was applied to remove slow signal drift. Regressors for each task block during each condition for each participant were then entered into a 2 (Task: RVIP, control) × 2 (Condition: satiated, abstinent) random effects ANOVA where the main effects of task, condition, and task × condition interactions were evaluated.
To examine transient activation, targets for each task block were binned into one of two categories: correctly identified targets or errors of omission. Errors of commission were also modeled. Each event was coded as a delta regressor and convolved with a canonical HRF plus a temporal derivative. Errors (omission and commission) during each task block and motion parameters were included in the model as nuisance covariates. A high-pass filter was applied to remove slow signal drift. Similarly to the sustained analyses, regressors for each task block during each condition were entered into a 2 (Task: RVIP, control) × 2 (Condition: satiated, abstinent) × 2 (Basis function: canonical, temporal) random effects ANOVA. Main effects of task, condition, and task × condition interactions were evaluated across basis functions. Across analyses, all activations were considered significant at p ≤ 0.001 (uncorrected) with a minimum cluster extent threshold of 8 contiguous voxels. Significant interactions were further evaluated by conducting two-tailed t-tests (α = .05) on extracted % signal change values.
Results
Sample characteristics
The final sample (n = 20) was 55.0% female (n = 11). Reported racial/ethnic group membership was 80.0% Caucasian (n = 16), 15.0% African-American (n = 3), and 5.0% Asian-American (n = 1). Mean age was 29.6 years (SD = 7.5). The sample reported smoking a mean of 17.9 cigarettes/day (SD = 3.8, min = 10, max = 27) for 12.4 years (SD = 6.6). Mean Fagerström test of nicotine dependence (Heatherton et al., 1991) score was 4.6 (SD = 1.4, min = 2, max = 7) suggesting the sample was moderately nicotine dependent. At screening, mean expired breath CO concentration was 26.1 (SD = 13.0) whereas mean salivary cotinine was 227.8 ng/ml (SD = 133.0).
Biochemical confirmation of abstinence
Expired breath CO concentrations indicated compliance with study requirements. In the satiated condition, mean CO level (ppm) was 24.5 (SD = 10.4) whereas in the 24-hr abstinent condition mean CO was 2.4 (SD = 1.3). Mean CO 24 hrs after the abstinent fMRI session was 2.0 (SD = 2.1).
The results of salivary nicotine analyses were consistent with CO results and also indicative of compliance. In the satiated and abstinent conditions, mean salivary nicotine (ng/ml) was 506.8 (SD = 359.9) and 19.8 (SD = 19.7), respectively. Mean salivary nicotine 24 hrs after the abstinent fMRI session was 21.6 (SD = 23.0), again indicating compliance with the instruction to maintain abstinence for 24 hrs following the abstinent condition scanning session.
Behavioral data
Performance during scanning was evaluated as a function of Condition (satiated, abstinent) and Task (RVIP, control). Across smoking conditions, accuracy was worse and RTs longer during RVIP as compared to Control task blocks, F(1,18) = 110.3, p<.001 and F(1,18) = 19.8, p<.001, respectively (see Figure 2). Across tasks, smoking abstinence significantly decreased accuracy, F(1,18) = 5.0, p = .038, but not RT. Task × Condition interactions were not observed.
Figure 2.
Mean and standard deviation for accuracy and reaction time (RT) data for the sustained attention task. Participants were more accurate (left panel) and had shorter RTs (right panel) during Control versus RVIP blocks (main effects Task; p’s < .001). Accuracy was worse in the satiated as compared to abstinent condition (main effect of Condition; p < .05).
fMRI Results: Task Activation
Compared to rest, sustained and transient activation during the RVIP and Control tasks was highly consistent with previous studies of SA and target detection (see Figure S1). In both tasks, sustained activation was observed in precentral gyrus, frontal, occipital and parietal cortices, supplementary motor area (SMA), and dorsal striatum. Likewise, transient activation was observed in frontal and parietal cortices, SMA, and striatum; but also insula, anterior and posterior cingulate, and thalamus.
fMRI Results: Sustained Activation
Across smoking conditions, activation during the two tasks was differentiated in only one region—the right angular gyrus. In this region, activation was greater during the control task relative to the RVIP task. Across tasks, sustained activation was greater in the satiated condition, compared to the abstinent condition, in right inferior and middle frontal gyri (see Table 1, Figure 3). Finally, a Task × Condition interaction was observed in left cerebellum where activation was greater during the RVIP task in the abstinent condition compared to all other Task × Condition combinations.
Table 1.
Sustained activation functional magnetic resonance (fMRI) results showing main effects of condition.
| Side | Brain Area | Brodmann Area |
Cluster Size (mm3) |
MNI x,y,z |
Zmax |
|---|---|---|---|---|---|
| Abstinent > Satiated | |||||
| no significant areas of activation | |||||
| Satiated > Abstinent | |||||
| R | Inferior Frontal Gyrus | 44 | 96 | 44, 14, 14 | 3.43 |
| R | Middle Frontal Gyrus | 46 | 152 | 40, 40, 10 | 3.43 |
Figure 3.
Sustained activation functional magnetic resonance (fMRI) results. A significant main effect of Condition (satiated, abstinent) was observed in inferior frontal and middle frontal gyri (IFG and MFG, respectively). In both regions, sustained activation was greater in the satiated as compared to abstinent condition across tasks.
fMRI Results: Transient Activation
Main effect of task
Relative to control targets, correctly identified RVIP targets evoked extensive transient activation in regions including frontal, cingulate and occipital cortices, thalamus, hippocampus, parahippcampus, insula, caudate and precuneus (see Table S1). Control targets, compared to RVIP targets, resulted in activation in left middle occipital gyrus, right supramarginal gyrus, right supplementary motor area and cuneus.
Main effect of condition
Across tasks, transient activation in response to correctly identified targets was greater in the abstinent as compared to satiated condition in frontal, parietal, occipital and temporal cortices, and cerebellum (see Table 2, Figure 4). Greater transient activations in the satiated versus abstinent condition were not observed.
Table 2.
Transient activation fMRI results.
| Side | Brain Area | Brodmann Area |
Cluster Size (mm3) |
MNI x,y,z |
Zmax |
|---|---|---|---|---|---|
| Main Effect of Condition | |||||
| Abstinent > Satiated | |||||
| L | Superior Parietal Gyrus | 5 | 80 | −34 −48 68 | 4.58 |
| L | Precuneus | 19 | 944 | −18 −48 0 | 3.84 |
| R | Superior Frontal Gyrus | 6 | 320 | 24 14 66 | 3.67 |
| L | Precentral Gyrus | 6 | 80 | −44 4 50 | 3.22 |
| R | Superior Occipital Gyrus | 7 | 352 | 20 −78 40 | 3.6 |
| L | Lingual Gyrus | 19 | 176 | −26 −52 −8 | 3.49 |
| R | Middle Temporal Gyrus | 21 | 112 | 58 −22 −10 | 3.46 |
| L | Middle Temporal Gyrus | 22 | 248 | −48 −52 20 | 3.42 |
| L | Cerebellum | 384 | −4 −70 −12 | 3.63 | |
| Satiated > Abstinent | |||||
| no significant areas of activation | |||||
| Task × Condition Interactions | |||||
| R | Inferior Frontal Gyrus | 44 | 648 | 42 28 14 | 3.77 |
| R | Angular Gyrus | 39 | 136 | 46 −78 34 | 3.62 |
| R | Middle Frontal Gyrus | 6 | 88 | 52 −2 52 | 3.43 |
| L | Cuneus | 31 | 64 | −18 −56 28 | 3.57 |
Figure 4.
Transient activation fMRI results. A significant main effect of Condition (satiated, abstinent) was observed in multiple brain regions (see Table 1) including the lingual (LG), superior frontal (SFG) and superior occipital gyri (SOG). In each region, activation was greater in the abstinent as compared to satiated condition across tasks.
Task × Condition interactions
Task × Condition interactions were observed in right middle and inferior frontal gyri, right angular gyrus and left cuneus (see Table 2 and Figure 5). As can be seen in Figure 5, smoking abstinence selectively increased transient % signal change in response to RVIP targets in each cluster.
Figure 5.
Transient activation results depicting significant Condition (satiated, abstinent) × Task (control, RVIP) interactions. Significant interactions were observed in inferior (IFG) and middle (MFG) frontal gyri, angular gyrus (AnG) and Cuneus (Cun). In each region, the amplitude of transient activation (in % signal change) was greatest in response to RVIP targets during the abstinent condition (red bars). * denotes a significant (p < .05) difference between conditions.
Discussion
The present study examined the neural basis of the effects of smoking abstinence on sustained attention (SA). A mixed-design imaging paradigm was used to dissociate sustained and transient activation during SA following smoking as usual and 24-hr abstinence. Compared with smoking as usual, smoking abstinence worsened accuracy, decreased sustained activation in right frontal regions but increased transient activation in response to targets across distributed cortical areas. In addition, in a small set of mostly right hemisphere cortical areas, smoking abstinence selectively increased transient activation during the more difficult RVIP task. These findings support the hypothesis that smoking abstinence decreases the efficiency of brain regions involved in the top-down control of SA but also suggest that additional transient operations necessary for target detection are recruited in an effort to maintain performance.
Sustained activation
The results of this study provide compelling evidence that abstinence-induced deficits in SA are mediated via disruption of right frontal lobe function. Right frontal regions specifically deactivated by abstinence in the present study were portions of the inferior and middle frontal gyrus that have been shown in previous studies to be active during SA (Coull et al., 1996) and SA-dependent tasks (e.g. working memory, Wager and Smith, 2003). Previous neuroimaging and human lesions studies have demonstrated that the right prefrontal cortex is a critical substrate of SA (Cabeza and Nyberg, 2000; Shallice et al., 2008; Sturm and Willmes, 2001) involved in the effortful initiation and control of tonic alertness or vigilance (Coull et al., 1996; Fan et al., 2005; Fernandez-Duque and Posner, 2001; Mottaghy et al., 2006; Pardo et al., 1991; Sturm and Willmes, 2001). Functionally, it has been proposed that right lateral frontal areas exert top-down control of tonic alertness via activation of the norepinephrine system via thalamic-brainstem pathways (Sturm and Willmes, 2001). Thus, our findings implicate a loss of right prefrontal-mediated control as a causal factor in abstinence-induced SA performance deficits.
Transient activation
Whereas smoking withdrawal decreased sustained activation, it increased transient (event-related) activation in regions dispersed across frontal, parietal, occipital and temporal cortices, regardless of level of task demands. A significant proportion of this activation was observed in visual information processing areas—lingual and occipital gyri—previously shown to be active in response to targets in event-related fMRI studies (Clark et al., 2000; Cohen et al., 1997). Additional activations were observed in areas that support memory and attention functions including the middle temporal gyrus and superior parietal gyrus, respectively. These findings are consistent with a previous neuroimaging studies in which placebo, compared to nicotine, was associated with increased transient activation to target stimuli (Hahn et al., 2009; Thiel and Fink, 2008; Vossel et al., 2008).
In addition to these main effects of smoking condition, smoking abstinence specifically increased transient activation in response to RVIP targets in right inferior and middle frontal gyri, right angular gyrus and left cuneus. Of these, the largest cluster was observed in right IFG, a region involved in inhibitory control (Aron and Poldrack, 2005; Garavan et al., 2006), but also strongly implicated in the making of responses to task-relevant stimuli, independent of inhibition (Hampshire et al., 2009). The greater involvement of right IFG and MFG following abstinence, but selectively during the more demanding RVIP task, suggests that transient executive processes are recruited when sustained activation in nearby areas is disrupted.
Interpreting the shift from sustained to transient activation
The results of this study can be interpreted within the context of the dual mechanisms of control (DMC) theory (Braver et al., 2009). Briefly, within this framework, proactive cognitive control involves the active, top-down initiation and maintenance of task-related processes over extended periods of time. By contrast, reactive cognitive control is transient and occurs in response to an imperative stimulus. Proactive control is mediated largely via prefrontal circuits, is dopamine dependent and is associated with sustained neural activation; reactive control is more widely distributed across the brain and is reflected by transient neural activity.
The finding that smoking abstinence results in a shift from frontal, sustained activation to more widely distributed transient activation is entirely consistent with DMC theory, which predicts increased reactive control in the face of disrupted proactive control. Recent studies supporting DMC have observed simultaneous decreased sustained but increased transient activation among high anxious (Fales et al., 2008) and older (Jimura and Braver, 2009) individuals. Though the exact cause for disruption of proactive control cannot be pinpointed in the present study, smoking withdrawal disrupts dopaminergic, noradrenergic and cholinergic activity in frontal cortex (Watkins et al., 2000) and these systems all play a critical role in the maintenance of frontally mediated arousal and/or sustained attention (Hahn et al., 2002; Levin et al., 2006). Studies that either measure (e.g. positron emission tomography) or probe these neurotransmitter systems via drug administration (e.g. DA agonist administration) can potentially identify factors leading to disruption of proactive control.
Sustained attention versus working memory effects
The RVIP is typically referred to as a SA task. However, the task places significant demands on working memory (WM) as identifying targets requires short-term storage and updating of information about the two preceding stimuli. Moreover, the task has been shown to activate a neuronal network that overlaps substantially with WM tasks including the n-back task (Coull et al., 1996; Lawrence et al., 2003). Whereas previous studies have examined the effects of nicotine and smoking abstinence on WM, the results of the present study are interpreted as SA effects for several reasons. First, to a large extent, findings were observed across the RVIP task and the Control task, which like the 0-back variant of the n-back, has little or no WM load. The exception was Task × Condition interactions in which greater transient activation was observed in response to RVIP targets in the abstinent condition in mostly right hemisphere regions. These findings might be indicative of greater WM processing during such conditions and would be consistent with a previous study finding greater frontal activation, albeit sustained activation, in frontal areas during smoking abstinence (Xu et al., 2005). Second, unlike studies of nicotine or smoking effects on n-back performance (Mendrek et al., 2006), we observed similar decrements in accuracy during abstinence for the RVIP and Control task. This again suggests that abstinence effects were on cognitive processes common (i.e. SA) to those two tasks. Finally, we hypothesize that the observed lack of differences between abstinence effects on RVIP and Control tasks may be due to the fact that participants were highly practiced on the RVIP task prior to scanning. Such practice may have significantly reduced demands on WM processes and resulted in similar patterns of activation between the two tasks (Garavan et al., 2000). Thus, while the RVIP has a significant WM component, based on the above we interpret the majority of effects observed in the present study as more indicative of abstinence effects on SA.
Individual differences in sustained and transient activation
In addition to the main analyses of the study, we also conducted a number of exploratory analyses examining relations between FTND score and abstinence-induced changes in sustained and transient activation in both cluster- and voxel-level data. Voxel-wise analyses produced a single cluster of positive correlation in right occipital cortex (36, −70, 6; 136 mm3) suggesting that greater dependence was associated with greater transient activation on the abstinent relative to satiated condition in this region. No other correlations were observed. The lack of such correlations may be due to the fact that the sample was selected to be moderate-to-high in nicotine dependence, that the manipulation was highly robust (thus restricting individual differences in response) or a lack of power. Future studies in samples with wider ranges of nicotine dependence severity and/or a wider range of abstinence manipulations (e.g., 2 hr, 12 hr) might help elucidate this question.
Limitations, Implications and Conclusions
Strengths of the present study include the use of a robust withdrawal manipulation (McClernon et al., 2009), an fMRI task that allows for modeling of both sustained and transient brain activation, and a standard, well-validated task. There were, however, several potential limitations. Because smokers were assessed only under conditions of satiety and abstinence, it is not possible to assess whether the observed effects are due to the effects of withdrawal (in the abstinent condition) or the effects of nicotine (in the satiated condition). Future studies that assess neurocognition beyond the withdrawal period or in non-smokers can help address this question. In addition, given the nature of the manipulation, the study was not blinded and as such, cognitive performance and brain activation may have been biased by smokers’ expectations regarding the effects of abstinence. The design lacked a nonsmoker control group. While comparisons to such a group might be of some interest, any observed group differences could be attributed to a number of factors including smoking history and drug state. In addition, the crossover nature of our design, while it controls for order effects, limits generalizability to the clinically relevant case of smoking cessation. Moreover, although we are unaware of evidence suggesting motivation to quit modulates cognitive performance, the inclusion of smokers not specifically interested in quitting smoking limits our ability to generalize to smoking cessation patients. Together, these factors leave open the question of whether shifts in the temporal dynamics of neurocognition would be observed following a quit attempt—a question that can be addressed with future research.
In contrast with a previous study (Lawrence et al., 2003) we did not observe robust differences in sustained activation between the RVIP and control tasks. One factor that may account for this is that in the present study, the RVIP task was practiced multiple times by participants prior to scanning and practice on similar cognitive tasks has been shown to result in decreases in brain activation (Garavan et al., 2000; Kelly and Garavan, 2005). Thus, while we do not have data from minimally practiced participants for comparison, we speculate that intensive practice may have decrease sustained activation on the RVIP relative to control task. Future studies can assess the interactive effects of task practice and smoking abstinence to more directly address this question.
Our findings suggest that smoking abstinence results in a qualitative change in neurocognition supporting sustained attention. These changes may apply to other forms of relevant cognition including working memory (Mendrek et al., 2006; Patterson et al., 2009), reactivity to drug cues (McClernon et al., 2009) and decision making (Baranger et al., in preparation). Smoking abstinence, for instance, has been shown to disrupt brain function during working memory (Xu et al., 2005), and to a greater degree among carriers of a specific polymorphism of the catechol-O-methyltransferase (COMT) gene (Loughead et al., 2009). These studies have only examined sustained activation which leaves open the question of whether compensatory transient activation is observed for working memory, and whether changes in transient and sustained activation represent two distinct phenotypes. Smoking cessation outcomes, for instance, may be differentially predicted by the degree to which smoking abstinence disrupts sustained, proactive versus increases transient, reactive cognition.
Smoking cessation significantly disrupts multiple forms of cognition including SA. The resulting deficits not only serve to negatively reinforce smoking behavior, but may also thwart attempts to actively engage in coping strategies aimed at maintaining abstinence. Moreover, these deficits may be of greater magnitude among individuals with psychiatric comorbidities including schizophrenia (Kumari and Postma, 2005) and attention deficit hyperactivity disorder (McClernon and Kollins, 2008) which may explain higher rates of smoking in these populations. The results of the present study provide new information regarding the neural mechanisms that underlie abstinence-induced deficits in cognition by examining the temporal dynamics of brain activation during smoking abstinence. We found that smoking abstinence results in a qualitative shift in neurocognition from sustained, frontally mediated processes to transient and more widely distributed processes. These findings are both predicted by, and provide additional support for DMC theory which proposes that transient, reactive cognitive control emerges when sustained, proactive control is disrupted. Future research can assess whether these two processes vary across smokers as a function of various factors including genetic variation and whether they are differentially predictive of smoking cessation outcomes.
Supplementary Material
Acknowledgements
We thank Natalie Goutkin and Amy Brightwood for their assistance with data acquisition.
This research was supported by NIDA grants K23DA017261 and R01 DA023516 to FJM and by an unrestricted research grant from Philip Morris USA, Inc to Dr. Rose.
Dr. Rose reports research funding from Vector Tobacco Co. and Philip Morris USA, Inc.; and receiving consulting fees from the National Institute on Drug Abuse, Novartis Pharmaceuticals, GlaxoSmithKline, Inc., Philip Morris International, Catalyst Pharmaceutical Partners, Lorillard, University of Kentucky, Medacorp, Pharmalink, Targacept, and Noble Medical Consulting Group. Dr. Froeliger reports having research funding from the National Institute on Drug Abuse. Dr. McClernon reports having research funding from the National Institute on Drug Abuse and the Atkins Foundation.
Footnotes
Ms. Kozink and Ms. Lutz report no conflicts of interest.
Authors contribution
FJM and JR were responsible for the study concept and design. AL and RK contributed to the acquisition of the data. RK and FJM were responsible for data analysis, interpretation of findings, and drafting of the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a stroop task. Neuropsychopharmacology. 2010;35:775–782. doi: 10.1038/npp.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in Working Memory. Oxford: Oxford University Press; 2009. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Marklund P, Eriksson E, Larsson A, Oman L, Annerbrink K, Backman L, Nilsson LG, Nyberg L. Influence of COMT Gene Polymorphism on fMRI-assessed Sustained and Transient Activity during a Working Memory Task. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21318. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Williams SC, Patel D, Michel TM, Nwaigwe A, Caceres A, Mehta MA, Anilkumar AP, Kumari V. Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–561. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Posner MI. Brain imaging of attentional networks in normal and pathological states. J Clin Exp Neuropsychol. 2001;23:74–93. doi: 10.1076/jcen.23.1.74.1217. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Human Brain Mapping. 1994;1:153–171. [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, Zuo Y, Huggenvik J, Botros N. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine Tob Res. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Estes SL, Welser R. Does noise stress modulate effects of smoking/nicotine? Mood, vigilance, and EEG responses. Psychopharmacology (Berl) 1997;129:382–389. doi: 10.1007/s002130050204. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Neurosci. 2009;9:103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Thaker GK, Stein EA. Nicotine Enhances but Does Not Normalize Visual Sustained Attention and the Associated Brain Network in Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jimura K, Braver TS. Age-Related Shifts in Brain Activity Dynamics during Task Switching. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Willmes K, Horwitz B, Muller HW, Krause BJ, Sturm W. Systems level modeling of a neuronal network subserving intrinsic alertness. Neuroimage. 2006;29:225–233. doi: 10.1016/j.neuroimage.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D. The multiple dimensions of sustained attention. Cortex. 2008;44:794–805. doi: 10.1016/j.cortex.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Fink GR. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152:381–390. doi: 10.1016/j.neuroscience.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Behavioral and neural effects of nicotine on visuospatial attentional reorienting in non-smoking subjects. Neuropsychopharmacology. 2008;33:731–738. doi: 10.1038/sj.npp.1301469. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.