Abstract
Recent data supports increased expression of PD-1, a negative regulator of immune function, is associated with T-cell exhaustion during chronic viral infection. However, PD-1 expression during acute infection and vaccination has not been studied in great detail in primates. Here we examine PD-1 expression on CD3+ T cells following DNA vaccination or lentiviral infection of macaques. Ex vivo peptide stimulation of PBMCs from DNA-vaccinated uninfected macaques revealed a temporal increase in PD-1 expression in proliferating antigen-specific CD8+ T cells. Following the initial increase, PD-1 expression steadily declined as proliferation continued with a concomitant increase in IFN-γ secretion. Subsequent examination of PD-1 expression on T cells from uninfected and lentivirus-infected non-vaccinated macaques revealed a significant increase in PD-1 expression with lentiviral infection, consistent with previous reports. PD-1 expression was highest on cells with activated memory and effector phenotypes. Despite their decreased telomere length, PD-1hi T-cell populations do not appear to have statistically significant uncapped telomeres, typically indicative of proliferative exhaustion, suggesting a different mechanistic regulation of proliferation by PD-1. Novelly, our data indicate PD-1 expression is increased as a result of T-cell activation during a primary immune response as well as during persistent immune activation in macaques.
Keywords: PD-1, HIV, SIV, exhaustion, vaccine
Introduction
Recent evidence suggests that the inability of T cells to clear chronic infections in mouse and humans is directly related to exhaustion of viral-specific CD8+ T cells. The nature of this exhaustion remains unclear. Barber et al. reported the expression of PD-1 on CD8+ T cells was correlated to an inability to mediate effective clearance of LCMV in chronically infected mice[1]. This effect could be abrogated by blockade of the PD-1/PD-L1 signaling pathway with an antibody against PD-L1, which subsequently restored CD8+ T-cell function in these animals[1]. Further studies in humans demonstrated that PD-1 is increased on human immunodeficiency virus- (HIV) specific T cells and is involved in the suppression of T-cell responses during chronic HIV infection[2–4]. Interestingly, Petrovas et al.[3] demonstrated that lower levels of cytokine production following PD-1 ligation were a result of suppressed proliferative abilities rather than a direct inhibition of cytokine secretion. Increased PD-1 expression correlated with higher spontaneous levels of apoptosis, which could be enhanced by the addition of agonist anti-PD-1 antibody[3]. Furthermore, PD-1 expression also augmented Fas-mediated apoptosis, suggesting there may be an interaction between the two pathways[3].
PD-1 expression on T cells specific for other human viral infections were also examined, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), and vaccinia virus (VV). These studies collectively show that EBV, but not CMV or VV, also had elevated levels of PD-1 on antigen-specific T cells, suggesting PD-1 expression is directly related to persistent antigen exposure[2–4]. PD-1 was also examined in relation to the state of T-cell activation and shown to be expressed on central memory (CM), effector memory (EM) and effector T-cell subsets[3], but not naive T cells[3, 4]. Consistent with these findings, Trautmann et al.[4] found that aviremic individuals on HAART therapy demonstrated a reduced level of PD-1 expression compared to viremic individuals, likely due to high ongoing levels of antigen exposure. This group also demonstrated that PD-1 expression increased in patients during HAART interruption and that this increase in PD-1 correlated with higher viral loads. Similarly, Day et al.[2] demonstrated that PD-1 expression inversely correlated with CD4+ T-cell counts, a clinical marker for immune health. Together, these very important data indicate that PD-1 expression is a marker for T-cell activation that correlates with HIV disease progression and negatively correlates with patient health.
Lentiviral infection of non-human primates (NHPs) currently provide the best models for HIV infection in humans[5]. However, there is limited information regarding PD-1 expression in macaques and the temporal relationship between T-cell activation and PD-1 expression has not been previously explored in detail, particularly in a vaccine setting. Here, we examined the expression of PD-1 on ex vivo-stimulated antigen-specific T cells from vaccinated macaques and on T-cell subsets from uninfected and infected macaques. Our data demonstrate PD-1 expression is associated with activation regardless of infection state and that infected macaques have overall higher levels of PD-1 expression compared to their uninfected counterparts. Finally, we demonstrate that T cells from infected macaques do not have significant levels of uncapped telomeres, which suggests the functional T-cell impairment observed during chronic infection may not be solely due to T-cell proliferative exhaustion, but possibly to more complex immunoregulatory control as a result of the continual activation of T cells. Understanding the underlying mechanisms driving PD-1 expression and their role in immune senescence may aid in the development of novel immune therapies for treatment of chronic infections, cancer, and autoimmune disease.
Results
Transient high-level PD-1 expression on CD8+ T cells following peptide stimulation
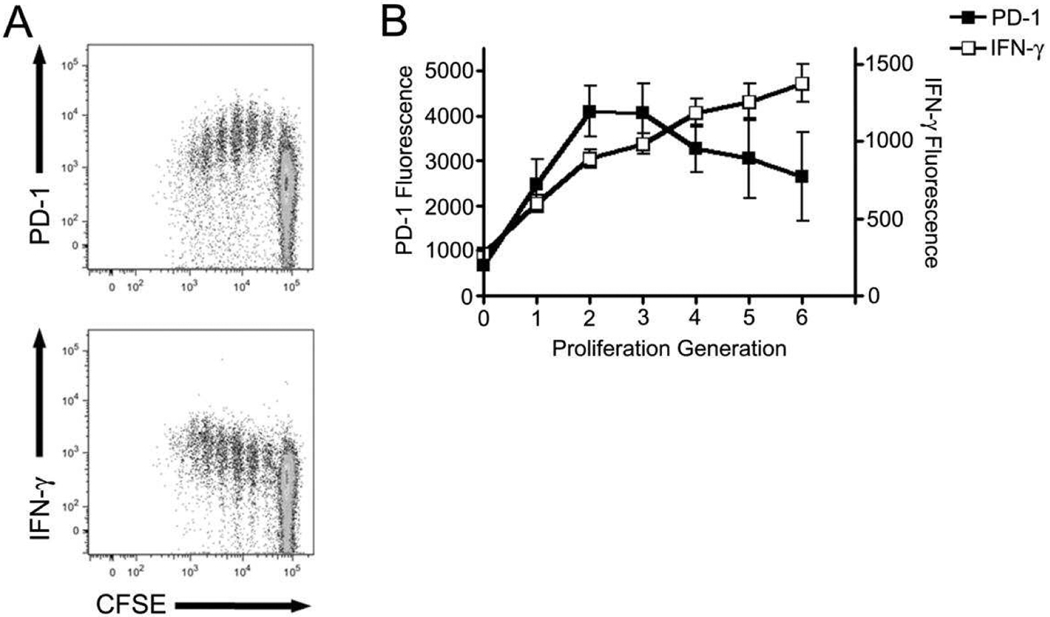
PD-1 expression has been described in mice[1], humans[2–4] and, most recently, non-human primates[6, 7]. However, to our knowledge, no one has examined the temporal relationship between activation-induced T-cell proliferation and PD-1 expression. We obtained PBMCs from cynomolgus macaques four months following a fourth immunization with poly-optimized plasmid DNA constructs expressing SIVgag, SIVenv, and SIVpol. Cells were labeled with CFSE and stimulated for five days with SIVpol peptides, with protein transport inhibitors added during the final six hours of incubation. Phenotypic and intracellular cytokine staining was then performed and samples analyzed by flow cytometry, gating on CD8+ T cells. We observe a transient increase in PD-1 expression on antigen-specific T cells through the first three divisions following peptide stimulation (Figure 1). This increase in expression is transient as demonstrated by the gradual decline of PD-1 on the surface of proliferating T cells. Interestingly, as PD-1 expression diminished, the secretion of IFN-γ increased (Figure 1). Proliferation observed was vaccine-specific as cells incubated without peptide failed to proliferate (Supplemental Figure 1A) and animals not vaccinated failed to proliferate in response to peptide (Supplemental Figure 1B). This pattern of PD-1 and IFN-γ staining appears to be stable as similar results were obtained on cells from monkeys 10 months following a final immunization (data not shown). Consistent with previous data demonstrating the immunoinhibitory function of PD-1 our data suggest that PD-1 expression inhibits the secretion of IFN-γ. Furthermore, the data also indicate the transient nature of PD-1 expression following antigenic stimulation.
Figure 1. Temporal PD-1 expression and IFN-γ secretion following antigenic stimulation ex vivo.
Peripheral blood mononuclear cells were isolated from six pSIVpol DNA-vaccinated cynomolgus macaques three months following a fourth DNA immunization. Cells were labelled with CFSE and stimulated for 5 days with SIVpol peptides, with the addition of golgi transport inhibitors added during the final 6 hours of incubation. (A) Cells were then stained for surface expression of PD-1 (top) and intracellular accumulation of IFN-γ (bottom). (B) Analysis was performed using FlowJo software to examine the fluorescence intensity of PD-1 (solid symbols) and IFN-γ (open symbols) for each proliferation generation and then graphed using Prism software. Error bars represent ± SD.
Chronic infection increases PD-1 expression
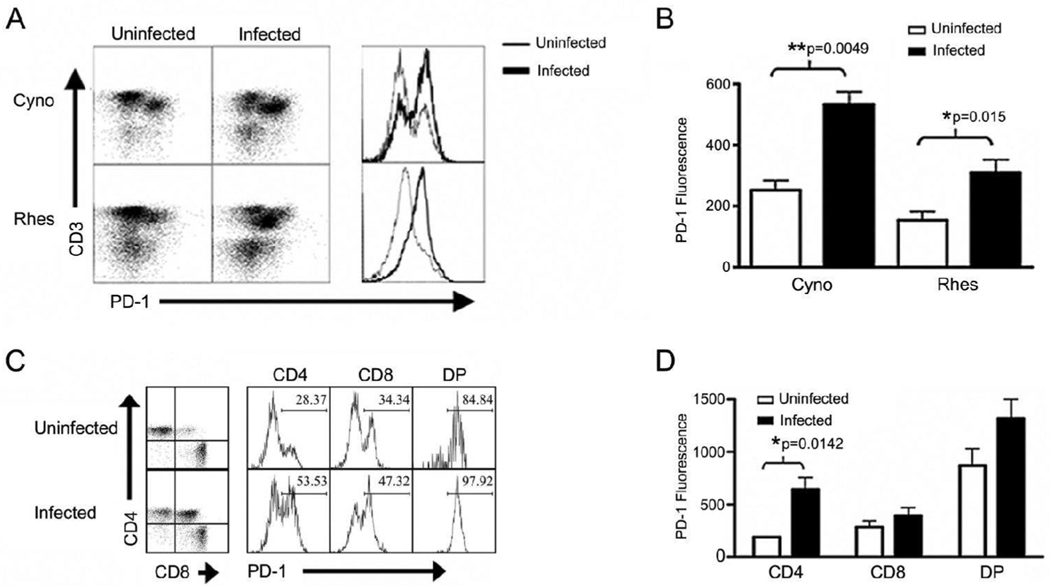
Increased expression of PD-1 on human PBMCs following stimulation has been previously described, and more recently was shown to be upregulated on HIV-specific CD8+ T cells[2–4, 8]. Because studies examining the expression of this marker in non-human primates limited, we investigated expression of PD-1 on PBMC from cynomolgus and rhesus macaques to determine if PD-1 expression is also increased in SHIV and SIV models of chronic infection (Figure 2A). PD-1 was expressed at significantly higher levels in lentivirus-infected monkeys compared to uninfected controls in both cynomolgus (p=0.0049) and rhesus (p=0.015) (Figure 2A–B) macaques. This data indicates that, similar to human lentiviral infection, PD-1 is upregulated in NHP lentiviral infection.
Figure 2. PD-1 expression in uninfected and infected macaques.
Peripheral blood mononuclear cells were stained for CD3, CD4, CD8, and PD-1 expression and analyzed by flow cytometry. PD-1 expression was evaluated on peripheral blood lymphocytes from uninfected (n=3) or SHIV-infected (n=3) cynomolgus and uninfected (n=5) or SIV-infected (n=5) rhesus macaques. (A) Dot plots show the PD-1 expression on lymphocytes from a representative animal. Histograms show the corresponding PD-1 fluorescence on CD3+ T cells, with the light and heavy lines representing uninfected and infected animals, respectively. (B) Quantification of mean PD-1 fluorescence on CD3+ T cells in uninfected (open bars) and infected (solid bars) cynomolgus and rhesus macaques using a two-tailed unpaired t test. (C) CD3+ T cells from uninfected or infected cynomolgus macaques were gated and CD4/CD8 expression used to evaluate PD-1 expression on T-cell subsets. Dot plots show the CD4/CD8 expression. Histograms show the PD-1 expression on the specific subsets from the representative animals; CD4+ CD8− (CD4), CD4− CD8+ (CD8), or CD4+ CD8+ (DP) T cells. Gates and numbers on histograms represent % PD-1+. (D) Quantification of PD-1 fluorescence on CD4+, CD8+, or DP T cells in uninfected and lentivirus-infected cynomolgus macaques using a two-tailed t test. Error bars represent ± SD.
To further characterize PD-1 expression in NHP, we examined PD-1 expression on T-cell subsets using multi-color flow cytometric analysis and determined that while there was a trend for increased PD-1 expression among all T-cell subsets in infected macaques the CD4+ T-cell compartment demonstrated a significant difference in PD-1 expression in cynomolgus macaques (p=0.0142) (Figure 2C–D) and rhesus macaques (p=0.0198) (data not shown). Interestingly, upon examination of peripheral blood T-cell subsets using flow cytometry, we observed high numbers of CD4bright CD8dim double-positive T cells in lentivirus-infected monkeys compared to uninfected controls (Figure 2C). Double-positive T cells have been described previously[9–13], with CD4bright CD8dim DP T cells arising from the upregulation of CD8 expression by CD4+ T cells[9, 13]. Surprisingly, we found that this population of T cells expresses very high levels of PD-1 in both infected and uninfected monkeys (Figure 2C–D). Importantly, expression of PD-1 was consistently higher on double-positive T cells regardless of the infection status of the monkeys. Together with our data from figure 1 the data suggest these cells have been recently activated due to their high PD-1 expression.
PD-1 expression increases in infected macaques during acute infection
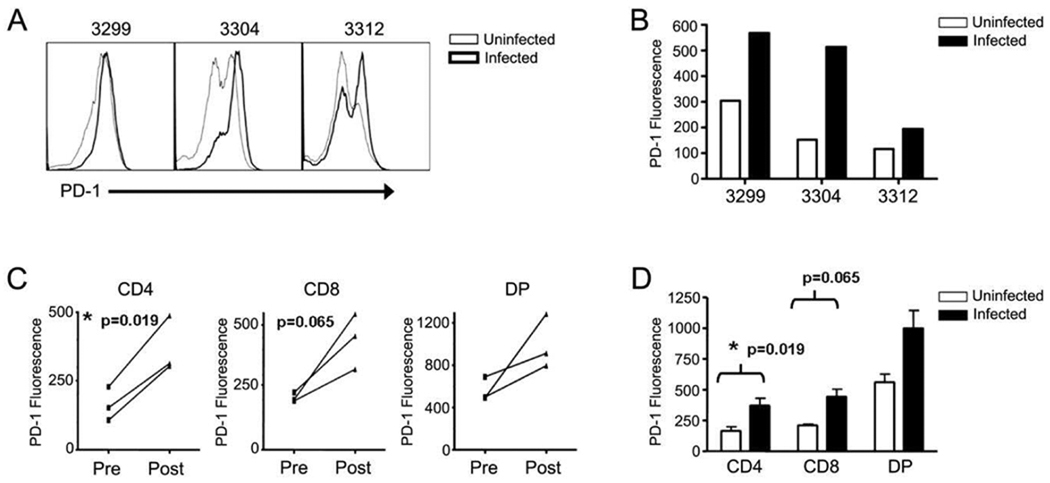
PD-1 expression was assessed in rhesus macaques prior to SIV infection and two weeks following infection. Analysis of total CD3+ T cells revealed that PD-1 expression increased on T cells during the acute phase of SIV infection (Figure 3A–B). In addition, PD-1 expression was examined on specific T-cell subsets using CD4 and CD8 expression. The data reveal that, as with chronic infection, CD4 T cells exhibited a significant increase in their levels of PD-1 following acute infection (Figure 3C–D). While obvious trends were observed in CD8+ and DP populations, significance was not reached. Together, the data indicate that PD-1 expression is increased early during infection and that this increase in PD-1 is not limited to chronic disease states.
Figure 3. PD-1 expression is upregulated during acute SIV infection.
Peripheral blood mononuclear cells from rhesus macaques prior to and two weeks following SIV infection were stained for CD3, CD4, CD8, and PD-1 and examined by flow cytometry. (A) Histrograms show the fluorescence intensity of total CD3+ T cells from uninfected (thin lines) and acutely infected (heavy lines) macaques. Numbers are animal identification. (B) Bars indicate the fluorescence intensity of total CD3+ T cells in uninfected (open bars) and acutely infected (solid bars) macaques. Numbers are animal identification. (C) CD3+ T cells were further divided into T-cell subsets based on expression of CD4 and CD8 as before. Lines represent the PD-1 fluorescence in T-cell subsets from individual animals both prior to infection (Pre) and two weeks post infection (post). (D) Quantification of PD-1 fluorescence on CD4+, CD8+, or DP T cells in uninfected (open bars) and acutely-infected (solid bars) rhesus macaques using a two-tailed paired t test. Error bars represent ± SD.
PD-1 expression on memory T-cell subsets
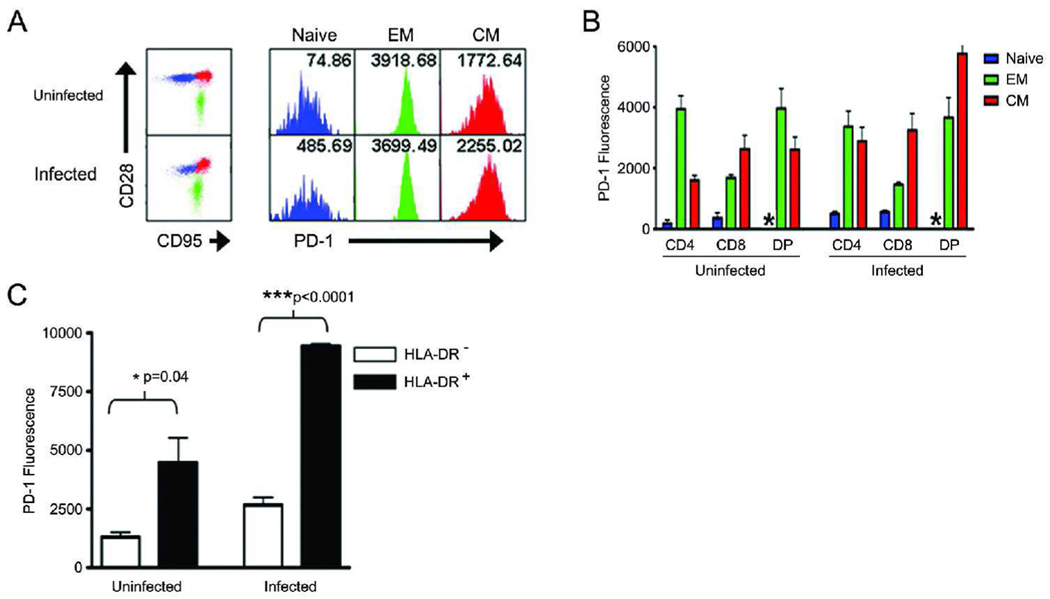
We next evaluated the expression of PD-1 on naïve and memory T-cell subsets using CD28 and CD95 expression as previously reported[14, 15]. In addition, we also labeled cells for expression of a classical CD4+ T-cell activation marker, MHC class II, and a human T-cell activation marker, CD38. As expected, naïve T cells express very low levels of PD-1 compared to both memory T-cell populations, with effector memory cells having the highest level of PD-1 expression (Figure 4A). PD-1 expression during lentiviral infection appears to be driven by an increase of PD-1 on central memory T cells rather than effector memory T cells (Figure 4B). As expected, DP T cells did not have a representative naïve population of cells, reflective of their activated state. We next examined the expression of PD-1 in relation to the expression of activation markers, such as MHC class II, on CD4+ T cells. PD-1 expression was significantly higher on HLA-DR+ cells compared to HLA-DR− cells (Figure 4C), indicating that PD-1 expression is increased upon T-cell activation. Interestingly, CD38 expression in NHPs does not correlate well with activation, as it does in humans. Indeed, naïve T cells were CD38hi, effector memory cells were CD38lo, and central memory populations were mixed CD38hi and CD38lo (data not shown). Further research is needed to clarify the role of CD38 expression in NHPs.
Figure 4. PD-1 is upregulated on memory T-cell subsets.
Periperal blood mononuclear cells were stained for CD3, CD4, CD8, CD28, CD95, HLA-DR, and PD-1 expression. (A) CD28 and CD95 expression were used to distinguish naïve (blue), effector memory (EM) (green), and central memory (CM) (red) T cells which were then evaluated for PD-1 expression (histograms). Numbers in histograms represent mean PD-1 fluorescence. (B) Quantification of PD-1 expression in T cell subsets, looking at naïve (blue), EM (green), and CM (red) populations within CD4, CD8, and DP subsets. (C) CD4+ T cells were examined for expression of HLA-DR and PD-1. Error bars represent ± SD.
Analysis of telomere length and dysfunction
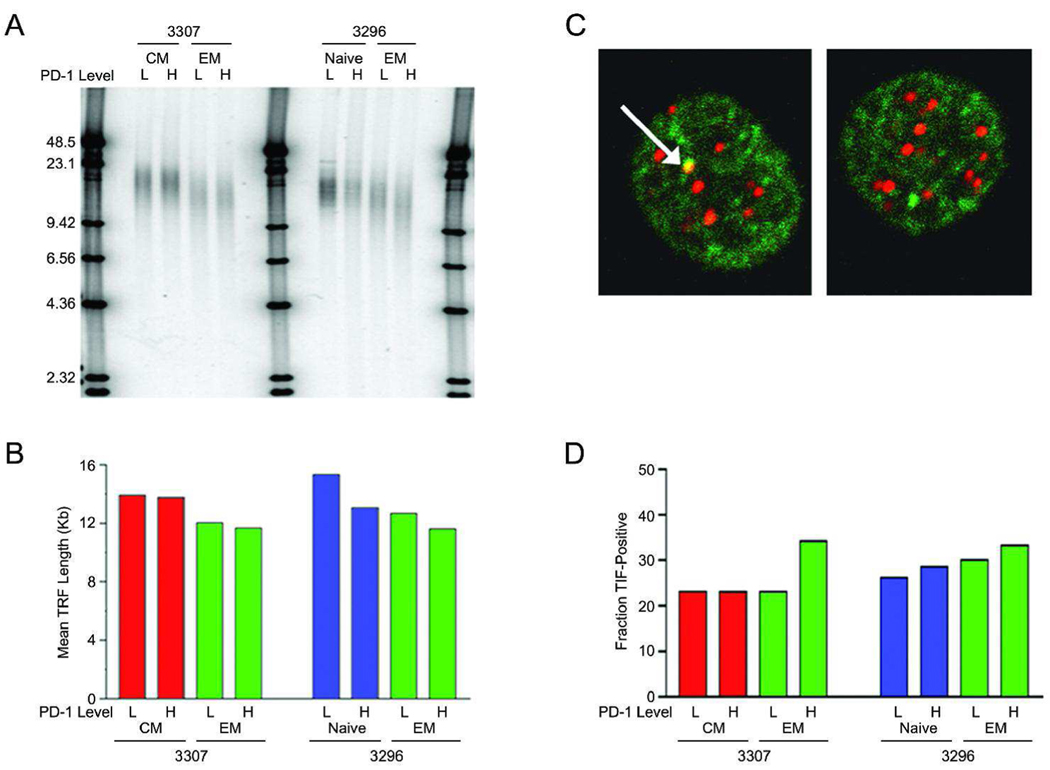
Recent data suggest PD-1 is strongly associated with the inhibition of antigen-specific T-cell proliferation during chronic infection[1–4], which could be interpreted as T-cell exhaustion. Therefore we investigated the relative level of exhausted T cells in our study by measuring telomere lengths to provide an indication of the proliferative history and possible replicative exhaustion of different T-cell subsets from infected cynomolgus macaques. Terminal restriction fragment (TRF) length analyses of naive (CD95−, CD28+), central memory (CD95+, CD28+) and effector memory (CD95+, CD28−) T lymphocytes, as well PD-1hi and PD-1lo subsets of each were examined (Figure 5A–B). As expected, naive cells had the longest telomeres and effector memory cells the shortest. Mean TRF lengths decreased by approximately two kilobases between the central and effector memory T cells in monkey 3307 and by the same amount between the naive and central memory T cells in monkey 3296 (Figure 5A–B). Assuming 50–100 bp of telomere sequence loss per cell division[16], this indicates that approximately 20–40 divisions have elapsed between these T-cell types in the infected macaques. In all cases, cells in PD-1hi fractions had shorter mean TRF lengths than PD-1low fractions. Indeed, mean TRF length was shortest in the PD-1hi subfraction of the effector memory type.
Figure 5. Telomere length measurements and TIF focus levels in T-cell subsets from infected macaques.
Peripheral blood mononuclear cells were stained for CD3, CD28, CD95 and PD-1, and separated by fluorescence activated cell sorting. (A) Genomic DNA from the different T-cell subsets was restriction digested to separate telomere repeat DNA from chromosome ends, products were separated by agarose electropheresis, denatured, and visualized with a telomere repeat probe. End-labeled DNA size markers are shown in kilobases. (B) Quantitation of mean telomere lengths for the samples in (A). 3307 are 3296 are two SHIV-infected cynomolgus macaques. (C) T-cell subsets were costained with a Cy3-labeled telomere repeat probe to visualize telomere ends and with FITC-labeled antibodies to visualize 53BP1. Overlap between the signals in TIF foci (yellow; arrowhead) was visualized by confocal microscopy. Examples of TIF-positive (left) and TIF-negative (right) cells are shown. (D) Quantitation of the fraction of nuclei containing one or more TIFs in the T-cells subsets. Differences between TIF frequencies were not statistically significant, although comparisons of data pooled from the two macaques showed a trend toward more frequent TIFs in the PD-1hi fraction versus the PD-1low fraction of effector memory cells (one tailed p-value = 0.15 by Fisher’s exact test).
To assess the functional status of telomeres in the different T cell subtypes, telomere dysfunction-induced foci (TIFs) were measured. Functional telomeres "cap" and thus prevent the ends of chromosomes from being recognized as double strand DNA breaks, but critically shortened telomeres lose capping function and thus recruit DNA repair factors, including 53BP1[17, 18]. Costaining for telomere repeat DNA and 53BP1 protein was therefore used to identify TIF foci (Fig 5C–D). TIFs were more abundant in the CD28− PD-1hi fraction from each infected monkey, consistent with these cells having the shortest mean TRF lengths (Figure 5A–B). However, the increased levels of TIFs in these cells were not statistically greater than in other T-cell subsets, and moreover, even in the CD28− PD-1hi fraction the majority of cells were TIF-negative. These results indicate that although CD28− memory cells have undergone the greatest number of cell divisions and have shorter telomeres, even in the case of the PD-1hi subfraction, replicative reserve remains.
PD-1 expression on antigen-specific T cells in SIV-infected macaques
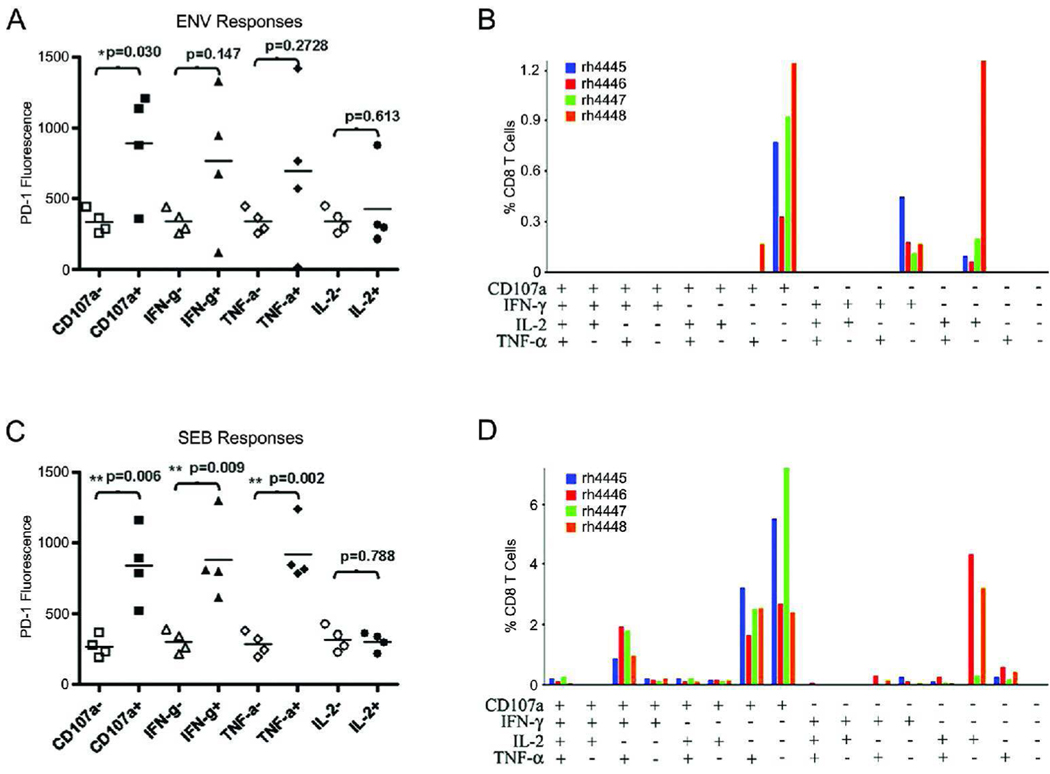
We next evaluated PD-1 expression on functionally active T cells from rhesus macaques that had been infected intravenously with SIVmac251 15 weeks prior to PBMC isolation. Functional analysis of CD8+ T cells by intracellular cytokine staining revealed an association between PD-1 expression and SIV-specific T cells. While only cells expressing CD107a demonstrated a significant difference in PD-1 expression compared to non-responding T cells, there was a similar trend observed in cells responding by IFN-γ and TNF-α. Interestingly, IL-2 secretion did not appear to be associated with PD-1 expression (Figure 6A). Polyfunctional analysis of SIV-specific analysis revealed a lack of polyfunctionality in infected animals, with the majority of responding cells expressing CD107a alone or secreting only IFN-γ or IL-2 (Figure 6B). While the differences between cells responding with IFN-γ and TNF-α. were not significant following antigenic stimulation, staphylococcal enterotoxin B (SEB) stimulation elicited very clear results, with CD8+ T cells responding by CD107a, IFN-γ, and TNF-α all having significantly higher levels of PD-1 compared to their non-responsive counterparts. Similar to what was seen with antigen-stimulated T cells, we observed no difference in PD-1 expression in cells responding by secreting IL-2 following SEB stimulation (Figure 6C). Figure 6D indicates that T cells from SIV-infected animals are capable of making polyfunctional responses with strong stimuli such as SEB.
Figure 6. Functionality of antigen-specific T cells in infected macaques.
PBMCs were collected from SIV-infected rhesus macaques (n=4) and stimulated using SIVenv peptides or SEB for 6 hours in the presence of golgi transport inhibitors. An enhanced stain was performed during the stimulation using anti-CD107a. (A,C) PD-1 expression was assessed on responding vs. non-responding CD8+ T cells following stimulation with SIVenv peptides (A) or SEB (C). Responding and non-responding cells are represented by solid and open symbols, respectively. Measurement of the differences of PD-1 expression levels between responding and non-responding T cells was measured by using a two-tailed t test. (B,D) Boolean gating was performed using FlowJo softeware to examine the polyfunctionality of the T cells from infected animals. The magnitude of the anti-SIV responses (B) or SEB responses (D) was then determined by using the Pestle and Spice programs, ignoring responses of less than 0.05%. The various bar colorations represent responses from individual animals as shown.
Discussion
In mice, PD-1 expression is known to increase on exhausted T cells and most likely exists as an immunoregulatory protein to prevent hyper activation of immune cells[1, 19–24]. Ligation of PD-1 by its receptors, PD-L1 and PD-L2, has been shown to inhibit T-cell proliferation and function[19, 21, 24–26]. Our data indicate that PD-1 upregulation occurs in the absence of infection on antigen-specific CD8+ T cells early in proliferative responses (Figure 1A–B) and following acute infection (Figure 3), suggesting this molecule plays an important regulatory function during normal T-cell activation. Our data is consistent with a very recent report by Wherry et al. which describes the upregulation of PD-1 on antigen-specific CD8+ T cells during both acute infection and chronic infection in LCMV-infected mice. Importantly, PD-1 expression was shown to diminish 10 days following acute infection while PD-1 expression on antigen-specific cells remained very high in chronically infected mice [27]. This report also demonstrates the upregulation of other inhibitory receptors on LCMV-specific T cells such as CTLA-4, suggesting there are multiple pathways responsible for the observed immune dysfunction during chronic infections [27]. Of note, recent literature also indicates PD-1 is upregulated during chronic lentiviral infection and on antigen-specific cells one week following vaccination. However, an increase in PD-1 expression was not observed on memory populations in these vaccinated uninfected monkeys[7]. Our data is not inconsistent with this data since we also observe that PD-1 expression decreases following early proliferative responses. In the absence of ex vivo peptide stimulation we did not observe the increase in PD-1 expression (Supplemental Figure 1A). Furthermore, our data demonstrating increased PD-1 expression on memory populations was not restricted to vaccine-specific responses. PD-1 expression on central memory populations was broad, suggesting a range of activation in these cells. Together, the data from both studies suggest the observed PD-1 expression in memory populations is likely due to persistent antigen presentation from other infectious agents. Also consistent with data from other labs[6, 7], we found that PD-1 expression is increased in cynomolgus macaques chronically infected with SHIV and rhesus macaques chronically infected with SIV (Figure 2). These results are particularly interesting considering the numerous reports documenting continual and sustained immune activation during lentiviral infection, and suggest that the increase in PD-1 expression we observed in infected macaques likely reflects the continued stimulation of T cells. This persistent activation likely prevents the downregulation of PD-1 expression that occurs following typical immune stimulation, resulting in immunosuppression.
Recent human studies have demonstrated that PD-1 expression is associated with decreased levels of cytokine secretion[2–4]. However, one study suggests that this decrease in cytokine secretion was a result of the suppression of proliferation rather than a direct effect on a cell’s ability to secrete cytokines[3]. Our data demonstrating the transient nature of PD-1 expression following ex vivo peptide stimulation along with our data indicating a delayed IFN-γ secretion suggests PD-1-mediated inhibition may have a direct effect on cytokine secretion. However, we cannot discount the possibility that PD-1 may inhibit proliferation as well. Indeed, several studies have demonstrated the inhibition of proliferation using PD-L1 blockade[1–4, 7, 28]. Interestingly, one report indicated PD-L1/PD-1 blockade failed to enhance antigen-specific proliferation in stimulated vaccine-specific T cells[7]. This is particularly interesting considering our data demonstrating a temporary increase in PD-1 expression following peptide stimulation of memory T cells and, together with our data, suggest short-term PD-1 expression may not result in the inhibition of T-cell proliferation.
PD-1 also increased with expression of CD95, a marker used for identifying memory T-cell subsets in monkeys, and tracked very well with HLA-DR expression in CD4+ T cells. Human data also indicate that PD-1 is upregulated on activated T cells[3, 4], with the highest expression found on central memory CD8+ T cells[3]. While our data indicate effector populations have the highest levels of PD-1 (Figure 4A), within the CD8+ T-cell subset we find that central memory cells have a higher PD-1 level (Figure 4B), consistent with the human studies. The failure of central memory cells to fully downregulate PD-1 expression does not appear to be a phenomenon exclusive to lentiviral infection, as uninfected macaques also have higher expression of PD-1 on CD8+ T cells. Further, it is important to note that even though PD-1 is downregulated following several rounds of proliferation on PBMCs from vaccinated macaques, the levels fail to drop back to baseline levels through six or seven proliferations and, based on memory T-cell expression, may persist albeit at lower levels than observed in recently activated T cells.
There are conflicting reports on the exact role of PD-1 in controlling immunity, with one group suggesting PD-1 may both inhibit and costimulate immune responses[29]. Our finding of higher PD-1 expression on CD8+ central memory cells in uninfected macaques suggests that PD-1 may play a costimulatory role in CD8+ T-cell populations. However, more work is necessary to determine if the role is truly costimulatory or whether PD-1 is preventing excessive activity of central memory CD8+ T cells. Nevertheless, our data clearly indicate high-level PD-1 expression as a marker for early T-cell activation.
Interestingly, the highest expression of PD-1 in infected macaques is associated with a distinct population of extrathymic CD4+ CD8+ double-positive T cells, previously shown to be highly-activated memory T cells[9–11, 13, 30]. Accordingly, double-positive T cells are known to have shorter telomere lengths compared to their single-positive counterparts[10]. Ultimately, shorter telomere length is associated with an eventual decrease in T-cell function and proliferative capacity[31, 32]. While some of these cells may be proliferatively exhausted the literature clearly demonstrates the majority of these cells are fully functional antigen-specific T cells with enhanced cytokine-secreting and cytotoxic capabilities[9–11, 13, 30].
The high level of PD-1 expression on CD4+ CD8+ double-positive T cells indicates that PD-1 expression in monkeys is a clear marker of T-cell activation. Interestingly, very recent evidence demonstrates that double-positive T cells found in the intestine express high levels of the SIV/HIV co-receptor CCR5, making them preferred targets for lentiviral infection[11]. Whether this PD-1hi DP T-cell population exists primarily as a result of lentiviral pathology driving a potential viral factory or represents the population of cells that comprises a last line of defense against a chronic infection remains an important area of study. Importantly, our data demonstrate that PD-1 is increased on T cells responding to antigenic peptides ex vivo. Therefore, PD-1 may be a useful marker for monitoring antigenic stimulation following vaccination in addition to monitoring HIV disease progression.
Our data demonstrating shortened telomere length in PD-1hi T cells is indicative of the proliferative history of these cells. Indeed, the data suggest a difference of 20–40 cell divisions separate naive and memory T-cell subsets. This may be an underestimate due to activity of telomerase, which is actively lengthening telomeres in these cells. Importantly, Weng et al.[16] observed about 14–28 divisions in humans (uninfected) between naïve and memory (CD45RA vs. CD45R0) CD4+ T cells, with a TRF difference of 1.4 kb. Whether the larger difference we see here is a result of lentiviral infection or natural differences between species remains to be determined.
Recent reports in humans indicate that PD-1 expression on antigen-specific T cells is correlated with a decrease in T-cell function and proliferation, which could be interpreted as T-cell exhaustion[2–4]. Despite the very short telomere lengths seen in our study, our data indicate that even effector memory PD-1hi cells are largely TIF-negative, consistent with earlier studies demonstrating that CD28− CD95+ T cell fractions in rhesus macaques contain actively cycling cells[14]. Therefore, while PD-1hi T cells may lose function sooner than their single-positive counterparts these cells are not mechanistically incapable of proliferating due to telomere-related issues. Together, our data suggest that defects seen in T-cell function are likely attributed to negative regulatory signals as a result of persistent high PD-1 expression rather than true T-cell proliferative exhaustion.
Interestingly, our data indicate a lack of polyfunctionality in SIV-specific T cells from infected macaques (Figure 6B,C), suggesting that while the cells are still functional, they may not be capable of responding using multiple T-cell functions when they encounter infected cells. Furthermore, the association of PD-1 expression with CD107a, IFN-γ, and TNF-α, but not IL-2 (Figure 6A,C), suggests that the relationship between PD-1 expression and functionality is rather complex. It is clear from our data and others that more research is necessary to understand these complex regulatory pathways that result, ultimately, in the activation or suppression of specific T-cell responses during chronic infections. Studies of acute infection in NHPs and studies examining the changes in PD-1 expression as infection progresses from acute phase to chronic phase may help to determine the exact role PD-1 plays in normal lymphocyte biology.
This manuscript adds to the current understanding of PD-1 biology in the following ways; 1) this is the first study to examine PD-1 expression in actively dividing cells, examining the level of expression as it relates to the number of proliferations, 2) this is the first study to examine the inverse relationship between PD-1 expression and IFN-γ secretion during T-cell proliferation, 3) this is the first study to indicate a relationship between double-positive expression of CD4/CD8 or HLA-DR expression on T cells, both markers of activation, and PD-1 expression in primates, and 4) this is the first study to examine terminal restriction fragment length and telomere dysfunction-induced foci, established markers of functional proliferative exhaustion, in relationship to PD-1 expression in non-human primates. In summary, the data presented provides valuable insight into the nature of PD-1 expression and its relationship to T-cell activation, function, and proliferation in non-human primates.
Materials and methods
Animals
Two sets of cynomolgus macaques were used in this study. The first set was housed at the Bristol-Myers Squibb (BMS) Lawrencville Facility (Princeton, NJ). The BMS Animal Care and Use Committee addresses animal facilities, contract facilities, and animal suppliers. The second set of cynomolgus macaques were housed at the Southern Research Institute and rhesus macaques were housed at Bioqual in Frederick, MD. These facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care International and meet National Institutes of Health standards as set forth in the Guidelines for Care and Use of Laboratory Animals. The first set of macaques were immunized 4 times over 3 months using poly-optimized SIVgag, SIVenv, and SIV pol DNA constructs by intramuscular immunization, 2mg per construct per immunization formulated in 0.15M citrate buffer and 0.25% bupivicaine at a pH of 6.5. The second set of cynomolgus macaques were part of a separate prophylactic vaccination study prior to SHIV infection. These animals were bled 73 weeks post infection and all had undetectable viral loads (<200 copies/mL). Two sets of rhesus macaques were used and were part of separate infection studies. Because rhesus macaques typically succumb to pathogenic SIV infection within 12 months, macaques from the first set were treated with FTC (50 mg/kg) and PMPA (20 mg/kg) (both from Gilead, Foster City, CA) once per day. Rhesus macaques were bled 63 weeks post infection. Two animals had undetectable viral loads (<40 copies/mL), while the other three had detectable viral loads (140, 720, and 3600 copies/mL) at the time of this study. For the second set of rhesus macaques, naive animals were infected with SIVmac251 intravenously 15 weeks prior to this study and maintained without antiretrovirals. Viral loads for these four monkeys were 2100, 1269560, 5467240, and 24016760 copies/mL at the time of the study.
Infection
Cynomolgus macaques were challenged with 300MID by the intravenous (IV) route with SHIV89.6P (kindly provided by Dr. Norman Letvin, Harvard University). Rhesus macaques were infected with 100 MID50 of SIVmac251 intravenously. The primates reached a peak viral load 2–6 weeks post infection and then set-point by week 12. Blood samples from cynomolgus macaques were collected approximately 30 months post SHIV infection and samples from rhesus macaques were collected 16 months post SIV infection.
Isolation of peripheral blood mononuclear cells (PBMCs)
10 mL blood from cynomolgus or rhesus macaques was collected in EDTA tubes and diluted 1:2 in Hank’s Balanced Salt Solution (HBSS). Diluted samples were overlayed on Percoll gradients and centrifuged for 40 min. at 1,200 RPM. Following centrifugation, PBMCs were collected from the interface and residual red cells lysed using ACK lysing buffer. Cells were washed with HBSS and counted.
Flow cytometry
Staining was performed using Pacific Blue-conjugated anti-CD3 (clone SP34-2) (BD Pharmingen), PerCP-conjugated anti-CD4 (clone L200) (BD Pharmingen, San Diego, CA), APC-conjugated anti-CD8 (clone SK1) (BD Biosciences, San Jose, CA), and biotin-conjugated anti-PD-1 (cat#BAF1086) (R&D Systems) and incubated with cells for 30 min on ice. After washing with PBS, cells were stained using streptavidin-PE (BD Pharmingen) for 20 minutes on ice. Cells were then washed 3 times in PBS and fixed with 1% paraformaldehyde. For polychromatic flow cytometry analysis of memory subsets, cells from uninfected or infected macaques were stained with Pacific Blue-conjugated anti-CD3, PerCP-conjugated anti-CD4, APC-conjugated anti-CD8, Biotin-conjugated anti-PD-1, FITC-conjugated anti-CD38 (clone AT-1) (Stem Cell Technologies, Vancouver, BC), APC-Cy7-conjugated anti-HLA-DR (clone L243) (Pharmingen), PE-Cy7-conjugated anti-CD28 (clone CD28.2) (eBioscience, San Diego, CA), and PE-Cy5-conjugated anti-CD95 (clone DX2) (Pharmingen). Cells were washed and then stained with streptavidin-PE. Following staining, cells were washed and fixed for flow cytometry. Data was collected using a LSRII flow cytometer (BD Biosciences). Flow cytometry data was analyzed using FlowJo software (Tree Star, Ashland, OR), gating on CD3+ lymphocytes.
T-cell proliferation assay
Freshly isolated PBMCs were stained with 5 µM CFSE (Invitrogen, Carlsbad, CA) in pre-warmed PBS for 10 minutes, washed 3 times, and suspended in RPMI 1640 containing 2 mM L-glutamine, 100 IU/mL penicillin and 100 IU/mL streptomycin and 10% fetal bovine serum (R10 medium). Cells were stimulated using either pooled peptides from SIVpol consisting of 15-mers overlapping by 11 amino acids (NIH Repository, Bethesda, MD) or 5 µg/mL Concanavalin A (ConA) and 5 µg/mL Phytohemagglutinin (PHA) (both from Sigma Chemical Co., St. Louis, MO) for 5 days, at which time balls of proliferating cells were apparent (snowballing). Six hours prior to the end of the incubation period, GolgiStop and GolgiPlug (both from BD Pharmingen) were added to the cells to inhibit protein transport. Cells were then washed in PBS and stained using the LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen) and subsequently stained using Pacific Blue-conjugated anti-CD14 (clone M5E2) (BD Pharmingen), Pacific Blue-conjugated anti-CD16 (clone 3G8) (BD Pharmingen), Pacific Blue-conjugated anti-CD19 (clone SJ25-C1) (Invitrogen), APC-Cy7-conjugated anti-CD3 (clone SP34-2) (BD Pharmingen), PerCP-Cy5.5-conjugated anti-CD4 (clone L200) (BD Pharmingen), Pacific Orange-conjugated anti-CD8 (clone RPA-T8) (kindly provided by Dr. Michael Betts at the University of Pennslvania), and biotin-conjugated anti-PD-1 (cat#BAF1086) (R&D Systems, Minneapolis, MN). Antibodies were incubated with cells for 30 min on ice. After washing with PBS, cells were stained using streptavidin-PE (BD Pharmingen) for 20 minutes on ice. Cells were then washed 3 times in PBS and intracellular staining was performed using BD Cytofix/cytoperm kit and Alexa Fluor 700-conjugated anti-IFN-γ (clone B27) (both from BD Pharmingen). Following staining, cells were washed twice in perm/wash buffer and fixed with 1% paraformaldehyde. For flow cytometry, cells were gated on singlets using FSC-H by FSC-A followed by gating on Pacific Blue-negative, CD3+ CD8+ CD4− T cells to examine CD8+ T-cell populations.
Telomere restriction fragment (TRF) length analysis
TRF analysis was performed essentially as described[33]. Briefly, 1 µg of genomic DNA was digested with a cocktail of AluI, HaeIII, HhaI, HinfI, MspI and RsaI, separated on a 0.5% agarose gel for 27 hours at 1 V/cm, and the gel was then dried and probed using the 32P-end labeled telomere repeat oligonucleotide (CCCTAA). The washed gel was visualized with a Molecular Dynamics Phosphoimager. Mean telomere length was calculated as the weighted average S(ODi)/S(ODi/Li), where ODi is the background-corrected intensity of telomere signal in interval i and Li is the average length of telomeres in interval i (each interval equal to a pixel), thus normalizing for the stronger signal emitted by longer telomeres. End-labeled lambda and HindIII-digested lambda DNA were used as markers, and sizes for individual sample lanes were derived from linear interpolations between markers in flanking lanes. Signals only between 4 and 48.5 kb were used for calculations.
TIF Analysis
Sorted subsets of T-cells were spotted onto poly-L-lysine coated 10-well test slides and fixed with 2% paraformaldehyde (PFA) for 10 minutes at room temperature. ImmunoFISH analysis was performed as described previously[17]. Briefly, cells were permeabilized and blocked with PBS containing 0.2% Tween and 4% BSA for 30 min at 37°C, and stained with anti-53BP1 (Novus Biologicals, Cat. #NB 100–304, 1:150 dilution) for 2 hr at 37°C. Secondary antibody staining was performed for 1 hr at 37°C with AlexaFluor-488 goat anti-rabbit (Molecular Probes, 1:800 dilution), and was followed by crosslinking to the sample with 4% PFA. Fluorescence in situ hybridization (FISH) was performed with a Cy3-conjugated PNA telomere probe (Applied Biosystems) overnight at room temperature, and was followed by tertiary staining with AlexaFluor-488 donkey anti-goat (Molecular Probes, 1:800) for 1 hr at 37°C. Cells were counterstained with DAPI (0.3 µg/ml). TIFs were visualized with a Zeiss LSM-510 Meta confocal microscope. Quantification was performed by analyzing z-stacks for co-localization of 53BP1 foci with telomeres. Any cell with at least one example of co-localization was considered TIF-positive. Between 13 and 42 cells were analyzed for each T cell subset.
Intracellular cytokine staining
Freshly isolated PBMC from infected rhesus macaques were obtained by gradient centrifugation and stimulated for 6 hours with either SIVmac239 peptides or SEB in the presence of golgi transport inhibitors. During the incubation, an enhanced stain with CD107a-FITC (Pharmingen) was performed. Following stimulation, cells were stained for viability and extracellular markers as in the T-cell proliferation assay. Cells were washed, fixed, and permeabilized as before and stained for intracellular expression of CD3, IFN-γ, TNF-α, and IL-2 (all from Pharmingen). Cells were then washed and fixed for analysis.
Statistical analysis
Data are expressed as means ± SD. Mean fluorescence intensity was used to evaluate PD-1 expression on CD3+ cells and specific T-cell subsets. Statistical significance of differences was assessed by a one-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test to evaluate differences between specific groups. An unpaired t-test was used to evaluate differences when only two groups were being compared. For studies examining changes in PD-1 expression during acute infection, a paired t-test was used to evaluate changes in expression. A two-tailed non-parametric Spearman correlation was used to assess relationships between sets of data. In all cases, a value of p<0.05 was considered to be significant. Statistical analyses and linear regressions were performed using Prism 4.02 software (GraphPad Software, San Diego, CA). For changes in PD-1 expression in infected rhesus macaques in relation to functionality, differences were assessed using a two-tailed unpaired t test. Polyfunctionality was assessed using Boolean gating with FlowJo software followed by analysis using Pestle and Spice software (kindly provided by Dr. Mario Roederer at the National Institutes of Health), ignoring responses below 0.05% of CD8+ T cells.
Supplementary Material
Acknowledgements
This work is supported by NIH training grant #2-T32-AI-007632-06, HIV Pathogenesis (Robert W. Doms, PI) (DH), NIH grant #5R01AG021521 (FBJ), and NIH training grant # T32 GM07229 (JS). DBW acknowledges support from the NIH including HIVRAD funding. We thank U. Herbig and J. Sedivy for help with TIF detection protocols and dedicate this work to the memory of Dr. Sanjeev Kumar.
Footnotes
Conflicts of interest
There are no conflicts of interest for this manuscript.
References
- 1.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 2.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 3.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 5.Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol. 2006;28:267–279. doi: 10.1007/s00281-006-0046-z. [DOI] [PubMed] [Google Scholar]
- 6.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res. 1997;232:25–28. doi: 10.1006/excr.1997.3493. [DOI] [PubMed] [Google Scholar]
- 9.Nam K, Akari H, Terao K, Shibata H, Kawamura S, Yoshikawa Y. Peripheral blood extrathymic CD4(+)CD8(+) T cells with high cytotoxic activity are from the same lineage as CD4(+)CD8(−) T cells in cynomolgus monkeys. Int Immunol. 2000;12:1095–1103. doi: 10.1093/intimm/12.7.1095. [DOI] [PubMed] [Google Scholar]
- 10.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 11.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 12.Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, et al. CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31:2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72:55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 18.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38:353–357. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 23.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 26.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 27.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Rio ML, Penuelas-Rivas G, Dominguez-Perles R, Ramirez P, Parrilla P, Rodriguez-Barbosa JI. Antibody-mediated signaling through PD-1 costimulates T cells and enhances CD28-dependent proliferation. Eur J Immunol. 2005;35:3545–3560. doi: 10.1002/eji.200535232. [DOI] [PubMed] [Google Scholar]
- 30.Weiss L, Roux A, Garcia S, Demouchy C, Haeffner-Cavaillon N, Kazatchkine MD, Gougeon ML. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis. 1998;178:1158–1162. doi: 10.1086/515674. [DOI] [PubMed] [Google Scholar]
- 31.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, et al. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. Aids. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 33.Steinert S, White DM, Zou Y, Shay JW, Wright WE. Telomere biology and cellular aging in nonhuman primate cells. Exp Cell Res. 2002;272:146–152. doi: 10.1006/excr.2001.5409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.