Abstract
The concept of using cholinesterase bioscavengers for prophylaxis against organophosphorous nerve agents and pesticides has progressed from the bench to clinical trial. However, the supply of the native human proteins is either limited (e.g., plasma-derived butyrylcholinesterase and erythrocytic acetylcholinesterase) or nonexisting (synaptic acetylcholinesterase). Here we identify a unique form of recombinant human butyrylcholinesterase that mimics the native enzyme assembly into tetramers; this form provides extended effective pharmacokinetics that is significantly enhanced by polyethylene glycol conjugation. We further demonstrate that this enzyme (but not a G117H/E197Q organophosphorus acid anhydride hydrolase catalytic variant) can prevent morbidity and mortality associated with organophosphorous nerve agent and pesticide exposure of animal subjects of two model species.
Keywords: countermeasures, nonconventional warfare agents, organophosphorous pesticides, protein engineering, transgenic plants
Butyrylcholinesterase (BChE) is the major cholinesterase (ChE) in the serum of humans (1, 2). Although the closely related enzyme acetylcholinesterase (AChE) is well described as the primary synaptic regulator of cholinergic transmission, a definitive physiological role for BChE has not yet been demonstrated (3). BChE is catalytically promiscuous and hydrolyzes not only acetylcholine (ACh), but also longer-chain choline esters (e.g., butyrylcholine, its preferred substrate, and succinylcholine) and a variety of non-choline esters, such as acetylsalicylic acid (aspirin) and cocaine (4, 5). Moreover, BChE binds most environmentally occurring ChE inhibitors as well as man-made organophosphorous (OP) pesticides and nerve agents (NAs) (6, 7–10).
The systemic biodistribution and affinity for ChE inhibitors allow endogenous BChE to provide broad-spectrum protection against various toxicants by their sequestration before they reach cholinergic synapses. However, under realistic high-dose exposure scenarios, BChE serum levels are too low to afford adequate protection, resulting in persistent cholinergic excitation due to irreversible inhibition of AChE and subsequent accumulation of ACh. Sublethal manifestations of this state include unregulated exocrine secretion and gastrointestinal hypermotility. Death usually results from unregulated stimulation at neuromuscular junction leading to hemodynamic instability and tetanic contraction of the respiratory muscles (11, 12).
Current OP poisoning therapy consists of atropine for muscarinic ACh receptor blockade and diazepam for symptomatic management of convulsions (12). Additionally, oxime therapy with 2-pralidoxime (2-PAM) can effectively reactivate some but not all OP-AChE adducts (13–15). This standard therapeutic approach can reduce mortality, but insufficiently prevents the incapacitation associated with OP toxicity (12, 16).
Prophylaxis by administration of exogenous ChEs has proven successful in reducing OP-associated morbidity and mortality, but requires the availability of relatively large amounts of these proteins because the ChE-OP interaction is stoichiometric and irreversible (17–20). Studies using plasma-derived human BChE (hBChE) provided proof of concept (21), but also raised concerns over the limited availability of hBChE, and the risk inherent to the use of a human blood product. Additionally, amino acid substitution variants of WT BChE (e.g., G117H/E197Q) have been identified that support the slow regeneration of the phosphorylated (or phosphonylated) active-site serine residue, thus creating a catalytic bioscavenger (22–25). Therefore the need for a sustainable source of WT and mutant ChEs prompted the search for recombinant protein production platforms, including transgenic goats and insect larvae (26–28).
As an alternative to animal-based technologies, we have evaluated the capacity of plant production of recombinant human cholinesterases (29–31) to meet this need. We report herein that human BChE assembles in planta into tetramers, unlike recombinant BChE from other sources. Further, plant-derived BChE (pBChE) can protect animals against otherwise lethal doses of pesticide and NA challenges.
Results
Oligomeric State of pBChE.
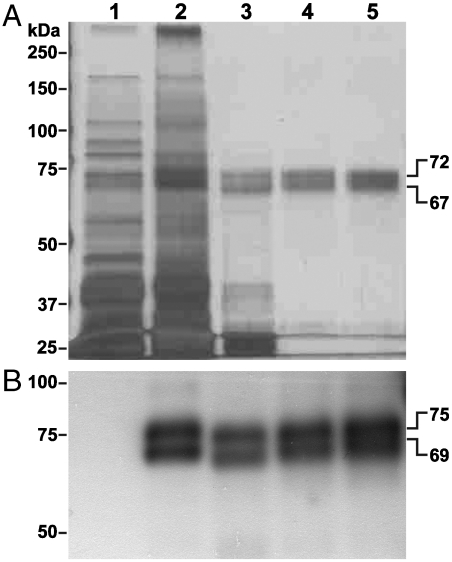
Highly purified preparations of WT and G117H/E197Q pBChEs were obtained by two sequential affinity chromatography steps (32). WT pBChE could be resolved by SDS-PAGE into two distinct protein bands with apparent molecular masses of 67–69 kDa and 72–75 kDa (Fig. 1), both smaller than the fully glycosylated ∼85 kD hBChE (33). WT pBChE was found to carry hybrid glycans with concanavalin A-reactive mannose terminal residues (32).
Fig. 1.
Highly purified preparation of pBChE. Water-soluble proteins were extracted from leaves of WT plants (lane 1) or transgenic plants that express pBChE (lane 2). The recombinant protein was purified from the extract by ammonium sulfate fractionation (30%–70% fraction, lane 3), and in tandem affinity chromatography steps using Con A-sepharose (lane 4) and procainamide (lane 5). Protein samples were resolved by SDS-PAGE and either (A) subjected to silver staining or (B) transferred to a PVDF membrane, immunodecorated by an anti-human BChE antibody followed by HRP-conjugated secondary antibody, and visualized by chemiluminescence. Gels were loaded based on equal BChE activity at 24 mU (A) or 2.4 mU (B). Crude extract samples contained equivalent amounts of total soluble proteins. One unit of enzyme will hydrolyze 1.0 µmol of butyrylthiocholine to thiocholine and butyrate per minute.
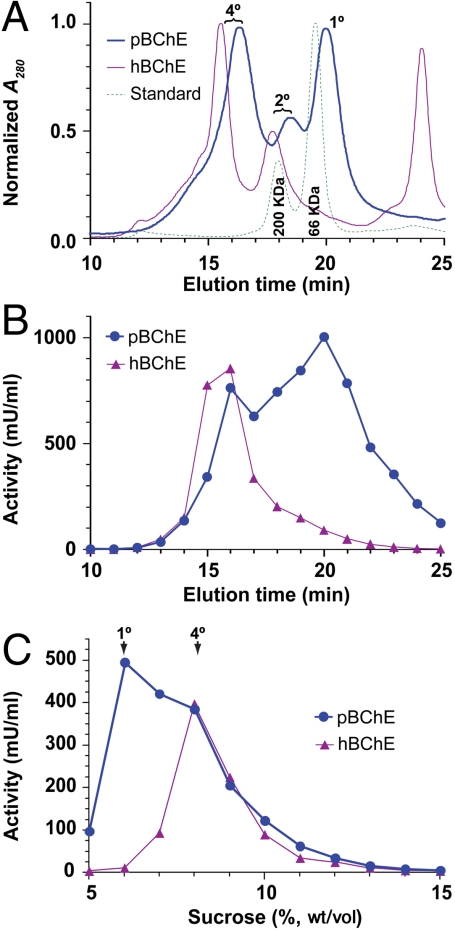
We used size-exclusion HPLC (SEC-HPLC) to separate the various molecular species that may be present in the pure preparations. Nearly half (48.7%) of pBChE was found to be organized into tetramers with smaller population of monomers (36.0%) and dimers (15.3%) (Fig. 2A). All pBChE species maintained catalytic activity irrespective of oligomerization state (Fig. 2B). The various oligomeric species of pBChE displayed decreased chromatographic mobility as compared to their respective hBChE counterparts, in agreement with the former’s lower apparent molecular masses (Fig. 2B). The presence of tetramers revealed by the SEC-HPLC data was further validated by sucrose density gradient sedimentation (Fig. 2C).
Fig. 2.
Tetramerization of pBChE. (A) SEC-HPLC analysis of preparations of pBChE and hBChE. Monomers—1°, dimers—2°, tetramers—4°. β-Amylase (200 kDa) and BSA (66 kDa) were used as size standards. (B) Fractions collected during SEC-HPLC analysis were assayed for BChE activity. (C) Sucrose density gradient sedimentation analysis of pBChE and hBChE preparations. Fractions were assayed for BChE activity.
Enzymatic Properties of pBChE.
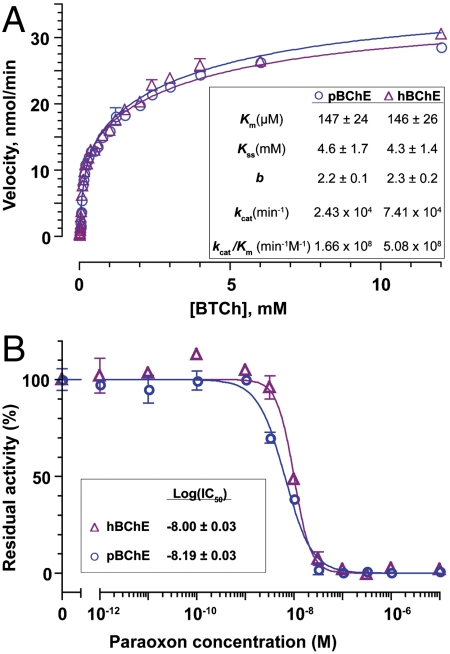
We compared the steady-state kinetics of substrate hydrolysis by WT pBChE to that of hBChE. Both enzymes displayed the typical substrate activation expected of this enzyme (34) (Fig. 3A). Nonlinear regression revealed the Km of pBChE for butyrylthiocholine (BTCh) to be 147 μM, practically identical to its plasma-derived counterpart (146 μM; Fig. 3A, Inset). Other kinetic parameters of the two enzymes, including the dissociation constant of BTCh at the peripheral binding site (Kss) and the activation factor, b, were also indistinguishable. On the other hand, the BTCh hydrolysis kcat value of hBChE was somewhat higher than that of pBChE.
Fig. 3.
BTCh hydrolysis and its inhibition by POX. (A) Steady-state kinetics of BTCh hydrolysis by pBChE and hBChE. (B) POX IC50 curves for pBChE and hBChE. Error bars indicate ± SEM.
To determine the in vitro OP scavenging ability of WT pBChE, we assayed its activity following incubation with paraoxon (POX), the activated form of the insecticide parathion and a model OP compound. The inhibition profiles of pBChE and hBChE were virtually indistinguishable with respective IC50 values of 0.65 × 10-8 M and 1 × 10-8 M (Fig. 3B).
Next, we examined the binding of pBChE to chemical warfare NAs. The final concentrations of NA were sufficiently high to establish pseudo-first-order reaction conditions for all pairings except WT BChE with soman (GD), in which a theoretical approximation for ki was calculated (Table 1). The results were in excellent agreement with previous observations with hBChE (35, 36) and were congruent with the results obtained for POX (Fig. 3B). The results indicated that inhibition rates of WT pBChE by GD and O-ethyl-S-(2-diisopropylaminoethyl) (VX) were 3- to 4-orders of magnitude higher than those of G117H/E197Q pBChE.
Table 1.
Inhibition rate constants (ki) for pBChE
| Variant |
Agent |
ki, M-1 min-1 |
| WT pBChE | VX | 4.3 × 106 |
| GD | 6.0 × 109* | |
| G117H/E197Q pBChE | VX | 1.8 × 103 |
| GD | 1.4 × 105 | |
| WT hBChE† | VX | 2.3 × 107 |
| GD | 4.0 × 107 |
*Value is estimated as pBChE activity was fully inhibited prior to initial measurement.
†From Cohen et al. (35).
It was previously proposed that ChE-based bioscavenger prophylaxis against NAs could be combined with administration of oximes to allow the rapid restoration of the ChE active site (19). This combination therapy would effectively turn a stoichiometric anti-OP bioscavenger into a “pseudocatalytic” one (19). We tested the potential of various oximes to reactivate WT pBChE following its inhibition by a panel of NAs (Table 2). Our analysis demonstrated that sarin (GB)- and VX-inhibited pBChE underwent slow reactivation with 2-PAM, as well as four other experimental oximes. Tabun-BChE and cyclosarin-BChE complexes were more resistant to oxime-mediated reactivation than their GB and VX counterparts. For all oxime–OP combinations tested, the plant-derived read-through isoform of human AChE (pAChE-R) was more amenable to oxime-mediated reactivation than pBChE.
Table 2.
Oxime-mediated reactivation of pBChE and pAChE-R
| Nerve agent | Oxime-mediated reactivation rates (k, min-1) |
||||
| HI-6 | 2-PAM | MMB-4 | TMB-4 | HS-6 | |
| pBChE | |||||
| Tabun | > 3.0 × 10-3 | > 3.0 × 10-3 | > 3.0 × 10-3 | > 3.0 × 10-3 | > 3.0 × 10-3 |
| Sarin | 8.7 × 10-2* | 1.5 × 10-2* | 1.0 × 10-2* | 1.9 × 10-3* | 1.9 × 10-3* |
| Cyclosarin | 1.9 × 10-3 | 1.9 × 10-3 | 1.9 × 10-3 | 1.9 × 10-3 | 1.9 × 10-3 |
| VX | 5.3 × 10-2 | 2.6 × 10-2 | 1.3 × 10-2 | 1.6 × 10-2 | 9.2 × 10-2 |
| pAChE-R | |||||
| Tabun | — | 4 × 10-3 | 2.5 × 10-3 | 2.7 × 10-2 | — |
| Sarin | 6.9 × 10-1* | 2.4 × 10-2* | 1.7 × 10-1* | 4.6 × 10-2* | — |
| Cyclosarin | 2.3 × 10-1* | — | 1.7 × 10-1* | 5.3 × 10-2* | 9.9 × 10-2* |
| VX | — | — | 2.4 × 10-2** | 3.1 × 10-2** | 6.3 × 10-2** |
Concentration of oximes was 1 mM except where noted (*0.1 mM; **0.01 mM).
Polyethylene Glycol Conjugation Improves the Pharmacokinetic Properties of Plant-Derived ChEs.
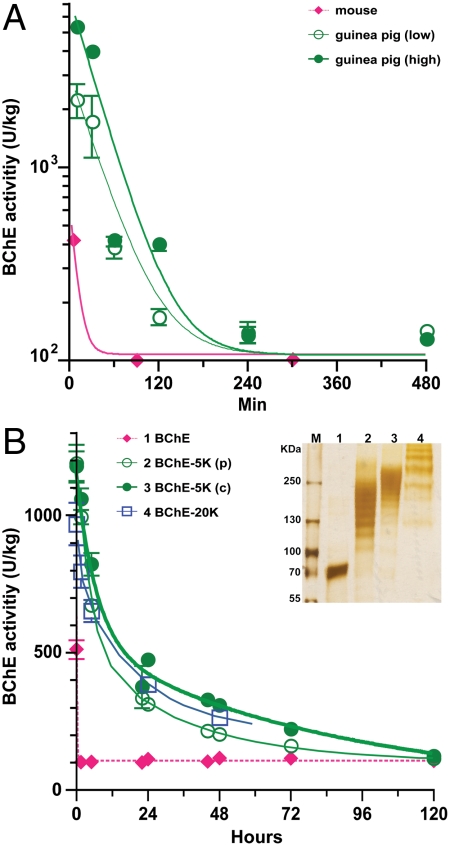
To determine the in vivo stability (and thus the therapeutic window) for pBChE, we undertook a pharmacokinetic analysis of the recombinant WT protein in mice and guinea pigs (GPs). Following i.v. administration into mice, WT pBChE and pAChE-R were rapidly cleared with t1/2 of 7 and 11 min, respectively (Fig. 4A and Table 3). In GPs (compared to mice), pBChE had a somewhat longer circulatory retention (t1/2 = 24 min), independent of dose (Fig. 4A and Table 3).
Fig. 4.
Pharmacokinetic profiles of pBChE and its PEGylated derivatives. (A) Unmodified pBChE was administered intravenously to mice at 2,000 units/kg (N = 10) or through a carotid catheter to GPs at 7,000 units/kg (low, N = 2) or at 14,000 units/kg (high, N = 2). (B) PEGylated BChEs were administered i.v. to mice: pBChE-5Kp (N = 10), pBChE-5Kc (N = 10), and pBChE-20K (N = 5). Plasma BChE levels were determined at the specified time points and converted to units per kilogram body weight assuming blood constitutes 7% of body weight in rodents. Control BChE levels were 100–130 units/kg in both mice and GPs. Data points represent mean ± SEM. (Insert) PEGylated pBChE was resolved by SDS-PAGE and protein bands were visualized by silver staining. Unconjugated pBChE (lane 1), or pBChE following conjugation to 5K PEG [2 h partial conjugation (lane 2), and 4 h complete conjugation (lane 3)], or 20K PEG (lane 4).
Table 3.
Pharmacokinetics of pBChE, pAChE-R, and derivatives thereof in mice and guinea pigs
| Half-life |
Residual activity |
|||
|
t1/2-F* |
t1/2-S† |
at 48 h |
||
| min | min (fold) | % | ||
| pBChE | Mouse | 7‡ (1) | 0 | |
| pBChE-5K (p) | 178 | 1,418 (213) | 9 | |
| pBChE-5K (c) | 281 | 3,475 (522) | 25 | |
| pBChE-20K | 53 | 876 (132) | 7 | |
| pBChE | Guinea pig | 24‡ | 0 | |
| pAChE | Mouse | 11‡ (1) | 0 | |
| pAChE-5k | 14 | 130 (12) | 0 | |
| pAChE-20k | 138 | 2669 (240) | 8 | |
*Half-life of the fast clearance phase.
†Half-life of the slow clearance phase.
‡Clearance was fast and appeared monophasic.
To increase the biological half-life of the plant-derived enzymes, we chemically conjugated them to linear activated PEG with average molecular masses of either 5 kDa (PEG-5K) or 20 kDa (PEG-20K). Chemical conjugation of pBChE to PEG-5K or PEG-20K decreased electrophoretic mobility proportionally to both the molecular weight of the PEG group and, in the case of PEG-5K, the duration of conjugation (Fig. 4B, Inset). Similar patterns were observed for pAChE-R (data not shown), which has fewer lysine residues (37).
PEG-conjugation dramatically prolonged the circulatory presence of the injected proteins, resulting in a marked biphasic clearance pattern: a rapid distribution phase with a half-life (t1/2-F) that was 8–19-fold shorter than that of the slower clearance phase (t1/2-S, Fig. 4B and Table 3). PEGylation dramatically increased t1/2-S of pBChE and pAChE-R. Conjugation with PEG-20K was most effective for pAChE-R, prolonging its t1/2-S from 11 min to almost 2 d (a 240-fold increase), but we observed a smaller increase for pBChE (Table 3). In contrast, PEG-5K had a particularly large effect (522-fold) on the t1/2-S of pBChE, but only modestly affected pAChE-R. PEGylation had a comparatively smaller effect over the rapid distribution phase, but in totum significant proportions of the injected ChEs remain in the circulation at 48 h after injection: 8% and 25% of PEGylated pACHE-R and pBChE, respectively.
pBChE Provides Effective Prophylaxis Against Pesticide and NA Poisoning.
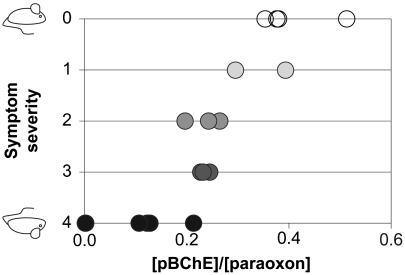
To test the in vivo efficacy of pBChE against OP poisons, we first used a mouse model of OP toxicity using POX (Fig. 5). Treatment with 1.1 × LD50 of POX (750 μg/kg) was lethal in 100% of animals. Prophylactic administration of pBChE prevented mortality at a pBChE/POX molar ratio as low as 0.15. However, these animals exhibited severe convulsions and increased respiratory effort. Symptom severity decreased with increasing pBChE dose and asymptomatic presentation was achieved at a molar ratio of 0.35 pBChE/POX.
Fig. 5.
Protection from acute symptoms of OP toxicity with pBChE. Mice (23–30 g) were i.v. injected with pBChE in saline (0–100 mg/kg). Actual BChE concentrations in blood were determined at 5 min after injection and ranged 0–58 mg/kg. At 10 min after BChE administration, mice were injected with POX (50–68 nmol per animal). Molar ratios ([BChE]/[POX]) were calculated and ranged between 0 and 0.51. Symptom score was 0, asymptomatic; 1, decreased motor activity; 2, tremors or fasciculation; 3, convulsions; 4, death.
To extend these results to chemical warfare NAs, GPs were administered WT pBChE at one of two doses, either 10.50 mg/kg (N = 2) or 26.15 mg/kg (N = 4). Five minutes following enzyme administration, animals administered 26.15 mg/kg of pBChE were challenged with 2 × LD50 of GD (56 μg/kg). The GPs administered 10.5 mg/kg were challenged with the same dose of GD at either 40 min or 22 h after pBChE administration. In the absence of prophylaxis, a 2 × LD50 dose of GD results in 100% lethality within 2–4 h of exposure, with onset of symptoms within 10–15 min (38). None of the animals (N = 4) pretreated with 26.15 mg/kg pBChE 5 min prior to GD exposure developed clinical signs for 2 h after exposure to a NA (Table 4). At 24 h after exposure, three of the four animals were alive and free of symptoms; one animal in this set was found dead at ∼18 h after exposure, possibly due to complications associated with the carotid catheter, or to unobserved late onset OP toxicity. Of the two GPs administered 10.5 mg/kg pBChE, the animal exposed to GD at 40 min after enzyme survived to 24 h and displayed no signs of NA intoxication. In contrast, the animal exposed to GD at 22 h after pBChE administration became symptomatic within 8 min and died within 20 min of NA injection.
Table 4.
WT but not G117H/E197Q pBChE protects guinea pigs from nerve agent challenge
| Group |
pBChE |
Agent |
||||||
| Dose | Time* | Dose | Time* | Survival after challenge† | ||||
| n | mg/kg | min | μg/kg | × LD50 | min | 1 h | 24 h | |
| WT pBChE (High) | ||||||||
| 4 | 26.15 | t = 0 | GD | 56 | 2 | t = 20 | 4/4 | 3/4 |
| WT pBChE (Low) | ||||||||
| 1 | 10.50 | t = 0 | GD | 56 | 2 | t = 40 | 1/1 | 1/1 |
| 1 | 10.50 | t = 0 | GD | 56 | 2 | t = 22 h | 0/1 | — |
| G117H/E197Q pBChE (High) | ||||||||
| 2 | 26.15 | t = 0 | GD | 56 | 2 | t = 5 | 0/2 | — |
| 2 | 26.15 | t = 0 | GD | 126 | 4.5 | t = 5 | 0/2 | — |
| 3 | 26.15 | t = 0 | VX | 18 | 2 | t = 5 | 0/3 | — |
| 2 | 26.15 | t = 0 | GB | 87 | 2 | t = 5 | 0/2 | — |
*Time of administration.
†Survivors per total tested.
All animals pretreated with G117H/E197Q pBChE and exposed to 4.5 × LD50 of GD (N = 2) or 2 × LD50 of GD (N = 2), VX (n = 3) or GB (N = 2) displayed signs of NA intoxication within 15 min of exposure and none survived to 24 h (Table 4).
Discussion
Human BChE has been a leading candidate for development as a bioscavenger of NAs to prevent OP poisoning. Current research has been conducted with enzyme prepared in limited quantities by purification from blood-banked plasma. It has proven to be an excellent bioscavenger that performed very well in animal challenge experiments and results from two recently completed Phase-Ia clinical trials (NCT00333528 and NCT00744146) are apparently forthcoming (21). A major remaining hurdle is to identify a reliable, safe, non-supply-limited and inexpensive source for BChE. With current technology, creating a small stockpile of plasma-derived BChE (1 kg ≈ 5,000 doses) would require dedicating the entire annual US supply of outdated plasma to a purification effort at a considerable cost (18). Recombinant DNA technology-based production systems are attractive alternatives. Among the various platforms for the production of recombinant proteins including transgenic goats (26), transgenic plants offer several unique advantages such as reduced costs of production, simple scaleup, enhanced biosafety and equivalent costs for purification and in vitro modifications (39, 40).
Unlike recombinant transgenic goat-milk-derived BChE (26), but similar to the plasma-derived enzyme, a substantial portion of the WT pBChE population is tetrameric (Fig. 2). Tetramerization of endogenously produced ChEs is mediated by noncovalent interactions between the conserved C-terminal Trp amphiphilic tetramerization (WAT) domains of individual BChE monomers with polyproline II helical domains of several proteins. For example, BChE and AChE at the neuromuscular junction form tetramers that are attached to the basal lamina through the proline-rich attachment domain of Collagen Q (41, 42). BChE tetramers in human serum were very recently shown by Lockridge and coworkers to associate with peptides derived from lamellipodin (43). Like the tetramerization itself, the interaction with lamellipodin depends on the C terminus, and is impaired in the K variant of BChE that carries a C-terminal substitution (44). To our knowledge, plants lack direct homologs of lamellipodin, but they do contain a fair number of proline-rich proteins, many of which are cell-wall associated (45). Proteolytic cleavage of some of these proteins gives rise to polyproline peptides (46, 47). Association of the WAT domain with such proteins or peptides may promote pBChE tetramerization.
Compared to their plasma-derived counterparts, pBChE tetramers and dimers have longer retention time upon SEC-HPLC, and this observation is congruent with the smaller apparent molecular mass of the monomer as observed by SDS-PAGE. We have previously observed that pBChE carries hybrid glycans with terminal mannose residues (facilitating the purification of pBChE) on their fucosylated N-acetylglucose amine cores (32). The nuanced disparity in the maturation of N-linked sugars in plants and humans did not affect the enzymatic properties of pBChE nor did it prevent its tetramerization.
Divergent glycosylation, especially the absence of terminal sialylation, explicates the relatively brief circulatory half-life of the non-PEGylated pBChE (Fig. 4 and Table 3), in agreement with rigorous studies by Shafferman et al. who showed that sialylation is the most important factor affecting the circulatory retention of recombinant ChEs (48). However, significant species-dependent variation was also noted especially when rodents were contrasted with primates, suggesting rodents may be poor predictors of ChE pharmacokinetics in humans (37, 49, 50). It is important to note that BChE tetramers were shown to have longer circulatory residence time than monomers suggesting that tetramerization may play a bigger role in enhancing stability of BChE as compared to AChE (51, 52). Our data here demonstrate an outstanding prolongation of the biological half-life of pBChE upon PEGylation as was previously shown for ChEs produced by other platforms (35, 49, 53). A more extensive coverage of more surface-exposed lysines, achieved with longer reaction time (i.e., allowing PEGylation with PEG-5K to continue to completion), was more effective in prolonging t1/2 than decoration with larger individual PEG moieties at fewer sites. Interestingly, when we compared the clearance profile of pBChE conjugated with PEG-20K to the similarly modified pAChE-R in mice, we noticed much more rapid clearance of the latter, in agreement with the finding that BChE is preferentially maintained over AChE in primate serum (49, 50).
Oximes are an integral part of the current treatment for exposure to some OPs, allowing partial recovery of cholinergic functions by reactivating synaptic AChE. Incorporating oximes into ChE prophylaxis may enhance the efficacy of a bioscavenger by allowing its regeneration thereby requiring a lower effective dose (19). Our analysis of oxime-mediated reactivation of plant-produced ChEs (Table 2) demonstrated that reactivation of pBChE was generally much less successful than reactivation of pAChE-R, which corroborates previous findings with ChEs derived from other sources (54, 55). It is important to note that the tested oximes were developed to fit the smaller active site of AChE for effective reactivation. For the successful implementation of ChEs as pseudocatalytic bioscavengers, optimizing oxime-ChE interactions is necessary and can be achieved by using recombinant AChE with currently available oximes, by modifying the BChE active site to allow efficient reactivation by such oximes, or by developing new BChE-compatible oximes.
We have successfully demonstrated here, in two animal models, that pBChE reduces mortality and morbidity associated with OP pesticide and NA exposure, thus providing dose-dependent protection against these poisons. It is important to note that mice (and other rodents but much less so for guinea pigs) have a high plasma concentration of carboxylesterase, allowing efficient scavenging of POX (56–58), and can provide an explanation for their resistance to poisoning by POX (and some other OPs) and to the better than 1∶1 stoichiometry observed here (Fig. 5).
We included the G117H/E197Q variant of pBChE in this study to test its feasibility as a catalytic scavenger of OP NAs. Previous in vitro studies suggested that the (modest) OP hydrolytic activity of G117H/E197Q BChE (22, 25) would result in higher protective ratios from a given dose of enzyme and/or reduce the amount of protein required to afford protection against a fixed dose of NA. The utility of the G117H/E197Q BChE variant as a catalytic bioscavenger was previously suggested but not tested in vivo. Here we demonstrate that this variant could not afford in vivo protection against nerve agents. The failure of the G117H/E197Q variant of pBChE to protect against GB, GD, or VX is most likely the consequence of its substantially reduced binding of OP NAs relative to WT pBChE; G117H/E197Q pBChE probably binds the NA too slowly to effectively scavenge it from the circulation before a lethal dose of NA distributes out of the bloodstream reaching its physiological targets. From an enzyme engineering (or directed evolution) point of view, this result suggests an important lesson: On the road to better catalytic efficiency (kcat/KM) some reduction in substrate affinity (i.e., increase in KM) can be expected and tolerated but this compromise cannot be too great.
Comparison of in vitro and in vivo protection data afforded by pAChE-R or WT pBChE demonstrated no significant difference in either OP scavenging or the ability to affect a clinically meaningful survival (29). As discussed above, in comparison to BChE, AChE’s more efficient oxime-mediated reactivation makes it more readily testable as a pseudocatalytic bioscavenger with currently available oximes. However, such differences must be balanced with the more favorable pharmacokinetic profile of BChE. Additionally, the ability of BChE to hydrolyze other toxins such as cocaine and succinylcholine makes the BChE a compelling “dual use technology” tool, providing a secondary market that is perhaps less applicable to AChE. The question of which enzyme is more effective as a bioscavenger remains open, with transgenic plants currently offering the only published source for both recombinant human AChE and BChE. The use of plant production of these enzymes will remove current limitations in protein availability and allow detailed studies of them toward human testing, including investigating the effect of custom glycosylation, potential improvements by mutagenesis, and evaluation of mixed isoform formulations to exploit the potentially positive attributes of enzyme synergy.
Materials and Methods
Plant Production of Human ChEs.
Purification of WT pBChE and pAChE-R from transgenic Nicotiana benthamiana plants was recently described (29, 31, 32). The G117H/E197Q variant of pBChE was transiently expressed in WT plants using the MagnICON vector system (59) and was purified following a similar protocol used for WT pBChE.
Biochemical Analyses and Manipulations.
Fractionation of pBChE molecular species using SEC-HPLC or sucrose gradient centrifugation is described in details in the SI Text. Conjugation of WT pBChE or pAChE-R (31) to PEG was performed essentially as described (53 and SI Text). Standard SDS-PAGE and immunoblot analyses were used. Rabbit polyclonal anti-hBChE Abs were the generous gift of Oksana Lockridge (University of Nebraska Medical Center, Omaha, NE).
Steady-state enzyme kinetics experiments are described in the SI Text. OP NAs tabun, GB, GD, cyclosarin, and VX were obtained from the US Army Edgewood Chemical Biological Center; all were found to be > 96% pure by 31P NMR analysis.
In Vivo Experiments.
In vivo experiments were approved by the Institutional Animal Care and Use Committees of Arizona State University and of the US Army Medical Research Institute of Chemical Defense. Full description of these experiments can be found in the SI Text.
Supplementary Material
Acknowledgments.
This work was funded in part by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke under the U-54-NSO58183-01 award—a consortium grant awarded to US Army Medical Research Institute of Chemical Defense and contracted to T.S.M. under the research cooperative agreement W81XWH-07-2-0023. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009021107/-/DCSupplemental.
References
- 1.La Du BN, Lockridge O. Molecular biology of human serum cholinesterase. Fed Proc. 1986;45:2965–2969. [PubMed] [Google Scholar]
- 2.Arpagaus M, et al. Use of the polymerase chain reaction for homology probing of butyrylcholinesterase from several vertebrates. J Biol Chem. 1991;266:6966–6974. [PubMed] [Google Scholar]
- 3.Ashani Y, et al. Butyrylcholinesterase and acetylcholinesterase prophylaxis against soman poisoning in mice. Biochem Pharmacol. 1991;41:37–41. doi: 10.1016/0006-2952(91)90008-s. [DOI] [PubMed] [Google Scholar]
- 4.Masson P, et al. Butyrylcholinesterase-catalysed hydrolysis of aspirin, a negatively charged ester, and aspirin-related neutral esters. Biochim Biophys Acta. 1998;1387:41–52. doi: 10.1016/s0167-4838(98)00104-6. [DOI] [PubMed] [Google Scholar]
- 5.Koetzner L, Woods JH. Characterization of butyrylcholinesterase antagonism of cocaine-induced hyperactivity. Drug Metab Dispos. 2002;30:716–723. doi: 10.1124/dmd.30.6.716. [DOI] [PubMed] [Google Scholar]
- 6.McGehee DS, et al. Cholinesterase inhibition by potato glycoalkaloids slows mivacurium metabolism. Anesthesiology. 2000;93:510–519. doi: 10.1097/00000542-200008000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Khan SB, et al. Butyrylcholinesterase inhibitory guaianolides from Amberboa ramosa. Arch Pharmacal Res. 2005;28:172–176. doi: 10.1007/BF02977710. [DOI] [PubMed] [Google Scholar]
- 8.Loizzo MR, Tundis R, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: An update. Curr Med Chem. 2008;15:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- 9.Decker M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur J Med Chem. 2005;40:305–313. doi: 10.1016/j.ejmech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Taylor P. Anticholinesterase Agents. In: Brunton LL, Goodman LS, Gilman A, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th Ed. New York: McGraw-Hill; 2006. Chap 8. [Google Scholar]
- 11.Greenfield RA, et al. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 2002;323:326–340. doi: 10.1097/00000441-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lee EC. Clinical manifestations of sarin nerve gas exposure. JAMA, J Am Med Assoc. 2003;290:659–662. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- 13.Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006;249:86–94. doi: 10.1016/j.jns.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Thiermann H, et al. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology. 2007;233:145–154. doi: 10.1016/j.tox.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25:297–323. doi: 10.2165/00139709-200625040-00009. [DOI] [PubMed] [Google Scholar]
- 16.Jokanovic M, Stukalov PV, Kosanovic M. Organophosphate induced delayed polyneuropathy. Curr Drug Targets: CNS Neurol Disord. 2002;1:593–602. doi: 10.2174/1568007023338879. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell DM, Brecht KM, Doctor BP, Wolfe AD. Comparison of antidote protection against soman by pyridostigmine, HI-6 and acetylcholinesterase. J Pharmacol Exp Ther. 1993;264:1085–1089. [PubMed] [Google Scholar]
- 18.Ashani Y. Prospective of human butyrylcholinesterase as a detoxifying antidote and potential regulator of controlled-release drugs. Drug Dev Res. 2000;50:298–308. [Google Scholar]
- 19.Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem Biol Interact. 2005;157–158:167–171. doi: 10.1016/j.cbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Lenz DE, et al. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: A mini review. Toxicology. 2007;233:31–39. doi: 10.1016/j.tox.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 21.Masson P, Lockridge O. Butyrylcholinesterase for protection from organophosphorus poisons: Catalytic complexities and hysteretic behavior. Arch Biochem Biophys. 2010;494:107–120. doi: 10.1016/j.abb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millard CB, Lockridge O, Broomfield CA. Organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase: Synergy results in a somanase. Biochemistry. 1998;37:237–247. doi: 10.1021/bi972057c. [DOI] [PubMed] [Google Scholar]
- 23.Millard CB, Lockridge O, Broomfield CA. Design and expression of organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase. Biochemistry. 1995;34:15925–15933. doi: 10.1021/bi00049a007. [DOI] [PubMed] [Google Scholar]
- 24.Lockridge O, et al. A single amino acid substitution, Gly117His, confers phosphotriesterase (organophosphorus acid anhydride hydrolase) activity on human butyrylcholinesterase. Biochemistry. 1997;36:786–795. doi: 10.1021/bi961412g. [DOI] [PubMed] [Google Scholar]
- 25.Broomfield CA, Lockridge O, Millard CB. Protein engineering of a human enzyme that hydrolyzes V and G nerve agents: Design, construction and characterization. Chem Biol Interact. 1999;119–120:413–418. doi: 10.1016/s0009-2797(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 26.Huang YJ, et al. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci USA. 2007;104:13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platteborze PL, Broomfield CA. Expression of biologically active human butyrylcholinesterase in the cabbage looper (Trichoplusia ni) Biotechnol Appl Biochem. 2000;31(Pt 3):225–229. doi: 10.1042/ba19990038. [DOI] [PubMed] [Google Scholar]
- 28.Li S, et al. High-level expression of functional recombinant human butyrylcholinesterase in silkworm larvae by Bac-to-Bac system. Chem-Biol Interact. 2010;187:101–105. doi: 10.1016/j.cbi.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 29.Evron T, et al. Plant-derived human acetylcholinesterase-R provides protection from lethal organophosphate poisoning and its chronic aftermath. FASEB J. 2007;21:2961–2969. doi: 10.1096/fj.07-8112com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geyer BC, et al. Translational control of recombinant human acetylcholinesterase accumulation in plants. BMC Biotechnol. 2007;7:27. doi: 10.1186/1472-6750-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geyer BC, et al. Purification of transgenic plant-derived recombinant human acetylcholinesterase-R. Chem Biol Interact. 2005;157–158:331–334. doi: 10.1016/j.cbi.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 32.Geyer BC, et al. Transgenic plants as a source for the bioscavenging enzyme, humanbutyrylcholinesterase. Plant Biotechnol J. 2010 doi: 10.1111/j.1467-7652.2010.00515.x. in press. [DOI] [PubMed] [Google Scholar]
- 33.Kolarich D, et al. Glycoproteomic characterization of butyrylcholinesterase from human plasma. Proteomics. 2008;8:254–263. doi: 10.1002/pmic.200700720. [DOI] [PubMed] [Google Scholar]
- 34.Masson P, Xie W, Froment MT, Lockridge O. Effects of mutations of active site residues and amino acids interacting with the Omega loop on substrate activation of butyrylcholinesterase. Biochim Biophys Acta. 2001;1544:166–176. doi: 10.1016/s0167-4838(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 35.Cohen O, et al. Comparison of polyethylene glycol-conjugated recombinant human acetylcholinesterase and serum human butyrylcholinesterase as bioscavengers of organophosphate compounds. Mol Pharmacol. 2006;70:1121–1131. doi: 10.1124/mol.106.026179. [DOI] [PubMed] [Google Scholar]
- 36.Ashani Y, Pistinner S. Estimation of the upper limit of human butyrylcholinesterase dose required for protection against organophosphates toxicity: A mathematically based toxicokinetic model. Toxicol Sci. 2004;77:358–367. doi: 10.1093/toxsci/kfh012. [DOI] [PubMed] [Google Scholar]
- 37.Cohen O, Kronman C, Lazar A, Velan B, Shafferman A. Controlled concealment of exposed clearance and immunogenic domains by site-specific polyethylene glycol attachment to acetylcholinesterase hypolysine mutants. J Biol Chem. 2007;282:35491–35501. doi: 10.1074/jbc.M704785200. [DOI] [PubMed] [Google Scholar]
- 38.Wetherell J, Hall T, Passingham S. Physostigmine and hyoscine improves protection against the lethal and incapacitating effects of nerve agent poisoning in the guinea-pig. Neurotoxicology. 2002;23:341–349. doi: 10.1016/s0161-813x(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 39.Fischer R, Emans N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000;9:279–299. doi: 10.1023/a:1008975123362. [DOI] [PubMed] [Google Scholar]
- 40.Mason HS, Warzecha H, Mor T, Arntzen CJ. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol Med. 2002;8:324–329. doi: 10.1016/s1471-4914(02)02360-2. [DOI] [PubMed] [Google Scholar]
- 41.Bon S, Ayon A, Leroy J, Massoulie J. Trimerization domain of the collagen tail of acetylcholinesterase. Neurochem Res. 2003;28:523–535. doi: 10.1023/a:1022821306722. [DOI] [PubMed] [Google Scholar]
- 42.Lee HH, et al. Transcriptional regulation of acetylcholinesterase-associated collagen ColQ: Differential expression in fast and slow twitch muscle fibers is driven by distinct promoters. J Biol Chem. 2004;279:27098–27107. doi: 10.1074/jbc.M402596200. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Schopfer LM, Masson P, Lockridge O. Lamellipodin proline rich peptides associated with native plasma butyrylcholinesterase tetramers. Biochem J. 2008;411:425–432. doi: 10.1042/BJ20071551. [DOI] [PubMed] [Google Scholar]
- 44.Podoly E, et al. The butyrylcholinesterase K variant confers structurally derived risks for Alzheimer pathology. J Biol Chem. 2009;284:17170–17179. doi: 10.1074/jbc.M109.004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon MC, et al. Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA. 2008;105:2226–2231. doi: 10.1073/pnas.0711980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce G, Siems WF, Bhattacharya R, Chen YC, Ryan CA. Three hydroxyproline-rich glycopeptides derived from a single petunia polyprotein precursor activate defensin I, a pathogen defense response gene. J Biol Chem. 2007;282:17777–17784. doi: 10.1074/jbc.M701543200. [DOI] [PubMed] [Google Scholar]
- 47.Chen YC, Siems WF, Pearce G, Ryan CA. Six peptide wound signals derived from a single precursor protein in Ipomoea batatas leaves activate the expression of the defense gene sporamin. J Biol Chem. 2008;283:11469–11476. doi: 10.1074/jbc.M709002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kronman C, Chitlaru T, Elhanany E, Velan B, Shafferman A. Hierarchy of post-translational modifications involved in the circulatory longevity of glycoproteins. Demonstration of concerted contributions of glycan sialylation and subunit assembly to the pharmacokinetic behavior of bovine acetylcholinesterase. J Biol Chem. 2000;275:29488–29502. doi: 10.1074/jbc.M004298200. [DOI] [PubMed] [Google Scholar]
- 49.Cohen O, Kronman C, Velan B, Shafferman A. Amino acid domains control the circulatory residence time of primate acetylcholinesterases in rhesus macaques (Macaca mulatta) Biochem J. 2004;378:117–128. doi: 10.1042/BJ20031305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronman C, Cohen O, Velan B, Shafferman A. Host-regulated disposition of mammalian AChEs. Chem Biol Interact. 2005;157–158:51–55. doi: 10.1016/j.cbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Duysen EG, Bartels CF, Lockridge O. Wild-Type and A328W mutant human butyrylcholinesterase tetramers expressed in chinese hamster ovary cells have a 16-hour half-life in the circulation and protect mice from cocaine toxicity. J Pharmacol Exp Ther. 2002;302:751–758. doi: 10.1124/jpet.102.033746. [DOI] [PubMed] [Google Scholar]
- 52.Saxena A, et al. Role of oligosaccharides in the pharmacokinetics of tissue-derived and genetically engineered cholinesterases. Mol Pharmacol. 1998;53:112–122. doi: 10.1124/mol.53.1.112. [DOI] [PubMed] [Google Scholar]
- 53.Cohen O, et al. Effect of chemical modification of recombinant human acetylcholinesterase by polyethylene glycol on its circulatory longevity. Biochem J. 2001;357:795–802. doi: 10.1042/0264-6021:3570795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007;233:166–172. doi: 10.1016/j.tox.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Jun D, Musilova L, Kuca K, Kassa J, Bajgar J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chem Biol Interact. 2008;175:421–424. doi: 10.1016/j.cbi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Bhat VB, Choi MH, Wishnok JS, Tannenbaum SR. Comparative plasma proteome analysis of lymphoma-bearing SJL mice. J Proteome Res. 2005;4:1814–1825. doi: 10.1021/pr0501463. [DOI] [PubMed] [Google Scholar]
- 57.Li B, et al. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Li B, Duysen EG, Carlson M, Lockridge O. The butyrylcholinesterase knockout mouse as a model for human butyrylcholinesterase deficiency. J Pharmacol Exp Ther. 2008;324:1146–1154. doi: 10.1124/jpet.107.133330. [DOI] [PubMed] [Google Scholar]
- 59.Santi L, et al. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc Natl Acad Sci USA. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.