Abstract
Individuals often eat calorically dense, highly palatable “comfort” foods during stress for stress relief. This article demonstrates that palatable food intake (limited intake of sucrose drink) reduces neuroendocrine, cardiovascular, and behavioral responses to stress in rats. Artificially sweetened (saccharin) drink reproduces the stress dampening, whereas oral intragastric gavage of sucrose is without effect. Together, these results suggest that the palatable/rewarding properties of sucrose are necessary and sufficient for stress dampening. In support of this finding, another type of natural reward (sexual activity) similarly reduces stress responses. Ibotenate lesions of the basolateral amygdala (BLA) prevent stress dampening by sucrose, suggesting that neural activity in the BLA is necessary for the effect. Moreover, sucrose intake increases mRNA and protein expression in the BLA for numerous genes linked with functional and/or structural plasticity. Lastly, stress dampening by sucrose is persistent, which is consistent with long-term changes in neural activity after synaptic remodeling. Thus, natural rewards, such as palatable foods, provide a general means of stress reduction, likely via structural and/or functional plasticity in the BLA. These findings provide a clearer understanding of the motivation for consuming palatable foods during times of stress and influence therapeutic strategies for the prevention and/or treatment of obesity and other stress-related disorders.
Keywords: corticosterone, anxiety-related behavior, synaptophysin, cAMP response element-binding protein, calcium/calmodulin-dependent protein kinase
The obesity epidemic is fueled by the easy availability of palatable, calorically dense foods amid an ever-escalating level of daily stress (1–3). Humans and rodents increase palatable food consumption when stressed (3–6), and the term “comfort food” is commonly used to signify possible stress-dampening properties of certain foods (particularly calorically dense foods containing high amounts of carbohydrates and/or fats). Indeed, comfort food intake in humans is linked with improved emotional states (7), and a high-carbohydrate diet is associated with reduced resting and stress-evoked cortisol levels (8–11).
Stress (a real or perceived threat to homeostasis or well-being) typically evokes both physiological and emotional responses (reviewed in ref. 12). The physiological responses include activation of the hypothalamic–pituitary–adrenocortical (HPA) axis and the sympathetic branch of the autonomic nervous system. Activation of the sympathetic nervous system has numerous effects, including increases in heart rate and blood pressure. Activation of the HPA axis results in elevations in circulating adrenocorticotropic hormone (ACTH) and glucocorticoids (e.g., cortisol in humans and corticosterone in rats). Glucocorticoids have widespread action on numerous physiological processes, including mobilization of stored energy and maintenance of vascular tone. Perceived stressors also engage emotional responses, recruiting fear, anxiety, and defense-related neurocircuitry to trigger context-appropriate behavioral responses. In toto, the stress response produces a new physiological and emotional state designed to optimize survival in the face of real or perceived adversity.
The mechanism providing stress buffering by comfort foods (6, 13) likely resides at the interface among stress, reward, and/or metabolic circuitry in the brain. We now demonstrate that rewarding properties of palatable foods can effectively buffer all major physiological and behavioral responses to stress, and we identify key neural circuits underlying the comfort food effect. Identification of these neural circuits provides potential strategies for intervening to prevent or curtail increasing rates of obesity and other metabolic disorders.
Results
Rewarding Properties of Palatable Food Dampen Collective Stress Responses.
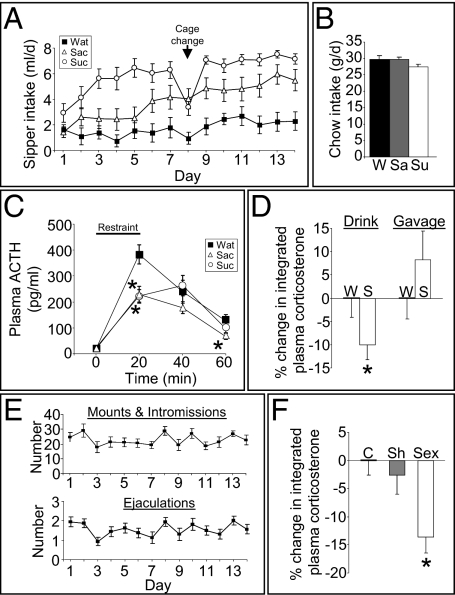
Rats given brief access to a small amount of palatable sucrose solution significantly reduce ACTH (Fig. 1A) and corticosterone (Fig. S1) secretion in response to an acute stress relative to controls that are given equivalent access to standard drinking water. Importantly, over the 2-wk, twice-daily sucrose-exposure regimen, rats consume ∼10% of their daily calories as sucrose (Fig. 1A) and compensate by reducing their intake of chow (Fig. 1B), thus maintaining normal caloric intake and body weight (Fig. S2). To determine whether the sweet taste per se is sufficient to reduce the subsequent response to restraint stress, another group of rats received twice-daily exposure to saccharin instead of sucrose. The saccharin-fed rats drank the sweetened drink in amounts approaching that of the sucrose-fed rats, but they did not reduce their chow intake, such that their caloric intake and body weight were the same as in the other groups (Fig. 1 A and B and Fig. S2). Saccharin-fed rats, like sucrose-fed rats, had attenuated HPA axis responses to acute stress (Fig. 1C), suggesting that the hedonic/rewarding properties of the “desserts” are sufficient to blunt the HPA axis response to stress. The plasma ACTH response was reduced by both sucrose and saccharin, whereas the plasma corticosterone response was reduced by sucrose and not saccharin (Fig. 1C and Fig. S1). Dissociations between circulating ACTH and corticosterone after stress are common. This dissociation could be caused by a reduction in adrenal responsivity to ACTH by palatable foods; however, palatable drink did not alter adrenal responsivity (Fig. S3), which suggests that the dissociation is likely because of (i) the timing of sample collection relative to the peak ACTH and corticosterone responses and/or (ii) differences between immunoreactive and bioactive ACTH (14, 15). Nonetheless, the present results demonstrate that, in this model, saccharin is sufficient to produce HPA axis dampening. Moreover, attenuation of the plasma corticosterone response to restraint by sucrose and saccharin is associated with reductions in corticotropin-releasing hormone mRNA expression in the hypothalamic paraventricular nucleus (13), suggesting, at least in part, a central site of action for palatable drink. Lastly, intragastric gavage of the same amount and schedule of sucrose did not reduce the plasma corticosterone response to stress (Fig. 1D and Fig. S4), providing further evidence that it is the hedonic, rather than the caloric, properties of palatable desserts that are sufficient for HPA axis dampening.
Fig. 1.
Natural rewards dampen the HPA axis response to stress. (A) Intake of rats given up to 4 mL of water (Wat), 0.1% saccharin (Sac), or 30% sucrose (Suc) drink twice daily for 14 d. (B) Chow intake for rats given water (W), saccharin (Sa), or sucrose (Su) drink. (C) Plasma ACTH response to a 20-min restraint stress. *P < 0.05 vs. water. (D) Percentage reduction in the plasma corticosterone response to restraint stress in rats given sucrose (S) versus water (W) via a drinking bottle or via intragastric gavage twice daily for 14 d. *P < 0.05 vs. water. (E) Sexual behavior [mounts and intromission (Upper); ejaculations (Lower)] of male rats given brief (30 min daily) access to a sexually receptive female rat. (F) Percentage reduction in the plasma corticosterone response to restraint stress in sexually active male rats (Sex) compared with undisturbed controls (C) and sham-sex males (Sh; exposed daily to a female rat that was confined within a wire mesh cage). *P < 0.05 vs. control, sham. Data are shown as mean ± SEM.
We next sought to determine whether other pleasurable/rewarding experiences similarly blunt stress responses. Sexually naïve male rats were given brief access to a sexually receptive female rat daily for 2 wk, over which period they rapidly learned to engage in sexual behavior (Fig. 1E). When these rats were given a subsequent restraint stress challenge, they too had a significantly blunted increase of plasma corticosterone (Fig. 1F and Fig. S5). These results suggest that HPA axis dampening by natural reward is a general phenomenon.
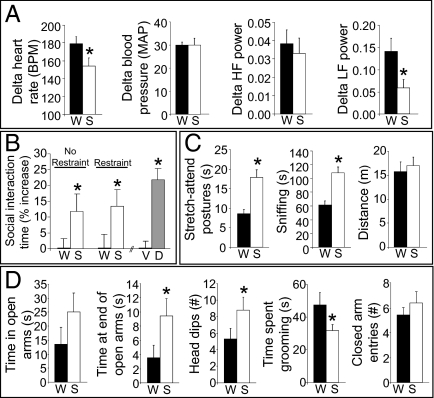
We next asked whether other responses to stress are similarly blunted. After 2 wk on the sucrose-dessert paradigm, rats significantly reduced restraint-induced tachycardia relative to controls (Fig. 2A). Analysis of heart rate variability in the frequency domain revealed preferential decreases in the low-frequency component (an index of sympathetic activity; Fig. 2A) in sucrose-fed rats, suggesting that the reduced tachycardia was primarily attributable to reduced sympathetic drive (as opposed to increased parasympathetic drive).
Fig. 2.
Sucrose dampens cardiovascular and behavioral responses to stress. (A) Restraint stress-induced increases in heart rate [beats per minute (BPM)], blood pressure [mean arterial pressure (MAP)], and the high-frequency (HF) and low-frequency (LF) domains of heart rate variability after a history of sucrose (S) drink relative to water (W). (B) Increase in time spent interacting in the social interaction anxiety test after a history of sucrose drink relative to water, in rats given either no stress immediately before testing (No Restraint) or a restraint stress immediately before testing (Restraint). For comparison, the increase in time spent interacting is also shown for a separate group of rats after diazepam (D; 1 mg/kg, i.p.) relative to vehicle (V; 2% Tween-20 in saline). (C) The amount of time spent in stretch-attend postures and sniffing and the total distance traveled in the open-field test after a history of sucrose drink versus water. (D) Total time spent in the open arms, time spent at the far end of the open arms, number of head dips/scans, time spent grooming, and the number of closed arm entries in the elevated plus-maze test after a history of sucrose drink or water. *P < 0.05 vs. control (W or V, respectively). Data are shown as mean ± SEM.
We then asked whether a history of consuming sucrose influences stress-related behaviors. When placed with an unfamiliar conspecific, sucrose-fed rats interacted more than controls did (Fig. 2B), which is suggestive of reduced behavioral anxiety (16). In an open-field test, sucrose-fed rats exhibited increased stretch-attend postures and sniffing, exploratory behaviors that negatively correlate with anxiety (17, 18; Fig. 2C) (general locomotion was not affected). Finally, in the elevated plus-maze test (Fig. 2D), ethological indices of exploratory behavior [e.g., time spent exploring the far end of open arms, time spent dipping head over edge to look down (19)] were increased in sucrose-fed rats, again with no effect on total locomotion. Collectively, these results demonstrate that a history of brief hedonically pleasurable events decreases neuroendocrine, sympathetic, and behavioral anxiety-like responses to stress.
Basolateral Amygdala (BLA) Is Necessary for Stress Dampening.
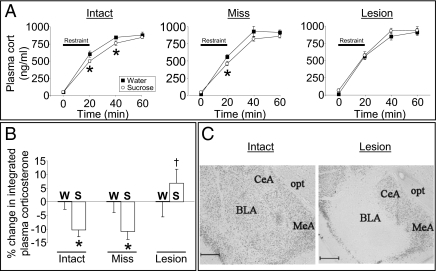
Because the hedonic/rewarding properties of sucrose, saccharin, or sex appear sufficient to dampen subsequent stress responses, we hypothesized that brain reward regions, such as the BLA, are likely to mediate these effects. Neuronal activity in the amygdala, including the BLA, is altered in humans and other animals while they are engaging in pleasurable behaviors, including eating appetizing foods (20–23). The amygdala is also activated in humans and other animals during stress (24, 25), and the BLA subregion is implicated in neuroendocrine and behavioral stress responses (26–28). We therefore asked whether neural activity in the BLA is necessary for the stress-blunting effect. Rats with bilateral ibotenate lesions of the BLA went through the 2-wk sucrose paradigm and subsequent stress challenge. Control rats that received vehicle into the BLA, and rats in which the ibotenate lesions missed the BLA (Fig. 3C), had an attenuated plasma corticosterone response to stress (Fig. 3 A and B), as expected. In contrast, sucrose-fed rats with confirmed bilateral lesions of the BLA (Fig. 3C) had a normal corticosterone response to stress (Fig. 3 A and B, and Fig. S6), despite an equivalent intake of sucrose over the 2-wk paradigm (Fig. S7), indicating that neural activity within the BLA is necessary for the HPA-dampening effect.
Fig. 3.
Neuronal activity in the BLA is necessary for HPA dampening by sucrose. (A) Time course of the plasma corticosterone response to restraint stress after a history of sucrose drink in rats with bilateral vehicle-injected, intact BLA (Intact), or bilateral ibotenate-injected, lesioned rats with lesions that missed (Miss) or hit (Lesion) the BLA. *P < 0.05 vs. water; n = 7–15 per group. (B) Percentage reduction in the integrated plasma corticosterone response by sucrose relative to water for BLA Intact, Miss, or Lesion rats. *P < 0.05 vs. water; †P < 0.05 vs. Intact-S and Miss-S. (C) Representative images of NeuN immunolabeling in the BLA of Intact and Lesion rats. CeA, central amygdala; MeA, medial amygdala; opt, optic tract. (Scale bar = 500 μm.) Data are shown as mean ± SEM.
Palatable Food Induces Structural Plasticity in the BLA.
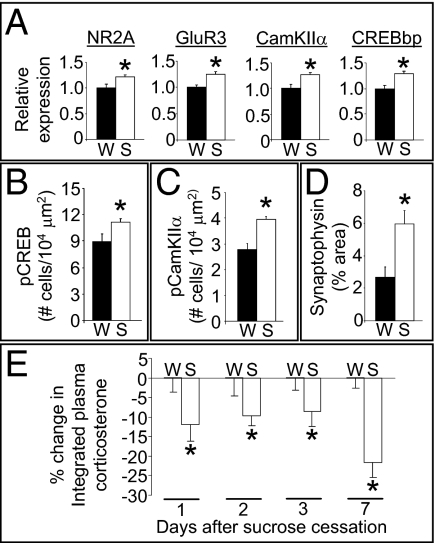
To determine potential molecular mediators within the BLA, an mRNA expression profile was performed on BLA tissue from rats with a history of the sucrose-dessert paradigm or on water-drinking controls. The microarray analysis identified 145 genes within the BLA whose mRNA expression was altered by sucrose exposure. Functional clustering analysis determined that these genes were significantly enriched in several biological pathways (Table S1), including intracellular calcium signaling and long-term potentiation. Identified genes of high interest in these pathways included ATP2B2 (Ca2+ transporting ATPase 2), CAMK2A [calcium/calmodulin-dependent protein kinase IIα (CamKIIα)], CREBBP [cAMP response element-binding protein (CREB) binding protein], GRIA3 [glutamate receptor AMPA 3 (GluR3)], GRIN2A [glutamate receptor NMDA 2A (NR2A)], PPP1R12A (protein phosphatase 1 regulatory subunit 12A), RAP2B (member of Ras oncogene family), and RYR2 (ryanodine receptor 2). The identification of these biological pathways suggests that structural plasticity may be occurring in the BLA, leading us to assess potential synaptic alterations in the BLA of rats after a history of consuming intermittent sucrose. Sucrose increased phosphorylated CREB (pCREB) and phosphorylated CamKIIα (pCamKIIα) immunoreactivity [postsynaptic indices associated with synaptic strengthening and remodeling (29–32)] and synaptophysin terminal densities in the BLA [presynaptic index of structural plasticity (33, 34)] (Fig. 4 B, C, and D and Fig. S8). Because increased structural plasticity can produce prolonged alterations in neural circuits (33, 35, 36), the BLA synaptic remodeling elicited by rewards (e.g., consumption of sucrose) may be expected to result in prolonged dampening of stress responses. To test this prediction, rats were given a restraint stress challenge at 1, 2, 3, or 7 d after 2 wk of consuming sucrose dessert or water. The plasma corticosterone response to restraint (Fig. 4E) was attenuated by sucrose in all groups, indicating that there is a prolonged dampening of stress responses (i.e., at least 7 d) that is likely mediated, at least in part, by structural plasticity in the BLA.
Fig. 4.
Indices of synaptic plasticity are up-regulated in the BLA by sucrose and are associated with persistent HPA dampening. (A) Relative mRNA expression for NR2A, GluR3, CamKIIα, and CREBBP (CREBbp) in the BLA are increased by sucrose (S) relative to water (W). (B) Density of BLA cells immunopositive for pCREB (S133) after a history of sucrose drink versus water. (C) Density of BLA cells immunopositive for pCamKIIα (T286) after a history of sucrose drink versus water. (D) Percentage of BLA image area occupied by synaptophysin immunoreactivity after a history of sucrose drink versus water. (E) The percentage reduction in the integrated plasma corticosterone stress response by sucrose relative to water in rats given restraint stress at 1, 2, 3, or 7 d after the cessation of sucrose drink. *P < 0.05 vs. water. Data are shown as mean ± SEM.
Discussion
Collectively, the present data demonstrate that the hedonic/rewarding properties of palatable foods have stress-buffering actions across numerous effector pathways (neuroendocrine, behavioral, and sympathetic nervous system). Stress-buffering actions of palatable foods can be mimicked by other types of naturally pleasurable activities (e.g., sexual activity). The data are consistent with a general stress-buffering role mediated by central reward circuitry. Moreover, components of reward circuitry, in particular the BLA, are critical for the stress-relieving properties of palatable foods. Stress buffering appears to be mediated by reward-induced structural plasticity in the BLA, triggered by long-term activation of the CamKIIα and CREB signaling pathways.
BLA Regulation of Stress Responses.
The BLA plays a complex role in stress regulation. The BLA appears to promote anxiety-related behaviors as well as sympathetic activation (37, 38). However, BLA lesion and inactivation studies have produced inconsistent effects on HPA regulation, showing either no effect or increased or decreased HPA activity (26, 37). Similarly, in the present studies, BLA lesion did not itself alter HPA activation in water-drinking rats (Fig. S6). Collectively, this work suggests that BLA output may have both HPA excitatory and inhibitory components. Previous experiences may produce a differential weighting of these opposing BLA components. For example, chronic stress may promote the BLA's stress excitatory output(s), whereas natural reward may promote the BLA's stress inhibitory output(s). In this manner, the BLA may be positioned as an interface that specifically relates information regarding previous emotionally salient experiences to the stress system.
The structure and anatomical connectivity of the BLA is ideally suited for subserving these dual stress-regulatory roles. The majority of BLA neurons (∼85%) are glutamatergic projection neurons, complemented by a variety of inhibitory interneurons that tonically inhibit projection neuron activity (39, 40). A history of natural reward could alter the excitability of the BLA via changing the level of interneuronal inhibition (27). Moreover, the BLA projection neurons innervate numerous stress-regulatory sites, including regions that are considered stress-excitatory (e.g., anteroventral bed nucleus of the stria terminalis, infralimbic cortex, medial amygdala) and stress-inhibitory (e.g., prelimbic cortex, hippocampus, lateral septum) (12, 41–44). Thus, natural rewards may inhibit stress responses via (i) preferentially increasing the glutamatergic BLA output to inhibitory stress-regulatory sites and/or (ii) preferentially decreasing glutamatergic BLA output to excitatory stress-regulatory sites. Importantly, synaptic remodeling provides an ideal mechanism to produce this type of preferential weighting by previous experiences. BLA plasticity can occur at both excitatory and inhibitory synapses (45–47) and is linked to altered function (48, 49). Moreover, some of the plasticity-related genes affected by sucrose are linked with synaptic remodeling at both excitatory and inhibitory synapses (e.g., NMDA receptor, CamKII) (50, 51), suggesting that multiple types of synaptic remodeling may be occurring after palatable food intake.
Role of Metabolic Versus Nonmetabolic Properties of Palatable Foods.
Highly palatable comfort foods have multiple attributes that can be crudely divided into two categories: metabolic properties (e.g., calories and macronutrient composition) and nonmetabolic properties (e.g., taste, hedonics, and reward). Previous work indicates that consuming large amounts of palatable foods (sucrose and lard) reduces stress responses, which may be associated with the metabolic properties (6, 52, 53). The present work shows that much smaller amounts of palatable foods also produce stress relief, mediated by rewarding properties acting on the BLA. Collectively, this work suggests that pleasurable behaviors (including consuming small amounts of sucrose or saccharin or engaging in sexual activity) may dampen stress via actions on brain reward circuitry. In contrast, when large amounts of palatable foods are consumed, the metabolic properties of the foods may provide additional stress relief, perhaps via actions on fat stores, brain metabolic circuitry, and/or the additional reward value provided by food macronutrients (52–56).
Physiological Relevance.
The presently described stress dampening by limited palatable food intake is likely of significant physiological relevance. Palatable drink decreased anxiety-related behaviors in multiple behavior tests, with effect sizes similar to those seen after other anxiolytic interventions (19), which is consistent with a meaningful effect on responses to anxiety-provoking stimuli. For the HPA axis and autonomic systems, palatable drink consistently reduced the response to stress by ≈10–20%. This attenuation seems relatively modest; however, it likely provides a cumulative physiological impact over long periods of time. Prolonged, subtle changes in HPA axis tone are associated with diminished bone density and cognitive function (57, 58). Similarly, relative modest changes in cardiovascular and/or autonomic function over long periods of time can have pronounced physiological consequences [e.g., borderline hypertension increases risk for cardiovascular disease and organ damage (59), and modest changes in heart rate variability after myocardial infarction significantly increase mortality (60)]. Thus, the cumulative stress-dampening effects of palatable foods on the behavioral, neuroendocrine, and autonomic systems can provide considerable stress-relieving effects throughout an individual's lifetime.
Lastly, although “self-medication” by consumption of highly palatable, calorically dense comfort foods clearly decreases collective stress responses, reductions can also be achieved by engaging in other naturally rewarding behaviors. The ability to engage reward systems in the face of stress may be exploited as a means to diminish the contribution of life stress to the obesity epidemic and other stress-related disorders.
Materials and Methods
Additional methodological details are provided in SI Materials and Methods.
Animals.
Adult male Long-Evans rats (∼225–350 g body weight at start of experiments) were supplied by either Harlan Labs or Charles River. All procedures were approved by the University of Cincinnati Animal Care and Use Committee.
Palatable Drink Paradigm.
Rats (n = 12–15 per group) with free access to water and normal chow were given additional access twice daily (at ≈09:00 and 15:00 h) to 4 mL of sucrose (30%; Sigma-Aldrich), the noncaloric sweetener sodium saccharin (0.1%; Sigma-Aldrich), or water, and the volume consumed was recorded. Body weight and chow intake were also monitored. In this paradigm, sucrose does not affect body weight gain or the weights of any individual fat depots (13). In one experiment, 4 mL of sucrose or water was administered to rats (n = 10–13 per group) via oral intragastric gavage twice daily to determine whether the caloric and other postingestive consequences of sucrose were sufficient to mediate stress dampening.
Restraint Stress and Plasma Hormone Measurement.
On the morning (0800 hours) after completion of the palatable drink paradigm (day 15), rats did not receive their respective drink solutions and were given a novel 20-min restraint stress challenge with assessment of plasma ACTH and corticosterone levels by RIA.
Sexual Activity Paradigm.
Sexually naïve male rats (n = 10–16 per group) were given once-daily brief (30 min) access to a sexually receptive female in their home cage for 14 d at 2 h before lights-on, and subsequent sexually activity was monitored under red light. Control rats either received no female rat into their cage, or received “sham sex” in which male rats received a female rat that was confined inside a small wire mesh box to prevent sexual interactions. On day 15, at 0–4 h before lights-on, all of the male rats were given a novel restraint stress challenge with blood collection as described above. The sexually receptive female rats were prepared as described in the SI Materials and Methods.
Telemetric Recording of Cardiovascular Parameters.
Rats (n = 11 per group) were anesthetized with isofluorane and implanted with telemetric devices (PA-C20; Data Sciences International) to measure physiological variables (e.g., blood pressure, heart rate).
Behavioral Assessments.
Rats (n = 12 per group) were given 14 d of limited, intermittent sucrose drink (vs. water-drinking controls) as described above. On the morning of day 15, rats were given a standard social-interaction test. Additional rats (n = 10–11 per group) were also given limited, intermittent sucrose drink (vs. water-drinking controls) as described above, and they subsequently were given standard open-field and elevated plus-maze behavioral tests.
BLA Lesions.
Rats (n = 12–15 per group) were anesthetized and given stereotaxic bilateral infusions of ibotenate (2.5 mg/0.5 μL per side, pH 7) or vehicle (PBS) targeted to the BLA (anterior–posterior = −2.7 mm, medial–lateral = ±5.0 mm, and dorsal–ventral = −7.65 mm from bregma with level skull). After at least 7 d recovery from surgery, rats were given access to limited, intermittent sucrose drink (versus water-drinking controls) twice daily for 14 d (as described above). On the morning (0800 hours) after completion of drink exposure (day 15), rats did not receive their respective drink solutions and were given a novel restraint stress challenge as described above. Lesions were verified with NeuN immunolabeling.
Microarray and Functional Clustering Analysis.
Rats (n = 12 per group) with free access to normal chow and water received additional limited intermittent sucrose (30% vs. water-drinking controls) as described above. On the morning (0800 hours) of day 15, the rats were killed by decapitation, and brains were quickly removed and placed into ice-cold PBS. The BLA was quickly dissected from the brains, placed into tubes containing RNAlater (Ambion), and stored at −80 °C until RNA was isolated with the RNeasy kit (Qiagen). RNA samples from two rats (randomly matched from within each group) were pooled to produce n = 6 RNA samples per group for microarray analysis.
BLA Immunolabeling.
Paraformaldehyde-perfused brains from rats with a history of 14 d of limited intermittent sucrose (vs. water) were immunolabeled for pCREB (S133; 1:500; 06-519; Millipore), pCamKIIα (T286; 1:100; ab5683; Abcam), and synaptophysin (1:300, 18-0130; Invitrogen) via standard immunolabeling procedures.
Image Analysis.
NeuN, pCREB, pCamKIIα, and synaptophysin immunolabeling were imaged and analyzed with a Zeiss Imager.Z1 microscope with Apotome, AxioCam camera, and AxioVision Rel. 4.6 software (Carl Zeiss). All analyses were performed by personnel unaware of rat group assignments.
Statistical Analyses.
Data are depicted as mean ± SEM. For comparisons of multiple groups, data were analyzed either by ANOVA (one-way or two-way, as appropriate) with repeated measures (when appropriate), followed by protected Fisher's post hoc analysis (SI Materials and Methods). Student's t tests were used for comparisons of two groups (SI Materials and Methods). Statistical significance was taken as P < 0.05.
Supplementary Material
Acknowledgments
We thank Neil Dazet, Daovy Soukkaseum, and Emily Colvin for technical assistance; the Cincinnati Children's Hospital Medical Center Gene Expression Microarray Core Facility for measurement of tissue gene expression; and the Cincinnati Children's Hospital Biomedical Informatics Core for statistical analysis of the gene expression data. This work was supported by National Institutes of Health Grants DK078906, DK067820, DK014877, DK054890, DK056863, MH069725, DK066596, and MH049698.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE20344).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007740107/-/DCSupplemental.
References
- 1.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 2.Coccurello R, D'Amato FR, Moles A. Chronic social stress, hedonism and vulnerability to obesity: Lessons from rodents. Neurosci Biobehav Rev. 2009;33:537–550. doi: 10.1016/j.neubiorev.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 5.Gibson EL. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 7.Dubé L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiol Behav. 2005;86:559–567. doi: 10.1016/j.physbeh.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Utter AC, et al. Effect of carbohydrate ingestion and hormonal responses on ratings of perceived exertion during prolonged cycling and running. Eur J Appl Physiol Occup Physiol. 1999;80:92–99. doi: 10.1007/s004210050563. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KE, et al. Diet-hormone interactions: Protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40:1761–1768. doi: 10.1016/0024-3205(87)90086-5. [DOI] [PubMed] [Google Scholar]
- 10.Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- 11.Deuster PA, Singh A, Hofmann A, Moses FM, Chrousos GC. Hormonal responses to ingesting water or a carbohydrate beverage during a 2 h run. Med Sci Sports Exerc. 1992;24:72–79. [PubMed] [Google Scholar]
- 12.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich-Lai YM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeland WC, Miller P, Gann DS. Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology. 1989;124:2978–2985. doi: 10.1210/endo-124-6-2978. [DOI] [PubMed] [Google Scholar]
- 15.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 16.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 17.Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 18.Walsh RN, Cummins RA. The Open-Field Test: A critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 19.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 20.Tabuchi E, et al. Spatio-temporal dynamics of brain activated regions during drinking behavior in rats. Brain Res. 2002;951:270–279. doi: 10.1016/s0006-8993(02)03173-6. [DOI] [PubMed] [Google Scholar]
- 21.O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 22.Scott TR, et al. Gustatory neural coding in the amygdala of the alert macaque monkey. J Neurophysiol. 1993;69:1810–1820. doi: 10.1152/jn.1993.69.6.1810. [DOI] [PubMed] [Google Scholar]
- 23.Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- 24.Gianaros PJ, et al. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor SE, et al. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic–pituitary–adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 27.Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 30.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tominaga-Yoshino K, Urakubo T, Okada M, Matsuda H, Ogura A. Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus. 2008;18:281–293. doi: 10.1002/hipo.20391. [DOI] [PubMed] [Google Scholar]
- 34.Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 35.Abraham WC, Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem. 2008;89:260–268. doi: 10.1016/j.nlm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Kleim JA, Vij K, Ballard DH, Greenough WT. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–721. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coover G, Ursin H, Levine S. Corticosterone and avoidance in rats with basolateral amygdala lesions. J Comp Physiol Psychol. 1973;85:111–122. doi: 10.1037/h0034858. [DOI] [PubMed] [Google Scholar]
- 38.Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 39.McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- 40.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- 41.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 42.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 43.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 44.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 45.Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 47.Aroniadou-Anderjaska V, Post RM, Rogawski MA, Li H. Input-specific LTP and depotentiation in the basolateral amygdala. Neuroreport. 2001;12:635–640. doi: 10.1097/00001756-200103050-00041. [DOI] [PubMed] [Google Scholar]
- 48.Läck AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE. Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci. 2010;30:4676–4686. doi: 10.1523/JNEUROSCI.6165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: Mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 51.Lüscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 52.Dallman MF, et al. Chronic stress and obesity: A new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 55.de Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 56.Ren X, et al. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinkelmann K, et al. Cognitive impairment in major depression: Association with salivary cortisol. Biol Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 58.Michelson D, Gold PW. Pathophysiologic and somatic investigations of hypothalamic-pituitary-adrenal axis activation in patients with depression. Ann N Y Acad Sci. 1998;840:717–722. doi: 10.1111/j.1749-6632.1998.tb09610.x. [DOI] [PubMed] [Google Scholar]
- 59.Houston MC. Hypertension and coronary heart disease risk factor management. Clin Auton Res. 1993;3:357–361. doi: 10.1007/BF01829453. [DOI] [PubMed] [Google Scholar]
- 60.Malik M, et al. Depressed heart rate variability identifies postinfarction patients who might benefit from prophylactic treatment with amiodarone: A substudy of EMIAT (The European Myocardial Infarct Amiodarone Trial) J Am Coll Cardiol. 2000;35:1263–1275. doi: 10.1016/s0735-1097(00)00571-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.