Abstract
People with cystic fibrosis (CF) exhibit growth defects. That observation has been attributed, in part, to decreased insulin-like growth factor 1 (IGF1) levels, and the reduction has been blamed on malnutrition and pulmonary inflammation. However, patients with CF already have a reduced weight at birth, a manifestation not likely secondary to poor nutrition or inflammation. We found that, like humans, CF pigs were smaller than non-CF littermates and had lower IGF1 levels. To better understand the basis of IGF1 reduction, we studied newborn pigs and found low IGF1 levels within 12 h of birth. Moreover, humerus length and bone mineral content were decreased, consistent with less IGF1 activity in utero. These findings led us to test newborn humans with CF, and we found that they also had reduced IGF1 levels. Discovering lower IGF1 levels in newborn pigs and humans indicates that the decrease is not solely a consequence of malnutrition or pulmonary inflammation and that loss of cystic fibrosis transmembrane conductance regulator function has a more direct effect. Consistent with this hypothesis, we discovered reduced growth hormone release in organotypic pituitary slice cultures of newborn CF pigs. These findings may explain the long-standing observation that CF newborns are smaller than non-CF babies and why some patients with good clinical status fail to reach their growth potential. The results also suggest that measuring IGF1 levels might be of value as a biomarker to predict disease severity or the response to therapeutics. Finally, they raise the possibility that IGF1 supplementation beginning in infancy might be beneficial in CF.
Patients with cystic fibrosis (CF) have well-documented growth defects (1–4). However, the causes of growth defects in people with CF are still being elucidated. Malnutrition and the consequences of chronic lung infection likely play important roles (for a review, see ref. 5). However, evidence suggests that even individuals with good clinical status do not reach their full growth potential (3). An appealing candidate for explaining this discrepancy is the somatotropic axis. Indeed, more than four decades ago, Green et al. reported that patients with CF had altered regulation of growth hormone (GH) (6). Subsequent studies also investigated GH release; however, the directionality of GH changes has not always been consistent, and the contribution of GH remains uncertain (7). Additional studies have suggested that reduced levels of insulin-like growth factor 1 (IGF1), the main effector of GH action on somatic growth, might be responsible, at least in part, for the growth defects in patients with CF (8, 9) and in CF mice (10).
Although much attention has been focused on growth deficits in children, adolescents, and adults, evidence suggests that individuals with CF are already different at birth. For example, several studies indicate that CF newborns weigh less, are shorter, and have a smaller head circumference than non-CF newborns do (2, 11, 12). The biological basis for these differences has not been identified.
We recently targeted the porcine CFTR gene and generated CFTR−/− and CFTR−/ΔF508 pigs (hereafter called “CF pigs”) (13, 14). Newborn CF pigs display defective Cl– transport, meconium ileus, pancreatic destruction, and early focal biliary cirrhosis that replicate disease in newborn humans with CF (14). At birth, lungs of CF pigs lack inflammation, but, with time, they spontaneously develop infection, inflammation, and remodeling (15). This model provided us with an opportunity to ask whether CF pigs also manifest a growth defect. When we found that they did, we tested the hypothesis that CF pigs have reduced IGF1 levels.
Results
CF Pigs Show Reduced Growth and Lower IGF1 Levels.
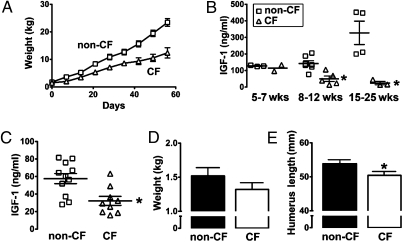
All newborn CF pigs have meconium ileus (14). In humans, surgery is often required to treat the intestinal obstruction, and we therefore gave CF pigs (and littermate controls) an ostomy to circumvent the obstruction (15). We measured weight in five CF and seven non-CF pigs over time and found that CF pigs gained less weight than non-CF controls did (Fig. 1A).
Fig. 1.
IGF1 deficits and growth parameters in CF pigs. (A) CF pigs (n = 5) gained less weight than did non-CF pigs (n = 7). Data are mean ± SE. (B) CF pigs (n = 5) had reduced plasma IGF1 levels compared with non-CF pigs (n = 7). Differences became more exaggerated over time. CF pigs, two females and three males; non-CF pigs, three females and four males. Data are for individual animals; line represents mean ± SE. *P = 0.003 compared with non-CF pigs at 8–12 wks and P = 0.016 at 15–25 wks versus non-CF pigs. (C) Plasma IGF1 levels in newborn piglets 6–12 h old indicated that CF piglets (five females and four males) had significantly reduced plasma IGF levels relative to non-CF piglets (seven females and four males); P = 0.005. Data are for individual animals; line represents mean ± SE. (D) Average birth weight was lower, but not statistically different, in CF compared with non-CF piglets (P = 0.12). (E) Humerus length of newborn CF piglets was shorter than that of non-CF animals (P = 0.04). For D and E, CF, 7 females and 11 males; non-CF, 7 females and 10 males. Data are mean ± SE.
Knowing the association between reduced IGF1 and growth in humans with CF, we measured serum IGF1 levels (Fig. 1B). Wild-type pigs showed the expected increase in IGF1 with time. In contrast, levels were lower in CF pigs.
Newborn CF Pigs Have Reduced IGF1, Bone Length, and Bone Mineral Content.
Although malnutrition caused by pancreatic insufficiency and chronic pulmonary inflammation might limit growth later in life (16, 17), these factors will not have contributed at the time of birth because fetal nutrition is from the mother and chronic lung infection and inflammation begin after birth. Moreover, IGF1 does not cross the placenta (18). Therefore, to learn whether lack of cystic fibrosis transmembrane conductance regulator (CFTR) might have a more direct effect on growth, we measured serum IGF1 levels at the time of birth and found that they were reduced in CF pigs compared with littermate controls (Fig. 1C).
Reduced IGF1 levels were predictors that newborn CF pigs would show evidence of impaired in utero growth. Although, on average, CF piglets weighed 14% less than their non-CF littermates did, the difference was not statistically significant (Fig. 1D). Because IGF1 is important for bone size (19), we also measured humerus length. Newborn CF pigs had a humerus length that was ∼9% shorter than that of non-CF controls (Fig. 1E). Likewise, bone mineral content and area were reduced in humeri from newborn CF pigs (Table 1). In contrast, bone density, which shows little relationship to cord blood IGF1 (20), did not differ by genotype. Decreased bone mineral density has been reported in CF mice, but these studies have tended to focus on older mice (21–24). Whether bone mineral density will decrease with time in CF pigs and whether IGF1 will contribute to this process remain to be determined. However, finding a growth defect in utero suggests a potential physiological consequence of decreased IGF1 in newborn CF piglets.
Table 1.
Humerus bone morphometry and density in newborn non-CF and CF pigs
| Parameter | Non-CF | CF | P value |
| Total content (mg/mm) | 30.8 ± 3.6 | 20.8 ± 2.1 | 0.02 |
| Total density (mg/cm3) | 270.0 ± 7.1 | 266.9 ± 5.2 | 0.73 |
| Total area (mm2) | 110.3 ± 11.0 | 78.2 ± 8.4 | 0.03 |
| Trabecular area (mm2) | 89.5 ± 10.1 | 60.6 ± 7.6 | 0.03 |
| Cortical/subcortical area (mm2) | 20.9 ± 1.1 | 17.6 ± 0.8 | 0.03 |
Pituitary of CF Pigs Shows Reduced GH Secretion.
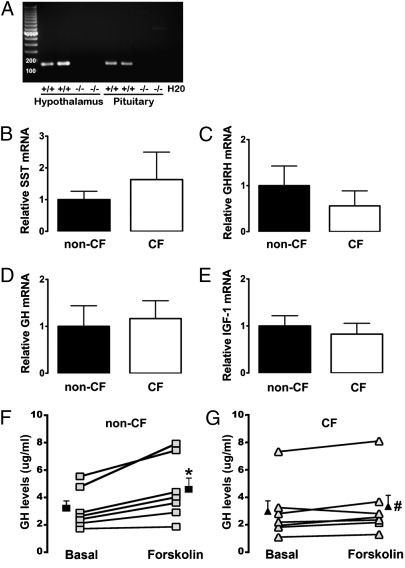
Circulating IGF1 is synthesized and released predominantly from hepatocytes; yet CFTR is primarily expressed in the apical membrane of bile duct cells (25). Thus, lack of CFTR in the liver seems unlikely to be the source of reduced circulating IGF1. Because IGF1 synthesis is under somatotropic axis control, we tested for CFTR mRNA and found it in hypothalami and pituitaries of newborn pigs (Fig. 2A) (26). This result suggested that expression of key somatotropic hormones might be altered; however, we detected no differences in mRNA for somatostatin or GH-releasing hormone in the hypothalamus, GH in the pituitary, or IGF1 in the liver (Fig. 2 B–E). Because previous data suggested that CFTR might affect neuropeptide release (27), we reasoned that GH release might be impaired. To test this hypothesis, we cultured organotypic pituitary slices from CF and non-CF pigs and measured GH release after adding forskolin to elevate cellular cAMP levels (27). Forskolin induced a greater GH increase in non-CF than in CF pituitary slices (Fig. 2 F and G). These data suggest that lack of CFTR impairs release of GH, which may explain, in part, the reduced IGF1 levels.
Fig. 2.
Decreased GH release in CF pituitary cultures. (A) Representative example of RT-PCR products of CFTR in hypothalamus and pituitary. (B–E) There were no differences in mRNA levels of key somatotropic hormones and IGF1 in newborn piglet samples. (B) Hypothalamic somatostatin (SST) mRNA (non-CF, four females and four males; CF, four females and six males; P = 0.39). (C) Hypothalamic GH-releasing hormone (GHRH) mRNA (non-CF, four females and five males; CF, three females and five males; P = 0.21). (D) Pituitary GH mRNA (non-CF, five females and four males; CF, five females and four males; P = 0.70). (E) Liver IGF1 mRNA (non-CF, three females and three males; CF, four females and four males; P = 0.62). Data are expressed relative to non-CF and represent mean ± SEM. (F and G) Forskolin increased GH content in media of pituitary slice cultures (non-CF, four females and three males; CF, three females and four males). Two male CFTR+/− heterozygotes were included as non-CF controls. Data are from individual animals; filled symbols are mean ± SE. *Relative to basal levels; P = 0.006. #Forskolin-induced increase in CF was different from that in non-CF; P < 0.02.
Human Newborns with CF Have Reduced IGF1 Levels.
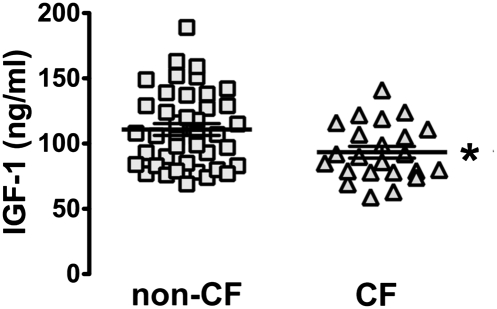
Our discovery that IGF1 levels are reduced in CF pigs at birth suggested that the same might be true for humans. In Iowa, a blood spot is collected from newborns for genetic screening for CF and other inherited diseases. Previous work demonstrated the utility and validity of measuring IGF1 in dried blood spots (28). Therefore, we measured IGF1 levels in blood spots and found that newborns with CF had a small but significant reduction in IGF1 levels relative to non-CF babies (Fig. 3). These findings may explain the long-standing observation that CF babies are smaller than non-CF babies are at birth (2, 11, 12).
Fig. 3.
IGF1 levels in CF infants. IGF1 levels in human newborns with CF (n = 23) were lower than in non-CF newborns (n = 41); *P = 0.016. Symbols indicate individual newborns; lines represent mean ± SE. We detected no relationship between the CFTR genotype and IGF1 levels (not shown), although our sample size may have been too small to detect one if it exists.
Discussion
IGF1 is critical for both fetal and childhood growth (29). Serum IGF1 concentrations in the fetus correlate with fetal size (30), and alterations in the genes encoding IGF1 and the IGF1 receptor retard fetal growth (31–34). Targeted disruption of the mouse IGF1 and IGF genes also markedly reduces fetal growth (35, 36). Although IGF1 levels are reduced in CF (8, 9), the temporal and/or causal relationships between lower IGF1 levels and growth defects and disease in CF have remained uncertain. Previous studies proposed that malnutrition and chronic pulmonary inflammation reduce IGF1 (37, 38). Our data do not argue against the contribution of these factors in older children and adults where IGF1 levels have previously been measured (8, 9, 39). However, our results in newborn pigs indicate that CFTR loss has an additional, more direct effect in reducing IGF1 levels. Moreover, in older patients and CF pigs, perhaps the combination of the effect we report plus the secondary effects on IGF1 levels related to disease progression might reduce IGF1 levels more than either alone.
Loss of CFTR function in the pituitary may contribute to reduced IGF1 levels in newborns (40). Consistent with this hypothesis, lack of CFTR expression decreased forskolin-stimulated release of GH from pituitary slice cultures. Although earlier studies postulated that older patients with CF have alterations in the somatotropic axis, the location of the defect is uncertain (41), and there has been disagreement about the direction of changes (7). Given the small differences we observed in GH release, and that GH levels can change rapidly and acutely, it is not surprising that this discrepancy exists. Our finding that CFTR affects GH release is consistent with an earlier study showing CFTR involvement in neuropeptide secretion (27). In addition, evidence suggests that CFTR functionally interacts with proteins important for vesicular release, including syntaxin1a, SNAP-23, and Munc18 (42).
It is important to note, however, that the role of GH in regulating intrauterine growth and IGF1 levels remains unsettled. Although GH expression begins during early gestation, the GH receptor is not expressed until late in gestation (29, 43). Studies of infants with congenital hypopituitarism, GH deficiency, and GH receptor mutations (Laron syndrome) have shown either reduced birth length or a birth length within the normal range, and the reasons for the differences are not certain (29, 43–47). An association between birth weight and polymorphisms at the transcriptional start site of pituitary GH has been reported (48), and a study of GH-deficient rats reported reduced fetal growth (49). However, other factors affect in utero IGF1 levels and growth, including nutrition and insulin levels. Thus, whether blunted GH release occurs in vivo in CF pigs and humans and whether it contributes in part, in whole, or not at all, to reduced IGF1 remains uncertain. Moreover, we cannot exclude other mechanisms that might reduce IGF1 levels in CF. Thus, substantial additional studies will be required to precisely elucidate how loss of CFTR function decreases IGF1 levels. In addition, measuring levels of IGF-binding proteins may be of value in future studies because they play important roles in determining the biological effects of IGF1.
Our findings have several implications and raise interesting questions. First, early diagnosis and an aggressive emphasis on nutrition have had important beneficial effects on increasing patient size and weight (50, 51). Yet, despite vigorous attempts, many patients with CF fail to reach their full growth potential (52). At 2 y of age, one-third of infants diagnosed by newborn screening (excluding those who had meconium ileus) failed to achieve their predicted weight (53). Moreover, the growth in height and weight of the 22% of CF infants who presented with meconium ileus was even less than that of infants identified by newborn screening (54). Might differences in IGF1 levels explain some of the variability in patient size and the response to maximal nutrition efforts?
Second, might IGF1 levels be of prognostic or predictive value in patients with CF? It is possible that measuring IGF1 at birth or within the first months of life, and then with time, could predict disease severity and/or response to therapeutics. Compared with GH, plasma IGF1 has an advantage as a biomarker because its concentrations are relatively stable, whereas GH shows marked pulsatile secretion and a short half-life. Thus, a single IGF1 measurement is thought to be as reliable as a protocol that obtains multiple samples of GH (55).
Finally, might IGF1 or GH supplementation be of benefit beginning in infancy? Previous studies suggested that GH administration may have beneficial effects in CF, including increased growth, increased bone mineral content, improved pulmonary function, and reduced hospitalization incidence (7, 56, 57). Those studies in older children, adolescents, and adults suggest that modifying the somatotropic axis may have therapeutic benefit. Recombinant human IGF1 was recently approved by the US Food and Drug Administration and the European Medicines Agency for treatment of severe primary IGF1 deficiency. One study examined the effects of 6 mo of IGF1 administered to CF adolescents (58); although there was no significant effect on growth rate or weight gain, it is possible that the targeted age group was beyond the therapeutic window to observe beneficial effects of replacing IGF1. Another study of 7 mo of IGF1 replacement is underway (trial NCT00566241, www.clinicaltrials.gov), but the age range is ≥18 y. However, we are not aware of studies administering IGF1 to infants, and, as discussed above, the consequences of IGF1 administration in this age group are not known. Perhaps newborn CF pigs might provide a model for testing this therapeutic approach. If additional larger studies reveal reduced IGF1 levels in newborn patients with CF and suggest a relationship with body size and disease severity, then it may be worth considering early replacement of IGF1 or GH for patients with CF, much as we replace their pancreatic enzymes and insulin.
Materials and Methods
Animals.
We previously reported generation of CFTR+/− and CFTR+/ΔF508 pigs, and production of CFTR−/− and CFTR−/ΔF508 pigs (13, 14). Animals were mated, and progeny was studied. Two different cohorts of animals were used for these studies. (i) Newborn piglets were used for studies of newborn IGF1 levels, birth weight, bone morphometric indices, and pituitary cultures. Only CFTR−/− pigs were used for studies at birth. Newborn piglets were separated from the sow within 6 h of birth. Piglets were weighed, sedated with ketamine and xylazine, and euthanized with an i.v. overdose of pentobarbital/phenytoin. (ii) Older pigs were used for studies of weight gain and IGF1 levels measured over time. Because of the limited number of animals surviving surgical correction of the meconium ileus, our studies of older pigs used both CFTR−/− (n = 3) and CFTR−/ΔF508 (n = 2) genotypes. Within 8–12 h after birth, control and CF piglets underwent placement of an ileostomy or cecostomy to prevent complications from meconium ileus. In the perioperative period, animals were maintained on i.v. fluids and prophylactic antibiotics. Thereafter, piglets were fed milk replacer and weaned to a solid diet. Animals received daily pancreatic enzymes, fat-soluble vitamins, an H2 blocker or a proton-pump inhibitor, and polyethylene glycol. Standard procedures for animal husbandry were used. All animal experiments were approved by the Institutional Animal Care and Use Committees of the Universities of Iowa and Missouri.
IGF1 Measurements.
Serum was obtained from newborn piglets within 6–12 h of birth and periodically from older pigs and stored at −20 °C until analysis of duplicate samples. In the active IGF1 ELISA, IGF1 was separated from its binding protein in serum by using an ethanol and HCl-based extraction solution provided by the manufacturer (Diagnostic Systems Laboratories). Extraction proceeded for 30 min followed by a neutralization step. The neutralized sample was then spun briefly for 3 min at 8,176 × g, and the clear supernatant was used for the ELISA. The minimal detection limit was 0.03 ng/mL The intra-assay and interassay coefficients of variation were 5.1% (n = 4) and 5% (n = 4), respectively.
IGF1 levels were measured in blood spots obtained from non-CF and CF infants born between 2007 and 2009. CF infants were detected by the Iowa Neonatal Metabolic Screening Program. All studies were approved by the University of Iowa Institutional Review Board and the Congenital and Inherited Disorders Advisory Committee of the Iowa Department of Public Health. A diagnosis of CF was confirmed by presence of two CFTR disease-causing mutations, elevated sweat chloride levels, and/or abnormal nasal voltage measurements. Of 23 CF infants, 8 were CFTRΔF508/ΔF508. Of the remaining 15 CF infants, 11 had one CFTRΔF508 allele, and 5 had at least one CFTR-R117H mutation. Control blood spots were randomly obtained from samples collected over the same time period, but they had normal immunoreactive trypsinogen levels. All blood spots were frozen from time of collection. The dried blood spot samples (8-mm punch) were extracted and analyzed for IGF1 by using Diagnostic Systems Laboratories ACTIVE nonextraction IGF-I ELISA. All measurements were performed by ZRT Laboratory.
Bone Morphometry Measurements.
Pig forelimbs were removed from 18 CF and 17 non-CF pigs and stored in 10% formaldehyde. The humeri were dissected and measured lengthwise from the humeral head to the groove between the medial and lateral epicondyle with digital calipers (Fisher Scientific).
Peripheral Quantitative Computed Tomography.
Humeri were placed in an XCT 3000 Research (STRATEC Biomedical Systems) scanner, and an axial scout scan was performed. A reference line was established at the distal end of the humerus, and a 2-mm-thick slice was positioned 9 mm from the proximal end of the bone (metaphyseal region). Bone mineral content, density, and area were calculated by using standard CalcBD and CortBD routines. Tissue measuring ≤200 mg/cm3 was classified as soft tissue. Trabecular (cancellous) bone threshold was between 200 and 500 mg/cm3. Cortical (compact) bone was represented by values higher than 700 mg/cm3.
RT-PCR.
The liver, pituitary, and brain were rapidly removed and placed into RNA later solution (Ambion). Two horizontal cuts on the ventral surface of the brain (one just caudal to the optic chiasm and another made rostral to the mammillary bodies) were made, and the hypothalamus was removed. Total RNA was isolated with the Qiagen Lipid Tissue RNeasy kit. RNA quality and concentration were assessed via Nanodrop. RNA was reverse-transcribed with the RT2 First Strand Kit (SA Biosciences). Quantitative RT-PCR was used to measure mRNA. The following primer sequences were used: somatostatin (forward, 5′-ATCGTCCTGGCTTTGGGC-3′; reverse, 5′-GCCTCATCTCGTCCTGCTCA-3′) (59); GH-releasing hormone (forward, 5′-TATGCAAATGCCATCTTC-3′; reverse, 5′-AAGCCGTACCCTTGCTCC-3′) (60); GH (forward, 5′-GTTTGGCACCTCAGACCG-3′; reverse, 5′-CCCAGCAACTAGAAGGCACAGC-3′) (60); IGF1 (forward, 5′-CACATCACATCCTCTTCG-3′; reverse, 5′-CTGGAGCCGTACCCTGTG-3′); and actin (forward, 5′-CTGCGGCATCCACGAAACT-3′; reverse, 5′-GTGATCTCCTTCTGCATCCTGTC-3′). Primer efficiency and validation were performed as described in ref. 61. PCR was performed in sample triplicates with RT2 SYBR Green qPCR Master Mixes (SA Biosciences) and a 7500 Fast Real-Time PCR System (Applied Biosystems), following manufacturers’ protocol. RNA not reverse-transcribed was used as control. Standard delta delta CT method with actin as a reference was used for analysis. For porcine CFTR, amplification consisted of 35 cycles at 94 °C for 5 s, 55 °C for 5 s, and 72 °C for 15 s, and a final extension cycle of 72 °C for 5 min with the following primers: forward, 5′-CTGGAGCCTTCAGAGGGTAAAAT-3′; reverse, 5′-AGTTGGCACGCTTTGATGACACTCC-3′. Products were separated on a 1.7% agarose gel.
Organotypic Pituitary Slice Culture and GH Measurement.
Whole pituitaries of newborn CF and non-CF pigs were quickly removed and dissected into 350-μm-thick coronal sections by using a manual tissue chopper. Slices were placed in polyethylene terephthalate–etched membrane culture inserts containing 1-μm pores (Fisher Scientific). Slices were cultured in 25% horse serum, 25% HBSS, 50% MEM, 2 mM glutamine, 1 mg/mL glucose, 44 mg/mL NaHCO3, and 10 units/mL penicillin-streptomycin in a 5% CO2 humidified incubator. On day 6 or 7, medium was changed and slices were incubated in serum-free medium (MEM replaced serum, and osmolarity was adjusted with bovine albumin fraction V to 1.5%) for 3 h. A 100-μL sample of culture medium was collected to measure basal GH levels, followed by addition of forskolin (0.5 μM) to stimulate release of GH (62). At 30 min later, another 100-μL sample of culture medium was collected. GH levels were measured by ELISA (Millipore) in duplicate samples. Porcine GH (obtained from A. F. Parlow of the National Institute of Diabetes and Digestive and Kidney Diseases’ National Hormone and Peptide Program) was used to construct standard curves. The minimal detection limit was 0.07 ng/mL The intra-assay and interassay coefficients of variation were 3.8% (n = 4) and 4% (n = 4), respectively.
Statistical Analysis.
Results are expressed as mean ± SE. For IGF1 analysis in older pigs, a one-way ANOVA was performed, followed by a Fisher's least significant difference post hoc test to determine the source of significance. For all other measurements, statistical differences between CF and non-CF were evaluated with a two-tailed unpaired t test. Statistical significance was determined by P ≤ 0.05. A Grubbs test was performed once to detect statistical outliers.
Acknowledgments
We thank Theresa Mayhew, Elizabeth Dowd, Dr. Sara Copeland (Director of the Iowa Neonatal Metabolic Screening Program), Dr. Stanton Berberich, Kimberly Noble Piper, the Iowa Department of Public Health (IDPH), the University of Iowa Hygienic Laboratory, and the Congenital and Inherited Disorders Advisory Committee of the IDPH for their assistance with the blood spot portion of this study. We also appreciate the help and comments of Peter Taft, Paula Ludwig, Emma Hornick, Mike Rector, Robert Hanfland, and Joel Shilyansky. This work was supported by the National Heart, Lung, and Blood Institute (HL91842) and the Cystic Fibrosis Foundation. D.A.S. is a Parker B. Francis Fellow and was supported by the National Institute of Allergy and Infectious Diseases (AI076671). M.J.W. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: Michael Welsh holds equity in Exemplar Genetics, which is licensing materials and technology related to this work.
References
- 1.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41:583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 2.Haeusler G, Frisch H, Waldhör T, Götz M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. Eur J Pediatr. 1994;153:158–163. doi: 10.1007/BF01958975. [DOI] [PubMed] [Google Scholar]
- 3.Hardin DS, et al. A retrospective study of growth hormone use in adolescents with cystic fibrosis. Clin Endocrinol (Oxf) 2005;62:560–566. doi: 10.1111/j.1365-2265.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein MN, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533–540. doi: 10.1016/s0022-3476(05)82478-3. [DOI] [PubMed] [Google Scholar]
- 5.Patel L, Dixon M, David TJ. Growth and growth charts in cystic fibrosis. J R Soc Med. 2003;96(Suppl 43):35–41. [PMC free article] [PubMed] [Google Scholar]
- 6.Green OC, Fefferman R, Nair S. Plasma growth hormone levels in children with cystic fibrosis and short stature. Unresponsiveness to hypoglycemia. J Clin Endocrinol Metab. 1967;27:1059–1061. doi: 10.1210/jcem-27-7-1059. [DOI] [PubMed] [Google Scholar]
- 7.Huseman CA, et al. Anabolic effect of biosynthetic growth hormone in cystic fibrosis patients. Pediatr Pulmonol. 1996;22:90–95. doi: 10.1002/(SICI)1099-0496(199608)22:2<90::AID-PPUL2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AM, et al. The relationship between insulin, IGF-I and weight gain in cystic fibrosis. Clin Endocrinol (Oxf) 1999;51:659–665. doi: 10.1046/j.1365-2265.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- 9.Switzer M, Rice J, Rice M, Hardin DS. Insulin-like growth factor-I levels predict weight, height and protein catabolism in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab. 2009;22:417–424. doi: 10.1515/jpem.2009.22.5.417. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59:191–195. doi: 10.1203/01.pdr.0000196720.25938.be. [DOI] [PubMed] [Google Scholar]
- 11.Ghosal S, et al. Disproportionate head growth retardation in cystic fibrosis. Arch Dis Child. 1995;72:150–152. doi: 10.1136/adc.72.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Festini F, et al. Gestational and neonatal characteristics of children with cystic fibrosis: A cohort study. J Pediatr. 2005;147:316–320. doi: 10.1016/j.jpeds.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Rogers CS, et al. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus–mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss RB. Cystic fibrosis: Pathogenesis, pulmonary infection, and treatment. Clin Infect Dis. 1995;21:839–849. doi: 10.1093/clinids/21.4.839. quiz 850–851. [DOI] [PubMed] [Google Scholar]
- 17.Street ME, et al. The IGF system and cytokine interactions and relationships with longitudinal growth in prepubertal patients with cystic fibrosis. Clin Endocrinol (Oxf) 2009;70:593–598. doi: 10.1111/j.1365-2265.2008.03387.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: Relation to fetal weight. J Endocrinol. 1991;129:459–464. doi: 10.1677/joe.0.1290459. [DOI] [PubMed] [Google Scholar]
- 19.Yakar S, et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24:1481–1492. doi: 10.1359/JBMR.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaid MK, et al. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J Bone Miner Res. 2004;19:56–63. doi: 10.1359/JBMR.0301211. [DOI] [PubMed] [Google Scholar]
- 21.Haston CK, Li W, Li A, Lafleur M, Henderson JE. Persistent osteopenia in adult cystic fibrosis transmembrane conductance regulator-deficient mice. Am J Respir Crit Care Med. 2008;177:309–315. doi: 10.1164/rccm.200705-659OC. [DOI] [PubMed] [Google Scholar]
- 22.Saeed Z, et al. Fenretinide prevents the development of osteoporosis in Cftr-KO mice. J Cyst Fibros. 2008;7:222–230. doi: 10.1016/j.jcf.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Dif F, Marty C, Baudoin C, de Vernejoul MC, Levi G. Severe osteopenia in CFTR-null mice. Bone. 2004;35:595–603. doi: 10.1016/j.bone.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Pashuck TD, et al. Murine model for cystic fibrosis bone disease demonstrates osteopenia and sex-related differences in bone formation. Pediatr Res. 2009;65:311–316. doi: 10.1203/PDR.0b013e3181961e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn JA, et al. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 26.Mulberg AE, Weyler RT, Altschuler SM, Hyde TM. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport. 1998;9:141–144. doi: 10.1097/00001756-199801050-00028. [DOI] [PubMed] [Google Scholar]
- 27.Weyler RT, et al. CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am J Physiol. 1999;277:C563–C571. doi: 10.1152/ajpcell.1999.277.3.C563. [DOI] [PubMed] [Google Scholar]
- 28.Schütt BS, Weber K, Elmlinger MW, Ranke MB. Measuring IGF-I, IGFBP-2 and IGFBP-3 from dried blood spots on filter paper is not only practical but also reliable. Growth Horm IGF Res. 2003;13:75–80. doi: 10.1016/s1096-6374(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld RG. Insulin-like growth factors and the basis of growth. N Engl J Med. 2003;349:2184–2186. doi: 10.1056/NEJMp038156. [DOI] [PubMed] [Google Scholar]
- 30.Lassarre C, et al. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–225. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 32.Walenkamp MJ, et al. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab. 2005;90:2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- 33.Vaessen N, et al. Association between genetic variation in the gene for insulin-like growth factor-I and low birthweight. Lancet. 2002;359:1036–1037. doi: 10.1016/s0140-6736(02)08067-4. [DOI] [PubMed] [Google Scholar]
- 34.Abuzzahab MJ, et al. Intrauterine Growth Retardation (IUGR) Study Group IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med. 2003;349:2211–2222. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- 35.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 36.Powell-Braxton L, et al. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12B):2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 37.Phillips LS, Vassilopoulou-Sellin R. Nutritional regulation of somatomedin. Am J Clin Nutr. 1979;32:1082–1096. doi: 10.1093/ajcn/32.5.1082. [DOI] [PubMed] [Google Scholar]
- 38.Fan J, Li YH, Bagby GJ, Lang CH. Modulation of inflammation-induced changes in insulin-like growth factor (IGF)-I and IGF binding protein-1 by anti-TNF antibody. Shock. 1995;4:21–26. doi: 10.1097/00024382-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AM, et al. Relation between insulin-like growth factor-I, body mass index, and clinical status in cystic fibrosis. Arch Dis Child. 1997;76:304–309. doi: 10.1136/adc.76.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pirazzoli P, et al. Developmental pattern of fetal growth hormone, insulin-like growth factor I, growth hormone binding protein and insulin-like growth factor binding protein-3. Arch Dis Child Fetal Neonatal Ed. 1997;77:F100–F104. doi: 10.1136/fn.77.2.f100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laursen EM, et al. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur J Endocrinol. 1999;140:315–321. doi: 10.1530/eje.0.1400315. [DOI] [PubMed] [Google Scholar]
- 42.Guggino WB, Stanton BA. New insights into cystic fibrosis: Molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 43.Ogilvy-Stuart AL. Growth hormone deficiency (GHD) from birth to 2 years of age: Diagnostic specifics of GHD during the early phase of life. Horm Res. 2003;60(Suppl 1):2–9. doi: 10.1159/000071219. [DOI] [PubMed] [Google Scholar]
- 44.Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): The personal experience 1958-2003. J Clin Endocrinol Metab. 2004;89:1031–1044. doi: 10.1210/jc.2003-031033. [DOI] [PubMed] [Google Scholar]
- 45.Gluckman PD, et al. Congenital idiopathic growth hormone deficiency associated with prenatal and early postnatal growth failure. The International Board of the Kabi Pharmacia International Growth Study. J Pediatr. 1992;121:920–923. doi: 10.1016/s0022-3476(05)80342-7. [DOI] [PubMed] [Google Scholar]
- 46.Wit JM, van Unen H. Growth of infants with neonatal growth hormone deficiency. Arch Dis Child. 1992;67:920–924. doi: 10.1136/adc.67.7.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pena-Almazan S, Buchlis J, Miller S, Shine B, MacGillivray M. Linear growth characteristics of congenitally GH-deficient infants from birth to one year of age. J Clin Endocrinol Metab. 2001;86:5691–5694. doi: 10.1210/jcem.86.12.8068. [DOI] [PubMed] [Google Scholar]
- 48.Adkins RM, Campese C, Vaidya R, Boyd TK. Association between fetal growth restriction and polymorphisms at sites -1 and +3 of pituitary growth hormone: A case-control study. BMC Pregnancy Childbirth. 2005;5:2. doi: 10.1186/1471-2393-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JD, Näntö-Salonen K, Szczepankiewicz JR, Rosenfeld RG, Glasscock GF. Evidence for pituitary regulation of somatic growth, insulin-like growth factors-I and -II, and their binding proteins in the fetal rat. Pediatr Res. 1993;33:144–151. doi: 10.1203/00006450-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Ramsey BW, Farrell PM, Pencharz P, The Consensus Committee Nutritional assessment and management in cystic fibrosis: A consensus report. Am J Clin Nutr. 1992;55:108–116. doi: 10.1093/ajcn/55.1.108. [DOI] [PubMed] [Google Scholar]
- 51.Farrell PM, et al. Wisconsin Cystic Fibrosis Neonatal Screening Study Group Nutritional benefits of neonatal screening for cystic fibrosis. N Engl J Med. 1997;337:963–969. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 52.Morison S, et al. UK Cystic Fibrosis Survey Management Committee Height and weight in cystic fibrosis: A cross sectional study. Arch Dis Child. 1997;77:497–500. doi: 10.1136/adc.77.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shoff SM, Ahn HY, Davis L, Lai H, Wisconsin CF Neonatal Screening Group Temporal associations among energy intake, plasma linoleic acid, and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatrics. 2006;117:391–400. doi: 10.1542/peds.2004-2832. [DOI] [PubMed] [Google Scholar]
- 54.Lai HC, et al. Nutritional status of patients with cystic fibrosis with meconium ileus: A comparison with patients without meconium ileus and diagnosed early through neonatal screening. Pediatrics. 2000;105:53–61. doi: 10.1542/peds.105.1.53. [DOI] [PubMed] [Google Scholar]
- 55.Barreca A, et al. Insulin-like growth factor I and daily growth hormone profile in the assessment of active acromegaly. Acta Endocrinol (Copenh) 1989;120:629–635. doi: 10.1530/acta.0.1200629. [DOI] [PubMed] [Google Scholar]
- 56.Hardin DS. GH improves growth and clinical status in children with cystic fibrosis—A review of published studies. Eur J Endocrinol. 2004;151(Suppl 1):S81–S85. doi: 10.1530/eje.0.151s081. [DOI] [PubMed] [Google Scholar]
- 57.Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F, German Cystic Fibrosis Growth Hormone Study Group A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics. 2007;119:e1230–e1238. doi: 10.1542/peds.2006-2783. [DOI] [PubMed] [Google Scholar]
- 58.Bucuvalas JC, et al. Effect of insulinlike growth factor-1 treatment in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2001;33:576–581. doi: 10.1097/00005176-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Goddard I, et al. Somatostatin inhibits stem cell factor messenger RNA expression by Sertoli cells and stem cell factor-induced DNA synthesis in isolated seminiferous tubules. Biol Reprod. 2001;65:1732–1742. doi: 10.1095/biolreprod65.6.1732. [DOI] [PubMed] [Google Scholar]
- 60.Zhu G, Liu S, Jiang Y, Yang H, Li J. Growth hormone pathway gene expression varies in porcine cumulus-oocyte complexes during in vitro maturation. In Vitro Cell Dev Biol Anim. 2008;44:305–308. doi: 10.1007/s11626-008-9130-0. [DOI] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 62.Ray KP, Gomm JJ, Law GJ, Sigournay C, Wallis M. Dopamine and somatostatin inhibit forskolin-stimulated prolactin and growth hormone secretion but not stimulated cyclic AMP levels in sheep anterior pituitary cell cultures. Mol Cell Endocrinol. 1986;45:175–182. doi: 10.1016/0303-7207(86)90145-0. [DOI] [PubMed] [Google Scholar]