Abstract
The risk of adverse cardiovascular events peaks in the morning (≈9:00 AM) with a secondary peak in the evening (≈8:00 PM) and a trough at night. This pattern is generally believed to be caused by the day/night distribution of behavioral triggers, but it is unknown whether the endogenous circadian system contributes to these daily fluctuations. Thus, we tested the hypotheses that the circadian system modulates autonomic, hemodynamic, and hemostatic risk markers at rest, and that behavioral stressors have different effects when they occur at different internal circadian phases. Twelve healthy adults were each studied in a 240-h forced desynchrony protocol in dim light while standardized rest and exercise periods were uniformly distributed across the circadian cycle. At rest, there were large circadian variations in plasma cortisol (peak-to-trough ≈85% of mean, peaking at a circadian phase corresponding to ≈9:00 AM) and in circulating catecholamines (epinephrine, ≈70%; norepinephrine, ≈35%, peaking during the biological day). At ≈8:00 PM, there was a circadian peak in blood pressure and a trough in cardiac vagal modulation. Sympathetic variables were consistently lowest and vagal markers highest during the biological night. We detected no simple circadian effect on hemostasis, although platelet aggregability had two peaks: at ≈noon and ≈11:00 PM. There was circadian modulation of the cardiovascular reactivity to exercise, with greatest vagal withdrawal at ≈9:00 AM and peaks in catecholamine reactivity at ≈9:00 AM and ≈9:00 PM. Thus, the circadian system modulates numerous cardiovascular risk markers at rest as well as their reactivity to exercise, with resultant profiles that could potentially contribute to the day/night pattern of adverse cardiovascular events.
Keywords: cardiovascular disease
Cardiovascular disease is the leading cause of mortality in both men and women in developed countries (1, 2). Epidemiological studies show a clear day/night pattern in risk for myocardial infarction (3–6), stroke (7), angina (5), ventricular arrhythmias (8), and sudden cardiac death (9) with primary peaks in the morning (≈6:00 AM–noon), secondary peaks in the evening (≈6:00–10:00 PM), and lowest vulnerability during the night (≈midnight–6:00 AM) (reviewed in ref. 10). The morning peaks in cardiovascular events cannot be fully explained by the day/night pattern in behavioral triggers such as activity (11). The circadian system, including the suprachiasmatic nucleus (SCN) in the hypothalamus and circadian oscillators in peripheral tissues, orchestrates endogenous circadian rhythms in physiology and behavior (12). Findings from animal work show that the SCN may influence the cardiovascular system via humoral factors and multisynaptic neural projections to the heart, adrenal cortex, adrenal medulla, kidneys, and vasculature (13) and that circadian clock mutations (Clock, Bmal1, Npas2) lead to abnormalities in sympathetic activity, vasculature, and blood pressure stress responses (14, 15). To assess the influence of the circadian system in humans, we used the forced desynchrony (FD) protocol, the gold standard to dissociate the separate influences of the circadian system and behavioral/environmental influences upon variables of interest (16). In addition, by scheduling individuals to live on non-24-h days, the FD protocol enabled the comparison of the effects of standardized behavioral stressors occurring at different phases of the circadian cycle. Based on these principles, we tested two hypotheses: (i) the circadian system modulates autonomic, hemodynamic, and hemostatic biomarkers at rest; and (ii) reactivity of these biomarkers to exercise is altered at different circadian phases. We sought to determine whether circadian rhythms in specific markers of cardiovascular risk match the day/night pattern in adverse cardiovascular events (primary morning peak, secondary evening peak, and overnight trough).
Results
Each of 12 healthy subjects was scheduled to live on recurring 20-h “days” for 240 h (FD protocol) in an individual laboratory suite while we repeatedly assessed a cardiovascular biomarker panel during rest and standardized exercise (Fig. 1). This approach enabled us to quantify the independent influences of the circadian system (endogenous cycle length close to 24 h) and behaviors (imposed cycle length of 20 h), and their interacting effects on the cardiovascular biomarker panel.
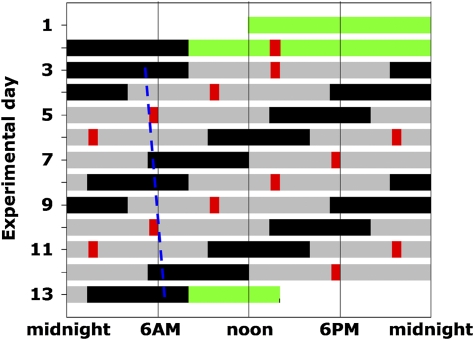
Fig. 1.
Forced Desynchrony Protocol. Solid back bars, scheduled sleep (0 lux); gray bars, wakefulness in dim light [≈1.8 lux (≈0.0048 W/m2)]; green bars, baseline days and discharge day in normal room light (≈90 lux); red bars, test batteries; dashed blue line, illustration of trajectory of circadian CBTmin throughout the FD protocol (circadian period of 24.09 ± 0.06 h in these subjects).
Circadian Phase and Period.
In the 2-wk period before entering the laboratory, average habitual waketime was 7:38 AM ± 15 min. In the laboratory, circadian period was 24.09 ± 0.06 h (mean ± SEM; range: 23.81–24.63 h), similar to prior reports (16, 17), and core body temperature minimum (CBTmin) occurred at 4:33 AM ± 19 min (3.08 ± 0.26 h before habitual waketime). For each individual, CBT was used as the circadian phase marker and the time of CBTmin was assigned as 0 circadian degrees, with one full circadian cycle spanning 360 circadian degrees. For clarity, when describing circadian time we also refer to the mean approximate corresponding time of day during normal entrainment in these subjects. Thus, 0° was ≈4:30 AM, 60° was ≈8:30 AM, and 120° was ≈12:30 PM, etc. in these subjects.
For each variable, we tested for the existence of circadian rhythmicity at rest (Hypothesis 1) and in reactivity to exercise (Hypothesis 2). We fitted the data to test each hypothesis by using cosinor analyses that included both a fundamental circadian cycle (≈24 h) and a cycle with twice the frequency (second harmonic; ≈12 h) (Fig. S1). Circadian rhythms in cardiovascular variables are described below by their peak-to-trough rhythm amplitudes and the phases at which their peaks and/or troughs occur. Our specific prediction was that cardiovascular risk markers would have circadian peaks (or troughs for ‘protective’ cardiac vagal markers) during rest and/or in response to exercise at times that would correspond to the most vulnerable window for adverse cardiovascular events (≈6:00 AM–noon).
Autonomic Nervous System and Cortisol.
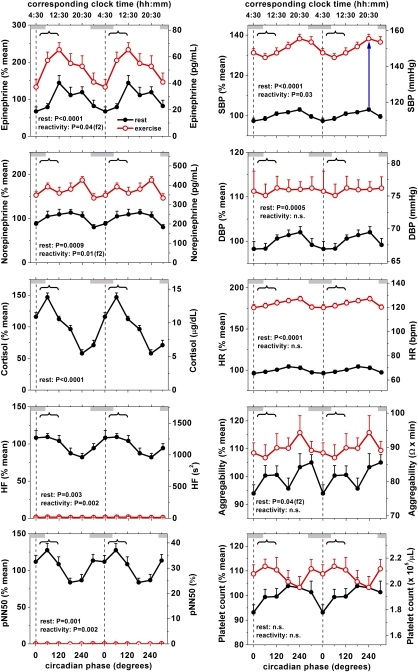
At rest, both plasma epinephrine and norepinephrine had large and significant circadian variations with average amplitudes of 70% (P < 0.0001) and 34% of the mean (P = 0.0009), respectively (Fig. 2, Left Top, black lines; Table 1 and Fig. S2). Both epinephrine and norepinephrine had troughs during the biological night and peaks across the biological day, without clear peaks in the primary vulnerable phase (≈9:00 AM, 70°). The circadian effect on catecholamine levels was large, being almost the same in magnitude as the effect of the standardized exercise protocol designed to sustain 60% of maximum heart rate (+88 and 67%, respectively; Fig. 2, difference between black and red lines). In fact—considering the underlying circadian rhythmicity—when subjects were at rest around midday, surprisingly, their epinephrine levels were as high as those during exercise at ≈4:30 AM (Fig. 2, Left Top).
Fig. 2.
Circadian rhythm in cardiovascular function at rest and during exercise. There were significant endogenous circadian rhythms at rest for plasma epinephrine, norepinephrine, cortisol, HF, pNN50, SBP, DBP, and HR. Platelet aggregability had two peaks across the circadian cycle. Platelet count had no significant circadian variation. Influence of exercise on cortisol could not be assessed reliably (see text) and is not displayed. The influence of the circadian system on reactivity to exercise is shown in Fig. S3. Data are expressed as a percentage of each individual's rest values across the FD protocol (left y axis) in addition to the absolute values (right y axis). Black circles, rest; red circles, exercise; error bars, SEM; gray bars, group average habitual sleep episodes; vertical dotted lines, CBTmin; curly brackets, most vulnerable period for adverse cardiovascular events observed in epidemiologic studies (≈6:00 AM–noon); P values, significance of circadian effect on resting values (rest) and in response to standardized exercise (reactivity) assessed by cosinor model; f2 (after P values), significance of second harmonic of circadian rhythm; blue upward arrow in Right Top Upper, example of reactivity to exercise at one particular circadian phase as analyzed in Fig. S3.
Table 1.
Circadian variation during rest
| Variable | P value circadian | P value harmonic | Timing trough, ° | Timing peak, ° | Amplitude | Amplitude, % |
| Epinephrine | <0.0001 | n.s. | 0 | 220 | 19.8 pg/mL | 70.3 |
| Norepinephrine | 0.0009 | n.s. | 330 | 190 | 77.0 pg/mL | 33.6 |
| Cortisol | <0.0001 | n.s. | 270 | 60 | 7.91 μg/dL | 84.9 |
| HF | 0.0028 | n.s. | 270 | 50 | 394 s2 | 34.1 |
| pNN50 | 0.0011 | n.s. | 200 | 40 | 6.08% | 20.9 |
| SBP | <0.0001 | n.s. | 10 | 230 | 6.16 mmHg | 5.5 |
| DBP | 0.0005 | n.s. | 30 | 200 | 2.78 mmHg | 4.1 |
| HR | <0.0001 | n.s. | 350 | 200 | 5.87 bpm | 8.6 |
| Aggregability | n.s. | 0.043 | 20; 190 | 110; 280 | 10.01 Ωxmin | 12.3 |
Platelet count was not significant and thus not shown. n.s., not significant.
In addition, the reactivity of epinephrine and norepinephrine to exercise varied significantly across the circadian cycle. However, rather than a simple ≈24-h rhythm, this effect was characterized by a ≈12-h rhythm with two large peaks at ≈7–10:30 AM (40–90°) and ≈8:00–10:00 PM (230–260°) for both epinephrine and norepinephrine (P = 0.039 and P = 0.011, respectively; Table 2 and Fig. S3). These 12-h rhythms in reactivity to exercise were similar to the 12-h component of the rhythms of the resting data (Fig. S1). Notably, there was a 2-fold larger exercise-induced increase in epinephrine at ≈8:30 AM (60°; +84 ± 15%; Fig. 2, Left Top Upper) compared with the least vulnerable period, i.e., the middle of the night (≈4:30 AM, 0°; +44 ± 11%) (P = 0.01, Student's t test). This difference was the largest among any two consecutive time windows, identifying 8:30 AM as a time of exaggerated physiological transition.
Table 2.
Circadian variation of the reactivity to the exercise stressor
| Variable | P value circadian | P value harmonic | Timing minimum response, ° | Timing maximum response, ° | Amplitude | Amplitude, % |
| Epinephrine | n.s. | 0.039 | 180; 350 | 90; 260 | 14.7 pg/mL | 52.1 |
| Norepinephrine | n.s. | 0.011 | 130; 320 | 40; 230 | 79.5 pg/mL | 34.6 |
| HF | 0.0023 | n.s. | 270 | 60 | 405 s2 | 35.1 |
| pNN50 | 0.0015 | n.s. | 200 | 40 | 6.06% | 20.8 |
| SBP | 0.034 | n.s. | 80 | 300 | 7.23 mmHg | 6.4 |
DBP, HR, aggregability, and platelet count were not significant and thus not shown; cortisol not shown, see text. n.s., not significant.
At rest, both frequency and time indices of cardiac vagal tone (HF power and pNN50, respectively: see Methods) had significant circadian rhythms. The amplitude was 34% for HF power (P = 0.003) and 21% for pNN50 (P = 0.001) (Fig. 2, Left Bottom; Table 1 and Fig. S2). These “protective” cardiac vagal markers had peaks at ≈7:00–8:00 AM (40–50°) and troughs around the time of the secondary peak in cardiovascular vulnerability (≈6:00–10:30 PM, 200–270°).
As hypothesized (Hypothesis 2), there were significant circadian rhythms in the reactivity of vagal markers to exercise (35% and 21% for HF and pNN50, respectively), with the greatest vagal withdrawal in the vulnerable ≈7–8:30 AM period (40–60°) (Table 2 and Fig. S3).
At rest, plasma cortisol had a pronounced circadian rhythm (P < 0.0001), with an amplitude of 85%, a sharp peak at ≈8:30 AM (60°) and a trough in the biological evening (≈10:30 PM, 270°) (Fig. 2, Left Middle; Table 1 and Fig. S2). We could not reliably analyze the effect of exercise on cortisol because exercise bouts were 15 min, whereas cortisol peaks ≈30 min after the start of exercise (18).
Hemodynamics.
At rest, both systolic blood pressure (SBP) and diastolic blood pressure (DBP) had significant circadian variations, with amplitudes of 5.5% and 4.1%, respectively (both P < 0.0001). Contrary to expectations, the peaks in SBP and DBP occurred at the end of the biological day (≈6:00–8:00 PM, 200–230°), the secondary vulnerable phase, rather than at the primary vulnerable phase of ≈8:30 AM (60°) (Fig. 2, Right Top; Table 1 and Fig. S2).
Exercise significantly increased SBP by 37.9 mmHg (P < 0.0001) and DBP by 7.6 mmHg (P = 0.0017). In addition, the reactivity of SBP to exercise, but not DBP, had a significant circadian rhythm (7.2 mmHg; 6.4%; Table 2 and Fig. S3). Again, contrary to expectations, the smallest SBP reactivity occurred around the time of the primary vulnerable phase (≈10:00 AM, 80°) and the greatest SBP reactivity occurred at the beginning of the biological night (≈midnight, 300°).
At rest, heart rate (HR) had significant circadian variation, with an amplitude of 8.6% (P < 0.0001), a peak during the middle of the biological day (≈4:00 PM, 200°) and a trough during the biological night (≈4:00 AM, 350°) (Fig. 2, Right Middle; Table 1 and Fig. S2). There was a significant increase in HR with exercise of 54.6 bpm (P < 0.0001), but there was no significant circadian rhythm in HR reactivity to exercise.
Hemostasis.
At rest, platelet aggregability had significant circadian variation, with an amplitude of 12% (P = 0.04), similar in magnitude to the effect of exercise (see below). Rather than a simple ≈24-h rhythm, platelet aggregability had a ≈12-h rhythm with peaks at both ≈noon (110°) and ≈11:00 PM (280°) (Fig. 2; Table 1 and Fig. S2). Exercise significantly increased platelet aggregability by 9% (P < 0.0001) without a significant circadian effect.
At rest, platelet count had no significant circadian variation (Fig. 2; Table 1 and Fig. S2). Exercise increased platelet count by 10% (P < 0.0072) but without significant circadian variation in reactivity to exercise.
Discussion
We found that, independent of environmental and behavioral changes, the endogenous circadian system modulates many components of cardiovascular control in humans at rest including sympathetic activity, cortisol, cardiac vagal modulation, HR, arterial blood pressure (≈24-h rhythms), and platelet aggregability (harmonic ≈12-h rhythm). Remarkably, the circadian system also modulated the vagal, sympathetic, and SBP reactivity to exercise.
Potential Mechanisms Involved in Circadian Variation in Cardiovascular Biomarkers.
The potential underlying mechanisms of the circadian variation in cardiovascular biomarkers may include humoral circadian rhythms driven by the SCN (e.g., ACTH), multisynaptic neural projections from the SCN to the organs (e.g., heart, vasculature, and adrenal gland), and/or peripheral oscillators located in these organs (13, 19, 20). Circadian rhythms in autonomic nervous system activity and humoral factors, in turn, could contribute to synchronization of peripheral oscillators in the heart and vasculature (21, 22). Surprisingly, we also found evidence for the existence of endogenous ≈12-h rhythm in cardiovascular biomarkers, specifically in platelet aggregability and in the reactivity to exercise of epinephrine and norepinephrine. Such 12-h patterns could be related to 12-h rhythms as have been demonstrated in animal studies, e.g., cardiac β-adrenergic receptor density and function (23), hormonal rhythms with a shaping role for the SCN (24), and gene transcription in various tissues, including liver, heart, kidney, and adrenals (25). Endogenous 12-h rhythms may have specific relevance to anticipating the daily transitions of dusk and dawn (25).
Comparison with Previous Studies.
There are clear day/night patterns of catecholamine release and in blood pressure in humans (10). However, few studies have standardized behavioral and environmental factors across the day/night cycle, which is a requirement to separate endogenous circadian from behavioral/environmental effects (e.g., induced by the daily rest/activity cycle). Linsell et al. (26) had indirect evidence of a circadian rhythm in plasma epinephrine (but not norepinephrine) based on 24-h fluctuations in epinephrine that could not be fully explained by sleep and/or posture. No daily rhythm in plasma or urinary epinephrine was found in a study in which subjects remained supine, but sleep/wake, feeding, and lighting conditions were not strictly controlled (27). However, in a previous study using well-established circadian techniques, i.e., the FD protocol, we observed an endogenous circadian rhythm in urinary epinephrine, but not in urinary norepinephrine, with a circadian peak at ≈4:00 PM (17), similar to the midpoint of the broad peak of plasma epinephrine in the current study (Fig. S1 and Fig. S2). Using plasma assessment of catecholamines, the current study confirms the circadian rhythm of epinephrine and identifies a circadian rhythm in norepinephrine. The circadian rhythm in plasma norepinephrine reflects whole body spillover of norepinephrine from sympathetic nerves and adrenal medulla into the circulation, which is likely a more important measure of sympathetic influence on the heart and vasculature than urinary norepinephrine that is affected more by kidney function (28).
Our finding of circadian rhythms in SBP and DBP contrasts with two studies that found no circadian rhythms in BP (29, 30). These previous studies might have suffered from some methodological limitations because their subjects: (i) were exposed to a relatively high luminance (up to 100 lux) that can greatly affect the circadian system (31); (ii) were permitted “bathroom breaks” that interrupted their semirecumbent posture; and (iii) had their BP data aligned based on habitual sleep times rather than a much more accurate circadian phase marker, such as CBTmin. Moreover, we recently confirmed the endogenous circadian rhythmicity of arterial blood pressure in humans in two additional complementary protocols (FD and constant routine protocols) (32).
The current findings of circadian rhythms in cardiac vagal markers (peaking during the biological night and morning), HR (biological day) and cortisol (≈9:00 AM) are consistent with other valid circadian studies that use constant routine or FD protocols (ref. 17; for review, see ref. 33). The circadian influence on platelet aggregability is consistent with animal experiments, showing effects of core molecular clock components on coagulation (34).
The circadian rhythm in reactivity of SBP and epinephrine in response to exercise is reminiscent of the daily rhythm in reactivity in response to restraint stress in mice that is circadian clock gene-dependent (Bmal1) (14). Furthermore, recent animal experimental data indicate increased susceptibility to cardiac ischemia/reperfusion at the sleep-wake transition that is mediated by the cardiomyocyte circadian clock, providing further support for the involvement of the circadian system in the timing of adverse cardiovascular events (35).
Potential Implications for Day/Night Pattern of Adverse Cardiovascular Events.
This study in healthy humans provides a unique dataset on circadian amplitude and phase of multiple cardiovascular biomarkers, both at rest and in the reactivity to a behavioral stressor. These data have possible implications for the day/night pattern of adverse cardiovascular events and provide a basis for comparison with studies of more vulnerable populations. For instance, the magnitude of circadian influences on many cardiovascular biomarkers at rest was considerable, with peak-to-trough differences of ≈35–85% for epinephrine, norepinephrine, cortisol, and cardiac vagal modulation. Superimposed on these observed rhythms during the resting state, there were large changes across the circadian cycle in physiological reactivity to exercise, in the range of ≈35–50% for our autonomic markers. Even the smaller circadian modulation of SBP of 6 mmHg (5%) at rest may be of clinical relevance, because this order of magnitude is comparable to that achieved by antihypertensive medications (36). Moreover, we found an additional 7 mmHg (6%) circadian rhythmicity in SBP reactivity to mild exercise that is superimposed on the underlying circadian SBP rhythm.
Even at rest, there are a number of risk factors that had their greatest potential circadian rhythm-related effects at the specific phase corresponding to the morning peak in adverse cardiovascular events (≈6:00 AM–noon), including the circadian peak in plasma cortisol, the first 12-h peak of platelet aggregability, and the greatest rise (i.e., rate of change, not peak) of epinephrine (Fig. 2 and Fig. S2). There are daily rhythms in sensitivity to catecholamines in different organ systems including the heart, due in part to the down-regulation of adrenergic receptors after stimulation (23, 37–39). The circadian-mediated rapid rise in epinephrine that we observed at ≈8:00 AM may be particularly problematic in the setting of the concomitant circadian peak in cortisol because cortisol has permissive effects on adrenergic receptors (40). Based on epinephrine's effects on blood clotting (41), myocardial oxygen demand, and vasoconstriction (42), we would speculate that the circadian epinephrine rhythm may contribute to the morning peak in adverse cardiovascular events (3–10).
In contrast, the peak in vagal cardiac markers and the trough in SBP and DBP at circadian phases corresponding to ≈6:00 AM–noon in these healthy control subjects may be protective (36, 43), possibly counterbalancing factors promoting cardiovascular risk at that circadian time. In contrast, because hypovagotonia is common in populations at risk for adverse cardiovascular events (44), the protective circadian peak in vagal tone in the morning may be reduced in patients who manifest the morning peak in cardiovascular risk. Of note, at circadian phases corresponding to the vulnerable time of ≈6:00 AM–noon, we observed relative coactivation of sympathetic and vagal activity. Sympathovagal coactivation may maximize precise control of end organ responses, increase cardiac output, but also increase risk for arrhythmia in vulnerable populations (45). Further studies are required to test the pathophysiological consequences of circadian influences on autonomic coactivation. Furthermore, loss of circadian rhythmic variation in vagal tone may tip the balance toward morning events during exercise.
Interestingly, the secondary peak in adverse cardiovascular events in the early evening (≈6:00–10:00 PM) coincides with, and therefore might be explained by, the peaks of norepinephrine, HR, SBP, and DBP, the second harmonic peak of platelet aggregability, and the second harmonic peak in reactivity of epinephrine and norepinephrine, as well as the trough in cardiac vagal modulation at a circadian phase corresponding to ≈5:00–10:30 PM.
As we hypothesized, the endogenous circadian trough in most of the cardiovascular risk biomarkers, as well as the circadian peak in “protective” vagal markers, occurred during the biological night (≈midnight–8:00 AM; ≈300°–50°) for most variables (except for cortisol: 10:30 PM; 270°; Table 1), possibly underlying the nighttime trough in adverse cardiovascular events in epidemiological studies (3–10).
Regarding the circadian effect on reactivity to exercise, the phase at which exercise resulted in the maximum vagal withdrawal and the first harmonic (≈12-h) peaks for epinephrine and norepinephrine corresponded to the most vulnerable time of day (6:00 AM–noon), with the epinephrine response to exercise doubling from ≈4:30 AM (0°) to ≈8:30 AM (60°). Thus, such a circadian rhythm in sympathovagal reactivity to behavioral stress could also contribute to the morning peak in adverse cardiovascular events (Fig. S4).
Integration and Summary.
Knowledge about circadian influences on mechanisms involved in cardiovascular risk is important for optimizing the timing of therapy, preventing behavioral triggers at potentially vulnerable circadian phases, and selecting the timing of diagnostic assessments. Furthermore, the interaction between the circadian system and behavioral influences may be especially important for the millions of night workers, jet travelers, and those affected by sleep disorders, in whom the temporal coupling between the endogenous circadian timing system and behavioral stressors is disturbed (46).
As expected from the complex physiology, no single variable exhibited a circadian profile that fully explained the timing of adverse cardiovascular events seen in population studies. Combinations of risk factors possibly summate to produce the day/night pattern in adverse cardiovascular events, as proposed in our simplified conceptual model (Fig. S4). Finally, the daily distribution of triggering behaviors may be the most important factor, although our data point to the fact that interactions between the circadian system and reactivity to behaviors need to be considered.
Methods
Subjects.
Twelve (6 male; 6 female) consenting healthy volunteers [mean ± SD (range); 25.8 ± 5.7 y (20–42 y); BMI: 23.6 ± 3.2 kg/m2 (19.9–29.6 kg/m2)] completed a 13-d inpatient local Human Research Committee-approved protocol at our Clinical and Translational Research Center. Subjects were healthy as confirmed by extensive history, physical, psychological, and laboratory examination. Subjects maintained a regular sleep/wake cycle of 8 h sleep per night for 2–3 wks before admission (verified by sleep/wake diaries, call-in times to a time-stamped voice recorder and wrist actigraphy). Subjects reported no shift work for 3 y and not crossing more than one time zone for 3 mo before the study. To minimize training and detraining effects while in the study, subjects maintained an exercise level equivalent to a 30-min brisk walk per day for the 2–3 wks immediately before admission. Toxicology screens upon admission confirmed that subjects were free of any drugs, including caffeine, alcohol, and nicotine.
Study Protocol.
Subjects spent 13 d in a private laboratory room free of time cues, including two baseline days and nights (16 h scheduled wakefulness and 8 h scheduled sleep) followed by a “forced desynchrony” protocol (FD) consisting of 12 20-h “days” (13:20 hours scheduled wakefulness; 6:40 hours scheduled sleep; maintaining a 1:2 sleep:wake ratio) in dim light (≈1.8 lux at the horizontal angle of gaze) to minimize influences on the circadian system (Fig. 1). Meals were given at 1:25 hours (breakfast), 6:45 hours (lunch), 10:45 hours (dinner) and 12:10 hours (snack) after scheduled waketime. Because the 20-h rest-activity cycle in dim light is outside the range of entrainment, the circadian timing system runs free at its inherent rate of close to 24 h (16). Thus, the sleep and wake episodes, and the exercise tests, were uniformly distributed across the circadian cycle. The exercise test was scheduled to start 5:25 hours after scheduled awakening, consisting of 25 min rest while seated on an ergometer and 15 min cycling on an ergometer (Cybex Semirecumbent Cycle Ergometer) at 60% maximum HR (see SI Methods for details). During each exercise test session, subjects maintained the same body posture (seated), and were asked to limit any unscheduled movement and not to talk. As opposed to in experimental animals, exercise appears to be a weak Zeitgeber for the circadian system in humans and does not affect circadian period (47). Indeed, the observed circadian period in the current study (average 24.09 h) was remarkably similar to that reported in previous forced desynchrony protocols (e.g., 24.18 h; ref. 16).
Measurements.
Blood was sampled from an i.v. catheter 15 min into the rest section and 10 min into the exercise section of each exercise test. Blood was analyzed for epinephrine, norepinephrine, cortisol, whole blood platelet aggregability, and platelet count (the latter only in the last six exercise tests per subject), totaling 1,392 assays across all subjects (see SI Methods for details).
Blood pressure was measured by automatic oscillometric cuff sphygmomanometer (Dinamap; Critikon) every 5 min during each rest section and every 3 min during each exercise section, and averaged for the rest and exercise sections.
For assessment of HR and heart rate variability (HRV), three-lead ECG was recorded on a Vitaport (Temec Instruments) at 256 Hz throughout each exercise test. HRV analyses were performed according to the standards of the Task Force (48). For the analysis of HRV, cardiac interbeat intervals were obtained by using a QRS wave detector based on amplitude and first derivative of ECG waveform and verified by a trained technician to ensure that only “normal-to-normal” R waves times were included (49). Power spectra were calculated by fast Fourier transform of 3.41-Hz cubic spline resampled data across time windows with stable signal of at least 5 min for each rest and exercise section. High frequency power (HF; 0.15–0.4Hz) and pNN50 (% of consecutive normal-to-normal R wave intervals differing >50 ms) were obtained as frequency- and time-domain vagal cardiac markers, respectively.
Statistical Analysis.
Based on pre hoc criteria to eliminate spurious outliers, values > 3 SDs from the mean for each condition (rest or exercise) within each subject across all wake periods were excluded from analysis. CBT was measured continuously throughout the 240-h FD protocol by using a rectal thermistor (Yellow Springs Instrument) and assessed by nonorthogonal spectral analysis technique to determine circadian phase (timing) and period (cycle length) as published (16). All data were assigned a circadian phase (0–359°) depending on the time from the fitted CBTmin (0°) and the individual's estimated circadian period (one full circadian period = 360°). Reactivity to exercise was calculated as the value of each variable during exercise minus the value during rest (reactivity = exercise − rest). The independent effects of the circadian cycle during rest (Hypothesis 1) and in the reactivity to exercise (Hypothesis 2) were assessed for each variable by using cosinor analyses including both the fundamental (≈24-h) and second harmonic (≈12-h) of circadian rhythmicity and two-factor mixed model analysis of variance with restricted maximum likelihood estimates of the variance components (JMP; SAS Institute) (50). Rather than assuming the data would fit a sinusoidal waveform (i.e., the fundamental component), the inclusion of a ≈12-h component (i.e., the second harmonic) permits fitting of a more complex function. For graphing purposes, to correct for any interindividual differences, data were expressed as a percentage of each individual's rest values across the FD protocol (left y axis in Fig. 2), in addition to the absolute values (right y axis in Fig. 2).
Supplementary Material
Acknowledgments
We thank the volunteers, research staff, and recruiters who participated in this study. This work was supported by National Institutes of Health Grants R01-HL76409, P30-HL101299, K24-HL076446, K99-HL102241, K24-HL093218, and M01-RR02635.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006749107/-/DCSupplemental.
References
- 1.Lloyd-Jones D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Allender S, et al. European Cardiovascular Disease Statistics 2008. British Heart Foundation and University of Oxford, Oxford; 2008. [Google Scholar]
- 3.Muller JE, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Manson JE, Buring JE, Muller JE, Hennekens CH. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation. 1990;82:897–902. doi: 10.1161/01.cir.82.3.897. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, et al. Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB) Am J Cardiol. 1997;79:253–258. doi: 10.1016/s0002-9149(97)00743-1. [DOI] [PubMed] [Google Scholar]
- 6.Willich SN, et al. ISAM Study Group. Increased morning incidence of myocardial infarction in the ISAM Study: Absence with prior beta-adrenergic blockade. Circulation. 1989;80:853–858. doi: 10.1161/01.cir.80.4.853. [DOI] [PubMed] [Google Scholar]
- 7.Casetta I, et al. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Tofler GH, et al. The CPI Investigators. Morning peak in ventricular tachyarrhythmias detected by time of implantable cardioverter/defibrillator therapy. Circulation. 1995;92:1203–1208. doi: 10.1161/01.cir.92.5.1203. [DOI] [PubMed] [Google Scholar]
- 9.Willich SN, et al. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 10.Shea SA, Hilton MF, Muller JE. In: Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. White WB, editor. Totowa, NJ: Humana Press Inc; 2007. pp. 251–289. [Google Scholar]
- 11.Krantz DS, et al. Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation. 1996;93:1364–1371. doi: 10.1161/01.cir.93.7.1364. [DOI] [PubMed] [Google Scholar]
- 12.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 13.Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 14.Curtis AM, et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anea CB, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 17.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanaley JA, Weltman JY, Pieper KS, Weltman A, Hartman ML. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab. 2001;86:2881–2889. doi: 10.1210/jcem.86.6.7566. [DOI] [PubMed] [Google Scholar]
- 19.Hastings MH, Reddy AB, Maywood ES. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 20.McNamara P, et al. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: A humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 21.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 22.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–1705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 23.Witte K, Parsa-Parsi R, Vobig M, Lemmer B. Mechanisms of the circadian regulation of beta-adrenoceptor density and adenylyl cyclase activity in cardiac tissue from normotensive and spontaneously hypertensive rats. J Mol Cell Cardiol. 1995;27:1195–1202. doi: 10.1016/0022-2828(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 24.Kalsbeek A, et al. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci. 2008;27:818–827. doi: 10.1111/j.1460-9568.2008.06057.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes ME, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210–1215. doi: 10.1210/jcem-60-6-1210. [DOI] [PubMed] [Google Scholar]
- 27.Cameron OG, et al. Circadian fluctuation of plasma epinephrine in supine humans. Psychoneuroendocrinology. 1987;12:41–51. doi: 10.1016/0306-4530(87)90021-7. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler MG, Morrissey EC, Kennedy B, Elayan H. Sources of urinary catecholamines in renal denervated transplant recipients. J Hypertens. 1990;8:927–931. doi: 10.1097/00004872-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kerkhof GA, Van Dongen HP, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens. 1998;11:373–377. doi: 10.1016/s0895-7061(97)00461-5. [DOI] [PubMed] [Google Scholar]
- 30.Van Dongen HP, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int. 2001;18:85–98. doi: 10.1081/cbi-100001178. [DOI] [PubMed] [Google Scholar]
- 31.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of endogenous circadian blood pressure rhythm that paradoxically peaks at night. Sleep. 2010;33:187. (abstr) [Google Scholar]
- 33.Rüger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertolucci C, et al. Evidence for an overlapping role of CLOCK and NPAS2 transcription factors in liver circadian oscillators. Mol Cell Biol. 2008;28:3070–3075. doi: 10.1128/MCB.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durgan DJ, et al. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: A meta-analysis. Lancet. 2001;358:1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 37.Vacas MI, Elverdin JC, Chiarenza AP, Luchelli MA. Daily changes in beta-adrenergic sensitivity of rat submandibular gland. Correlation with beta-adrenoceptor rhythm. Auton Neurosci. 2001;89:1–6. doi: 10.1016/s1566-0702(01)00226-0. [DOI] [PubMed] [Google Scholar]
- 38.Tsujimoto G, Honda K, Hoffman BB, Hashimoto K. Desensitization of postjunctional alpha 1- and alpha 2-adrenergic receptor-mediated vasopressor responses in rat harboring pheochromocytoma. Circ Res. 1987;61:86–98. doi: 10.1161/01.res.61.1.86. [DOI] [PubMed] [Google Scholar]
- 39.Collins HE, Rodrigo GC. Inotropic response of cardiac ventricular myocytes to beta-adrenergic stimulation with isoproterenol exhibits diurnal variation: Involvement of nitric oxide. Circ Res. 2010;106:1244–1252. doi: 10.1161/CIRCRESAHA.109.213942. [DOI] [PubMed] [Google Scholar]
- 40.Davies AO, Lefkowitz RJ. Regulation of beta-adrenergic receptors by steroid hormones. Annu Rev Physiol. 1984;46:119–130. doi: 10.1146/annurev.ph.46.030184.001003. [DOI] [PubMed] [Google Scholar]
- 41.Lin H, Young DB. Opposing effects of plasma epinephrine and norepinephrine on coronary thrombosis in vivo. Circulation. 1995;91:1135–1142. doi: 10.1161/01.cir.91.4.1135. [DOI] [PubMed] [Google Scholar]
- 42.Stone PH. Unraveling the mechanisms of ambulatory ischemia. How and why. Circulation. 1990;82:1528–1530. doi: 10.1161/01.cir.82.4.1528. [DOI] [PubMed] [Google Scholar]
- 43.Vanoli E, et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji H, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 45.Paton JF, Nalivaiko E, Boscan P, Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: Observations and functional implications. Clin Exp Pharmacol Physiol. 2006;33:1245–1250. doi: 10.1111/j.1440-1681.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 46.Couch RD. Travel, time zones, and sudden cardiac death. Emporiatric pathology. Am J Forensic Med Pathol. 1990;11:106–111. doi: 10.1097/00000433-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Cain SW, Rimmer DW, Duffy JF, Czeisler CA. Exercise distributed across day and night does not alter circadian period in humans. J Biol Rhythms. 2007;22:534–541. doi: 10.1177/0748730407306884. [DOI] [PubMed] [Google Scholar]
- 48.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 49.Fraden J, Neuman MR. QRS wave detection. Med Biol Eng Comput. 1980;18:125–132. doi: 10.1007/BF02443287. [DOI] [PubMed] [Google Scholar]
- 50.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.