Abstract
The amygdala is a sexually dimorphic brain region critical for the regulation of social, cognitive, and emotional behaviors, but both the nature and the source of sex differences in the amygdala are largely unknown. We have identified a unique sex difference in the developing rat medial amygdala (MeA) that is regulated by cannabinoids. Newborn females had higher rates of cell proliferation than males. Treatment of neonates with the cannabinoid receptor agonist, WIN 55,212–2 (WIN), reduced cell proliferation in females to that of males and a wide range of WIN doses had no effect on cell proliferation in males. The effect of WIN on cell proliferation in the MeA was prevented by coinfusions of a CB2 but not CB1 receptor antagonist. Females had higher amygdala content of the endocannabinoid degradation enzymes, fatty acid amid hydrolase, and monoacylglycerol lipase than males, and lower amounts of the endocannabinoids 2-arachidonoylglycerol and N-arachidonylethanolamide (anandamide). Inhibition of the degradation of 2-arachidonoylglycerol in females occluded the sex difference in cell proliferation. Analyses of cell fate revealed that females had significantly more newly generated glial cells but not more newly generated neurons than males, and treatment with WIN significantly decreased glial cell genesis in females but not males. Finally, early exposure to cannabinoids masculinized juvenile play behavior in females but did not alter this behavior in males. Collectively, our findings suggest that sex differences in endocannabinoids mediate a sex difference in glial cell genesis in the developing MeA that impacts sex-specific behaviors in adolescence.
Keywords: sexual differentiation, neurogenesis, development
The medial amygdala (MeA) is a sexually dimorphic nucleus critical for modulating sex differences in juvenile rough-and-tumble play (1), and regulation of adult social behaviors, including mating, parenting, aggression, and territoriality (2). The overall size of the rat MeA is larger in adult males than females (3) and is responsive to steroids in adulthood (4). Most of the well-characterized volumetric sex differences in the brain are the result of differential cell death during a perinatal-sensitive period, with more cells dying in one sex than the other (5).
A large body of evidence has accumulated in the last 10 y supporting the important role of cannabinoid receptors and their endogenous ligands in regulation of synaptic strength (6). It is clear, in particular, that endocannabinoid signaling, via CB1 receptor activation, subserves activity-dependent, retrograde signaling in many brain regions. Cannabinoid receptors and endogenous cannabinoid ligands are present and active early in brain development (7), and the tissue contents of the primary endocannabinoids N-arachidonylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) vary substantially throughout brain development. The 2-AG content peaks on the day of birth and dramatically decreases from there, whereas AEA content gradually increases throughout life (8). Cannabinoid signaling has been implicated in cell proliferation (9), neurogenesis (10), neuronal differentiation (11), and synaptogenesis (12). Neural progenitors have functional CB1 and CB2 receptors and can synthesize endocannabinoids (13), demonstrating that endocannabinoid signaling can have dramatic influences on neural development. To date, most studies have focused on the CB1 receptor, with minimal investigation of the CB2 receptor (14). This focus is in part because CB2 receptor distribution was considered limited to peripheral tissues and immune cells, although a functional role is emerging for CB2 receptors in neural stem cell proliferation (14, 15). In the current study, we report a unique female-biased sex difference in cell proliferation in the developing MeA that is mediated by the CB2 receptor and accompanied by a sex difference in endocannabinoid content. Administration of cannabinoid receptor agonist, WIN 55,212–2 (WIN) to newborn female rats induced masculinization of juvenile play behavior, supporting the notion that endocannabinoid-mediated reduction of cell proliferation within the MeA is a critical determinant of sexual dimorphism in this behavior. To our knowledge, this study is unique in reporting a role for endocannabinoids in the establishment of a previously unexplored sex difference in cell proliferation in the developing brain, with functional implications.
Results
Activation of Cannabinoid Receptors Occludes a Sex Difference in Cell Proliferation in the Developing Amygdala.
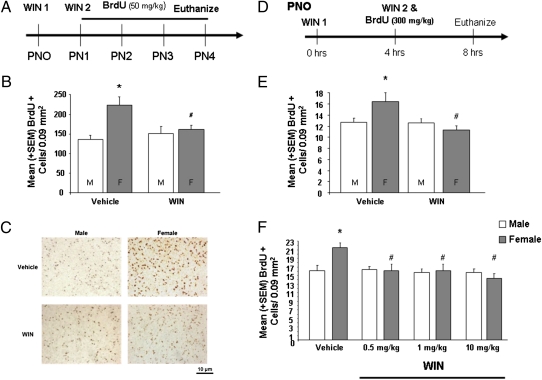
Injection of the cell cycle marker BrdU from postnatal day (PN)1 to PN4, revealed that females had more new cells in the developing amygdala than males on PN4 (Fig. 1). Treatment with the CB1/2 receptor agonist, WIN (1 mg/kg), on PN1 and PN2 occluded the observed sex difference on PN4, such that females treated with WIN had the same number of new cells as both control and WIN-treated males [F(3,12) = 4.64, P < 0.05] (Fig. 1 B and C). WIN treatment had no effect in males. It has been reported previously that adult males have larger MeA volumes than adult females (3, 16), which we have replicated in 33-d-old animals [F(1,14) = 6.39, P < 0.05] (Fig. S1). To ensure that sex differences in cell genesis during the first postnatal week are not the result of bias introduced by overall volume differences, we also compared the MeA volume at PN4 in male and female animals. There was no sex difference or effect of treatment on MeA volume in 4-d-old animals [F(1,21) = 0.12, P > 0.05) (Fig. S1). To distinguish between sex differences in cell proliferation versus differential survival of new cells in males versus females, we used a time-course sufficiently short to preclude a contribution from cell death. Females had significantly more BrdU+ cells than males 8 h after treatment with WIN and 4 h after BrdU, indicating a sex difference in cell proliferation. This sex difference was occluded by WIN [F(1,19) = 4.27, P < 0.05], which reduced the density of new cells in females to that of males (Fig. 1E). There was no sex difference in the density of pyknotic (i.e., dying) cells in the developing amygdala at PN4 [F(1,11) = 0.12, P < 0.05] (Fig. S2). To determine whether males have reduced sensitivity to exogenous cannabinoids, we conducted a dose-response analysis and found that three doses of WIN (0.5, 1.0, or 10 mg/kg) significantly decreased cell proliferation in the developing amygdala of females but had no effect in males [F(3,41) = 3.00, P < 0.05] (Fig. 1F).
Fig. 1.
Activating cannabinoid receptors occludes a sex difference in cell proliferation in the developing amygdala. (A). Rat pups were treated once daily from PN1 to PN4 with BrdU and vehicle or WIN (1 mg/kg) on PN0 and PN1, and their brains collected on PN4 for immunohistochemical detection of BrdU. (B). Data are expressed as mean ± SEM [n (total sample size) = 15]. Females have greater BrdU+ cell density than males (*P < 0.05) in the MeA at PN4. Activation of CB1/2 receptors with WIN on PN0 and PN1 obscures the sex difference by reducing the density of new cells in females (#P < 0.05). (C). Representative photomicrographs of BrdU+ cells in the MeA in males and females treated with vehicle or WIN. (D). Newborn males and females were treated with vehicle or WIN at 0 and 4 h in combination with a single injection of BrdU (300 mg/kg) and brains collected at 8 h and processed for detection of BrdU. (E). Data are expressed as mean ± SEM (n = 23). Females have greater BrdU+ cell densities in the MeA than males (*P < 0.05). Treatment with WIN decreased BrdU+ cell density in females (#P < 0.05) but not males (P > 0.05). (F). Data are expressed as mean ± SEM (n = 50). Again, females have greater BrdU+ cell density in the MeA than do males (*P < 0.05). Treatment with WIN (0.5, 1, or 10 mg/kg) decreased the BrdU+ cell density in females (#P < 0.05) but not males.
CB2 Receptor Activation Reduces Cell Proliferation in the Developing Female Amygdala to That of Males.
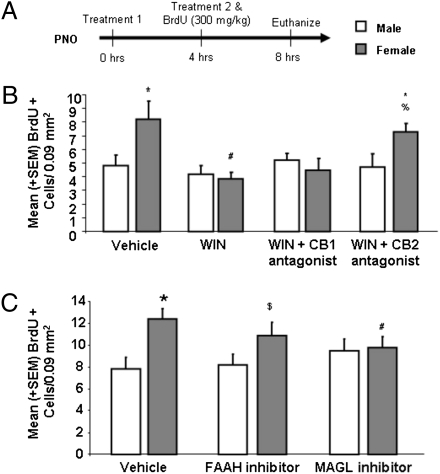
WIN is a nonselective CB1/2 receptor agonist; therefore, we determined the receptor mediating the effects of WIN by combining treatment with either a selective CB1 or CB2 receptor antagonist to explore which of these receptors is involved in the regulation of amygdala cell genesis. There was no effect of either antagonist alone and so the treatment groups were collapsed (Fig. S3). Coinfusions of WIN and the CB2 receptor antagonist AM-630 (3 mg/kg) prevented the WIN-induced decrease in BrdU+ cell density in females [F(7, 50) = 2.40, P < 0.05] (Fig. 2B). In contrast, coinfusion of WIN and the CB1 receptor antagonist AM-281 (3 mg/kg) did not prevent the WIN-induced decrease in BrdU+ cell density in the MeA of females (P > 0.05).
Fig. 2.
CB2 receptor activation reduces cell proliferation in the developing amygdala to that of males. (A) Newborn males and females were treated with vehicle, WIN (1 mg/kg), the CB1 receptor antagonist AM-281 (3 mg/kg), the CB2 receptor antagonist AM-630 (3 mg/kg), or combinations of WIN and either the CB1 or CB2 receptor antagonist at 0 and 4 h in combination with a single injection of BrdU and brains were collected at 8 h and processed for detection of BrdU. Data are expressed as mean ± SEM (n = 59). In a separate experiment, newborn males and females were treated with vehicle, the FAAH inhibitor JNJ (20 mg/kg), or the MAGL inhibitor NAM (1 mg/kg) at 0 and 4 h in combination with a single injection of BrdU and brains were collected at 8 h and processed for detection of BrdU. Data are expressed as mean ± SEM (n = 67). (B). There were more BrdU+ cells in the female MeA than male (*P < 0.05) and treatment with WIN decreased BrdU+ cell density in females (#P < 0.05) but not males. Combined treatment with WIN and AM-630, but not AM-281 prevented the sex difference in BrdU+ cell density (*P < 0.05, vs. male-vehicle; %P > 0.05, vs. female-vehicle). (C). There were again more BrdU+ cells in the female amygdala than male (*P < 0.05) and inhibition of MAGL decreased the number in females (#P < 0.05) but not males. Inhibition of FAAH attenuated BrdU+ cell densities in females ($P > 0.05 vs. control females or males).
Females Have More Endocannabinoid Degradative Enzyme and Lower Endocannabinoid Levels than Males in the Developing Amygdala.
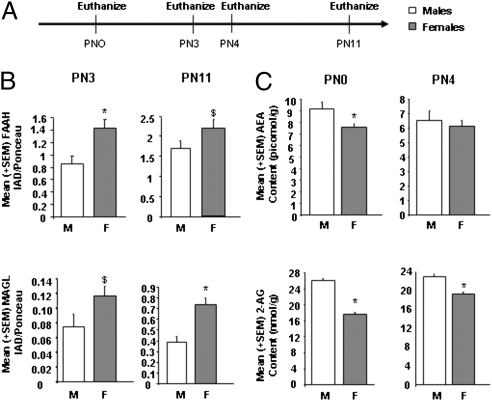
After determining that both males and females have detectable CB2 receptor protein in the MeA (Fig. S4), we hypothesized that lower endogenous cannabinoid signaling mediates the enhanced cell genesis in the MeA of females. The endocannabinoids are made-on-demand, so their tissue contents are thought to reflect their signaling concentrations (17). In support of our hypothesis, newborn males had higher 2-AG content in the amygdala than females on both PN0 and PN4 [PN0: F(1,16) = 23.15, P < 0.05; PN4: F(1,14) = 6.66, P < 0.05] (Fig. 3C). Newborn males also had higher AEA content in the amygdala on the day of birth [F(1,16) = 4.74, P < 0.05] but not on PN4 [F(1,14) = 0.11, P > 0.05] (Fig. 3C). There were no sex differences in the amygdala contents of oleoylethanolamide and palmitoylethanolamide, N-acylethanolamines that do not bind cannabinoid receptors (Fig. S5). Endocannabinoid levels are regulated by both biosynthetic and catabolic processes (17). We determined the amounts of the primary catabolic enzymes for AEA and 2-AG, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively, using Western blot analysis. Females had significantly more FAAH protein than males in the amygdala at PN3 [t(10) = 6.25, P < 0.05] but only a statistical trend at PN11 [t(11) = 4.30, P = 0.06] (Fig. 3B). Females also exhibited a statistical trend toward more MAGL protein than males in the amygdala at PN3 [t(12) = −1.65, P = 0.08] and a difference that reached significance at PN11 [t(11) = −11.46, P < 0.05] (Fig. 3B). These findings are consistent with the hypothesis that males have higher endocannabinoid-mediated signaling than females and suggest further that higher expression of the catabolic enzymes in female amygdala contributes to this difference. We examined this notion by treating newborn pups with either a FAAH (JNJ 1661010) or a MAGL [N-arachidonylmaleimide (NAM)] inhibitor before measurement of the density of BrdU+ cells. Treatment with the MAGL inhibitor, but not the FAAH inhibitor, significantly reduced the density of BrdU+ cells in females but not in male pups [F(5,61) = 3.90, P < 0.05] (Fig. 2C). These data support the hypothesis that the concentration of 2-AG in the developing amygdala is greater in males than females, in part because of lower MAGL activity, and that this results in reduced cell genesis.
Fig. 3.
Females have more endocannabinoid degradative enzyme and lower endocannabinoid levels than males in the developing amygdala. (A) The amygdala of males and females were collected and processed for Western blot analysis of FAAH and MAGL protein levels and HPLC quantification of AEA and 2-AG at multiple developmental time points. (B) Data are expressed as mean ± SEM (n = 14 at PN3; n = 12 at PN11). There was significantly more FAAH (*P < 0.05) and a tendency toward more MAGL ($P = 0.07) in the female amygdala on PN3, and this sex difference persisted until PN11 for MAGL (*P < 0.05) but only tended to persist for FAAH ($P = 0.06). (C) Data are expressed as mean ± SEM (n = 18 at PN0; n = 16 at PN4). There was significantly more AEA and 2-AG in male amygdala at PN0 (*P < 0.05), and the sex difference in 2-AG persisted until PN4 (*P < 0.05).
Cannabinoids Reduce Astrocyte Density in the Developing Female Amygdala to That Seen in Males.
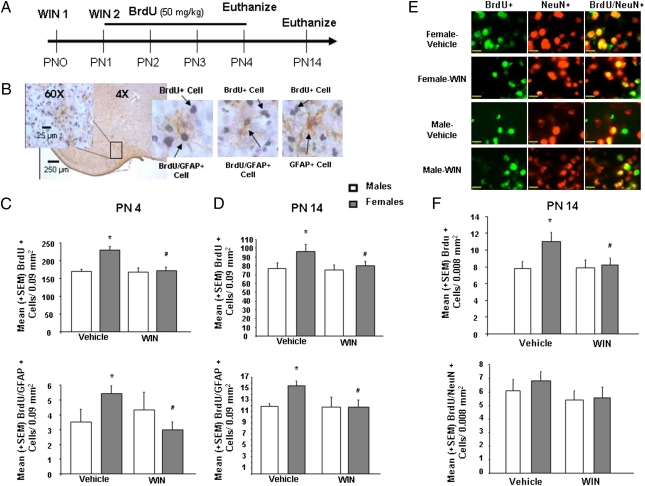
To determine the mature phenotype of the newly proliferating cells being impacted by cannabinoids in the developing MeA, we used double-label immunohistochemistry for BrdU and GFAP, a marker for mature astrocytes (Fig. 4B). Consistent with our other findings, newborn females had more BrdU+ cells in the MeA than males at PN4, and this was still true at 2 wk of age (P < 0.05 compared with males for both time points). Treatment with WIN significantly decreased the number of BrdU+ cells in females but not males at both time points [PN4: F(3,23) = 11.68, P < 0.05; PN14: F(3,31) = 3.53, P < 0.05] (Fig. 4 C and D). The overall density of GFAP+ cells was not affected by sex or treatment at either time point. However, the number of cells in which BrdU and GFAP were colocalized was significantly higher in females and decreased by WIN treatment in females but not males at both times [PN4: F(3,23) = 3.82, P < 0.05; PN14: F(3,31) = 3.62, P < 0.05] (Fig. 4 C and D). The number of newly generated neurons was assessed by confocal microscopy of double-label immunofluorescence for BrdU and the neuronal marker, NeuN (Fig. 4E). Although females had more BrdU+ cells in the MeA than males (P < 0.05) and treatment with WIN decreased BrdU+ cells in females but not males [F(1,40) = 4.18, P < 0.05] (Fig. 4F), the density of NeuN+ cells and BrdU+/NeuN+ cells were not affected by sex or WIN treatment (Fig. 4F).
Fig. 4.
Cannabinoids reduce the number of astrocytes in the developing female amygdala to that seen in males. (A) Males and females were treated PN1-4 with BrdU (50 mg/kg) and on PN 0 and PN1 with vehicle or WIN (1 mg/kg) to determine whether WIN treatment would decrease the number of BrdU, GFAP, BrdU/GFAP, NeuN, and BrdU/NeuN immunoreactive cells in the rat MeA at PN4 or PN14. (B) Representative photomicrographs of BrdU+, GFAP+, and BrdU/GFAP+ cells in the developing MeA. [Scale bars, 250 μm (4×) or 25 μm (60×).] (C) Data are expressed as mean ± SEM (n = 29). On PN4, females had more BrdU+ and BrdU/GFAP+ cells in the MeA than males (*P < 0.05) and activation of CB1/2 receptors eliminated the sex difference (#P < 0.05, vs. female-vehicle). (D) Data are expressed as mean ± SEM (n = 35). On PN14, females still had more BrdU+ and BrdU/GFAP+ cells than males (*P < 0.05, vs. male-vehicle), and this sex difference was again eliminated by prior treatment with WIN (#P < 0.05, vs. female-vehicle). (E) Representative photomicrographs of BrdU+, NeuN+, and BrdU/NeuN+ cells in the developing MeA. Cells were visualized by fluorescent confocal microscopy on a Grid Confocal at 100×. (Scale bars, 17 μm.) (F) Data are expressed as mean ± SEM (n = 44). Quantification of fluorescently detected BrdU also revealed that females had more BrdU+ cells in the MeA than males (*P < 0.05, vs. male-vehicle) and activation of CB1/2 receptors again eliminated the sex difference (#P < 0.0, vs. female-vehicle). There was no sex difference or effect of neonatal WIN treatment on the density of NeuN+ or BrdU/NeuN+ cells in the MeA.
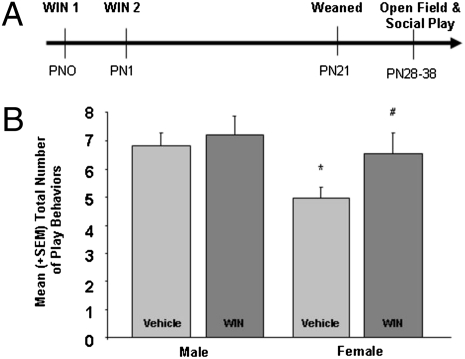
Neonatal Cannabinoid Treatment Masculinizes Play Behavior in Juvenile Females.
As exogenous cannabinoid agonists abolish the sex difference in cell genesis in the MeA, we examined the effects of neonatal WIN treatment on sex differences in juvenile social play, anxiety, and locomotion in the open-field test. As expected, males exhibited more social play behavior than females. However, treatment with WIN increased the duration of play in females to that of males [F(3,36) = 2.98, P < 0.05] (Fig. 5B). There was no significant effect of sex or WIN treatment in either anxiety or locomotor responses in the open-field test (Fig. S6).
Fig. 5.
Neonatal cannabinoid treatment masculinizes play behavior in juvenile females. (A) Male and female rats were treated with vehicle or WIN at PN0 and PN1, weaned on PN21, and housed in same-sex groups of three until testing. (B) Data are expressed as mean ± SEM (n = 41). Control males played more frequently than females (*P < 0.05) but WIN treatment increased the total occurrence of play behavior in females (#P < 0.05 vs. control females) to the level seen in males; WIN treatment had no effect in males.
Discussion
We report here that more new cells are born in the amygdala of neonatal females than males and that this sex difference is a result of higher rates of cell proliferation in females. Furthermore, the sex difference in cell proliferation was caused by enhanced astrocyte cell genesis in female animals, with no differences in neurogenesis between males and females. Our pharmacological and biochemical data support the hypothesis that greater CB2 receptor activation in males, possibly as a result of decreased endocannabinoid catabolism, underlies the sex difference in astrocyte proliferation. In accord with this hypothesis, we have found that treatment of neonatal females with exogenous CB receptor agonists induces a male profile of juvenile play behavior.
The current report of more proliferating cells in the female than male MeA at PN4 at first appears in contrast to the findings in adult rats, where the male MeA is larger than that of females (3, 18), with more and bigger neurons (16, 19), greater dendritic length, and higher spine density (16, 20). To date, the majority of sex differences in the size of a particular brain region have been attributed to differences in the total number of neurons as a result of differential cell death (5). The findings reported here are surprising because the bias is toward more cell proliferation in females and because very few sex differences in cell proliferation have been reported (21), suggesting that sex differences in proliferation may not mediate the establishment of sex differences in volume of particular brain regions. Moreover, sex differences in cell proliferation during the early postnatal and juvenile period could be specific to those developmental ages and underlie functional differences associated with development, such as the correlation we observed here between cell proliferation and juvenile play behavior.
Our observation that the sex difference in proliferation were cells that coexpressed an astrocyte—not neuronal—cell marker, could mediate the known sex difference in synaptic patterning within the mature MeA (18, 22), as has been seen for other brain regions (23). There are conflicting reports on sex differences in astrocytes in the adult MeA, with some finding higher GFAP content in the MeA of females (24); others report fewer and less complex astrocytes in females (25). Elevated hormones associated with puberty also impact the final differentiation of the brain (26), including sex differences in adolescent neurogenesis in the MeA (27).
Our data support the hypothesis that differences in endocannabinoid/CB2 receptor signaling underlie the sex difference in cell proliferation in the MeA. First, we found that activation of CB1/2 receptors with WIN had no effect on proliferation in males but caused a significant reduction in females. The effect of WIN was reversed by coadministration of a CB2, but not a CB1, receptor antagonist, suggesting that cell proliferation in the female MeA is suppressed by CB2 receptor activation. These results could be explained in two ways: (i) cell proliferation in males is insensitive to CB2 receptor activation, or (ii) CB2 receptor regulation of cell proliferation occurs in both males and females but endogenous CB2 signaling is maximally activated in males, thereby occluding effects of WIN. In support of the second possibility, Western blot analyses indicate that both males and females have CB2 receptor protein. More importantly, male pups had significantly higher amounts of the endocannabinoid that activates CB2 receptors, 2-AG, than females, which is consistent with the hypothesis that the CB2 receptor in males is maximally activated. Males also had less of the primary catabolic enzyme for 2-AG, MAGL, and inhibition of MAGL in females prevented the sex difference in cell proliferation in the MeA. These data suggest that 2-AG is the endocannabinoid involved and that differences in MAGL activity underlie the sex difference observed. These findings do not rule out a role for AEA in cell genesis because MAGL inhibition could also affect AEA content (28); however, the lack of effect of a FAAH inhibitor argues against a role for AEA.
Contrary to expectation, treatment of male pups with the CB2 receptor antagonist, AM-630, did not increase cell proliferation. One possibility is that males have delayed maturation of the endocannabinoid system and subsequently delayed cell proliferation in comparison with females. This possibility seems unlikely, given males have greater endocannabinoid content, and we detected CB2 receptor protein in males by Western blot, although this does not speak to the functionality of CB2 receptors in males versus females. More likely is that activation of CB2 receptors is sufficient to serve as a stop signal for cell proliferation, but multiple signaling steps in addition to CB2 receptor silencing are required to enhance cell proliferation. Thus, pharmacological antagonism of the CB2 receptor is not sufficient to induce cell proliferation in the developing amygdala of males, but instead requires engagement of other as-yet-unidentified parameters, such as the presence of growth factors and activation of signaling cascades important for cell genesis.
In adulthood, females are more susceptible to cannabinoid-induced antinociception, locomotion, and memory deficits (29, 30). Thus far, the majority of sex differences in the endocannabinoid system or cannabinoid-induced behavioral effects have been attributed to fluctuations in gonadal steroid levels (31, 32). However, many sex differences in the adult brain are the result of the organizational action of neonatal androgens from the testis and brain synthesis of estrogens (33). Neonatal exposure to steroid hormones during a sensitive period permanently alters the morphology and synaptic patterning of the brain and results in sex differences in physiology and behavior in juveniles and adults (33). The play of juvenile males is more frequent and of longer duration than females and neonatal treatment of females with estradiol or testosterone masculinizes these parameters of play (34, 35). Data indicate that the effects of the steroids are mediated by the amygdala (35) and correlate with increased excitatory synapses (22). In the present study, we found that early exposure to cannabinoids masculinizes social play in females, implicating cannabinoid signaling in the establishment of normal sex differences in the brain. This is in contrast to studies in which prenatal cannabinoid exposure is associated with enduring impairments in cognitive and social functions (36). An important next step will be determining the effects of gonadal hormones on the endogenous cannabinoid system and cell genesis to further place these findings in the context of organization of sex differences during normal brain development.
In summary, we have identified a unique sex difference in cell proliferation that favors females in the developing MeA. Furthermore, these data indicate a pivotal role of endocannabinoids and CB2 receptors in cell proliferation and astrocyte genesis in females. Developmental cannabinoid exposure masculinizes astrocyte numbers in the MeA and juvenile social play behavior. These findings are unique in implicating cannabinoid regulation of cell proliferation and cell type as a mechanism for sexual differentiation of the developing brain and behavior. Elucidating the normal function of endogenous signaling molecules that are readily mimicked by drugs of abuse is essential to understanding the consequences of inappropriate exposure, and how those consequences can be avoided or best remedied.
Materials and Methods
Subjects.
Timed pregnant Sprague-Dawley rats (Harlan) mated in our facility were allowed to deliver normally under standard laboratory conditions. Food and water were available ad libitum. The University of Maryland, Baltimore Institutional Animal Care and Use Committee approved all animal procedures (SI Materials and Methods).
Drug Preparation and Administration.
Male and female rat pups were given intraperitoneal injections of BrdU (50 or 300 mg/kg; Sigma) or subcutaneous injections of vehicle (0.1% DMSO in sterile saline, pH = 7.0), the CB1/2 receptor agonist WIN 55–212,2 mesylate (0.5, 1, or 10 mg/kg), the CB1 receptor antagonist AM-281 (3 mg/kg;), the CB2 receptor antagonist AM-630 (3 mg/kg), the FAAH inhibitor 4-(3-phenyl-[1,2,4]thiadiazol-5-yl)-piperazine-1-carboxylic acid phenylamide (JNJ 1661010) (20 mg/kg), or the MAGL inhibitor NAM (1 mg/kg). All drugs, with the exception of BrdU, were purchased from Tocris Cookson, Inc.
Immunohistochemistry and Immunofluorescence.
Briefly, coronal brain sections (45 μm thick) through the rostral-caudal extent of the extended amygdala were double-labeled with BrdU and an astrocyte specific marker, GFAP, or the mature neuronal maker neuronal nuclear antigen (NeuN). Free-floating tissue sections were processed for BrdU or BrdU/GFAP immunohistochemistry using protocols our laboratory has previously published using monoclonal anti-BrdU antibody (1:10,000 in PBS-T; Caltag Laboratories) and a polyclonal antibody against GFAP (1:10,000; Sigma) and diaminobenzidine (DAB)- and nickel-DAB- H2O2 substrate (21). For detection of new neurons, tissue sections were incubated simultaneously in rat anti-BrdU (1:200; Abcam) and mouse monoclonal anti-NeuN (1:1,000; Chemicon). The BrdU primary antibody was used in conjunction with biotinylated anti-rat secondary (1:500; Vector) for 2 h and Streptavidin Alexa488 (1:500; Invitrogen). NeuN antibody was used in conjunction with secondary goat anti-mouse Alexa Fluor568 (1:500; Invitrogen). See SI Materials and Methods for more information.
Immunohistochemical Analysis.
Quantification of DAB stained tissue was performed with a Nikon microscope at 60× magnification and Neurolucida software (MicroBrightField, Inc.). The density of BrdU+ and GFAP Sigma cells was determined in a counting frame (300 × 300 μm) in both hemispheres of the MeA. The density of immunopositive cells was averaged from four sections (45 μm thick) of each brain.
Confocal Microscopy.
The density of BrdU and NeuN+ cells was determined using a Volocity Grid Confocal system (SI Materials and Methods). Confocal z-stacks (10–12 μm) were collected sequentially in the two channels at 0.5-μm intervals using a 100× oil objective. The BrdU+ cells were examined in orthogonal views (0, 90, 180°) to determine whether BrdU+ cells are colocalized with NeuN. The density of immunopositive cells in the MeA was averaged from both hemispheres of four sections (45 μm) of each brain using an 80 × 100-μm counting frame.
Western Blot Analysis.
All animals were euthanized by rapid decapitation and amygdala tissue was dissected and processed for Western blot analysis (SI Materials and Methods). The membranes were incubated in the primary antibody, anti-FAAH (1:500; Santa Cruz) or anti-MAGL (1:500; Axxora) and an anti-rabbit HRP-linked secondary antibody (1:3,000; Cell Signaling Technologies). The immunoreactive bands were detected using chemiluminesence and the integrative grayscale pixel area-density (IAD) was analyzed with National Institutes of Health image software. The membranes were then incubated in a Ponceau S (Sigma) stain and the density of the largest band on the membrane was used as a loading control to standardize the total protein values.
Endocannabinoid Extraction and Analysis.
Brain regions were subjected to a lipid extraction process. Briefly, tissue samples were weighed and placed into borosilicate glass culture tubes containing two ml of acetonitrile with 84 pmol of [2H8]anandamide and 186 pmol of [2H8]2-AG. Tissue was homogenized in glass culture tubes and lipids were extracted and stored in methanol until analysis. The contents of the two primary endocannabinoids, AEA and 2-AG, within lipid extracts in methanol from brain tissue were determined using isotope-dilution, liquid chromatography−mass spectrometry (SI Materials and Methods).
Behavioral Assessment.
Play behavior.
The social play testing was carried out at PN28 to PN38. Briefly, males and females were weaned on P21 and housed in same-sex groups of three. Six animals from different sex and treatment conditions were video-recorded in a neutral arena for four 2-min intervals per day over 5 d. The total number of aggressive play behaviors were scored, summed, and then averaged per interval by an observer blind to treatment (SI Materials and Methods).
Open field.
The number of central, intermediate, outer, and total grid crossings in the open-field arena were recorded for 5 min by an experimenter blind to treatment (SI Materials and Methods).
Statistical Analyses.
The data were expressed as means and SEMs and analyzed using Student's t tests, one-way ANOVA, or univariate two-factor ANOVA as appropriate (SI Materials and Methods). Tukey HSD or Fisher LSD post hoc tests were used to detect group differences (See SI Results for detailed analyses). An α-level of P < 0.05 was required for statistical significance.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants R01 MH052716 (to M.M.M.) and R21 DA022439 (to C.J.H.), and a Cellular and Integrative Neuroscience Training Grant to the University of Maryland T32 NS07375 (to D.L.K.-K.); M.N.H. was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005003107/-/DCSupplemental.
References
- 1.Meaney MJ, Dodge AM, Beatty WW. Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiol Behav. 1981;26:467–472. doi: 10.1016/0031-9384(81)90175-x. [DOI] [PubMed] [Google Scholar]
- 2.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 3.Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- 4.Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrendero F, et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Ruiz J, Berrendero F, Hernández ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- 9.Aguado T, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26(1):1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galve-Roperh I, Aguado T, Rueda D, Velasco G, Guzmán M. Endocannabinoids: A new family of lipid mediators involved in the regulation of neural cell development. Curr Pharm Des. 2006;12:2319–2325. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- 11.Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguado T, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- 14.Goncalves MB, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Palazuelos J, et al. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- 16.Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- 17.Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: An historical overview and some recent developments. Lipids. 1999;34(Suppl):S319–S325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- 18.Nishizuka M, Arai Y. Organizational action of estrogen on synaptic pattern in the amygdala: Implications for sexual differentiation of the brain. Brain Res. 1981;213:422–426. doi: 10.1016/0006-8993(81)90247-x. [DOI] [PubMed] [Google Scholar]
- 19.Hermel EE, Ilha J, Xavier LL, Rasia-Filho AA, Achaval M. Influence of sex and estrous cycle, but not laterality, on the neuronal somatic volume of the posterodorsal medial amygdala of rats. Neurosci Lett. 2006;405(1–2):153–158. doi: 10.1016/j.neulet.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: A novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol. 2009;69(2–3):141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasia-Filho AA, Xavier LL, dos Santos P, Gehlen G, Achaval M. Glial fibrillary acidic protein immunodetection and immunoreactivity in the anterior and posterior medial amygdala of male and female rats. Brain Res Bull. 2002;58(1):67–75. doi: 10.1016/s0361-9230(02)00758-x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511:599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burston JJ, et al. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–553. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430(1):41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- 30.Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol. 2007;18:563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54(3):159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 32.González S, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- 35.Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398:324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- 36.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.