Abstract
The major cell surface glycoconjugate of Leishmania major, a putative parasite receptor for macrophages, is a lipophosphoglycan containing 81.6% (wt/wt) carbohydrate, 17.0% (wt/wt) phosphate, and 1.4% (wt/wt) lipid. It has been purified to homogeneity by hydrophobic chromatography and consists of a polydisperse family of molecules with Mr 5000-40,000. It contains galactose, mannose, glucose, arabinose, glucosamine, and inositol in the molar ratio of 51:21:5:6:1:1. The lipophosphoglycan has a complex structure, consisting mainly of tri- and tetrasaccharide units linked by phosphodiester bonds, which are cleaved by HF hydrolysis. The phosphate groups are located on the 6-hydroxyl of both galactose and mannose residues. The lipophosphoglycan is anchored to the parasite surface by a 1-O-alkyl-sn-glycero-3-phosphoinositol moiety. This conclusion is supported by analysis of the products of nitrous acid deamination, HF hydrolysis, and Staphylococcus aureus phosphatidylinositol specific-phospholipase C treatment. The 24:0 and 26:0 alkyl chains accounted for 93% of the ether-linked fatty acids in the lipid anchor. The results are also consistent with a glycosidic linkage between the inositol and a non-N-acetylated glucosamine residue. The lipophosphoglycan membrane anchor shares limited structural homology with the glycosylphosphatidylinositol anchors of several eukaryotic proteins, indicating that this type of membrane anchor is not limited to proteins. Vaccination of mice with the purified L. major lipophosphoglycan in liposomes induced resistance against cutaneous leishmaniasis.
Full text
PDF
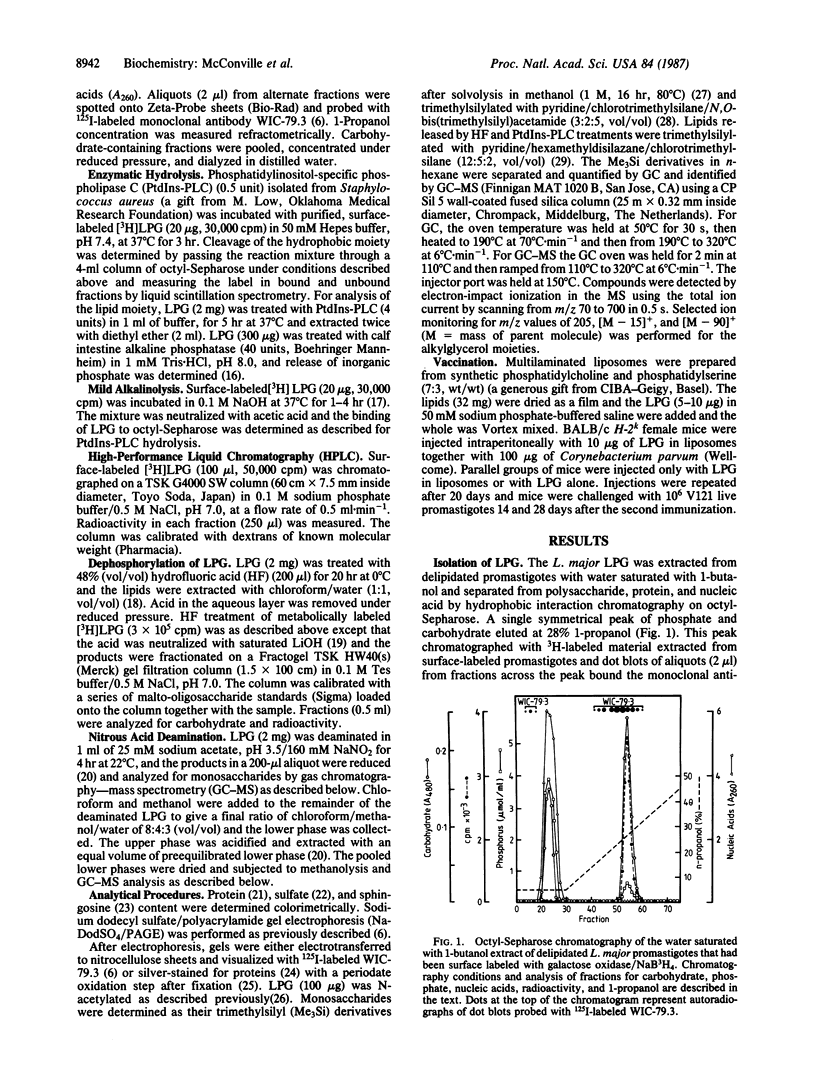
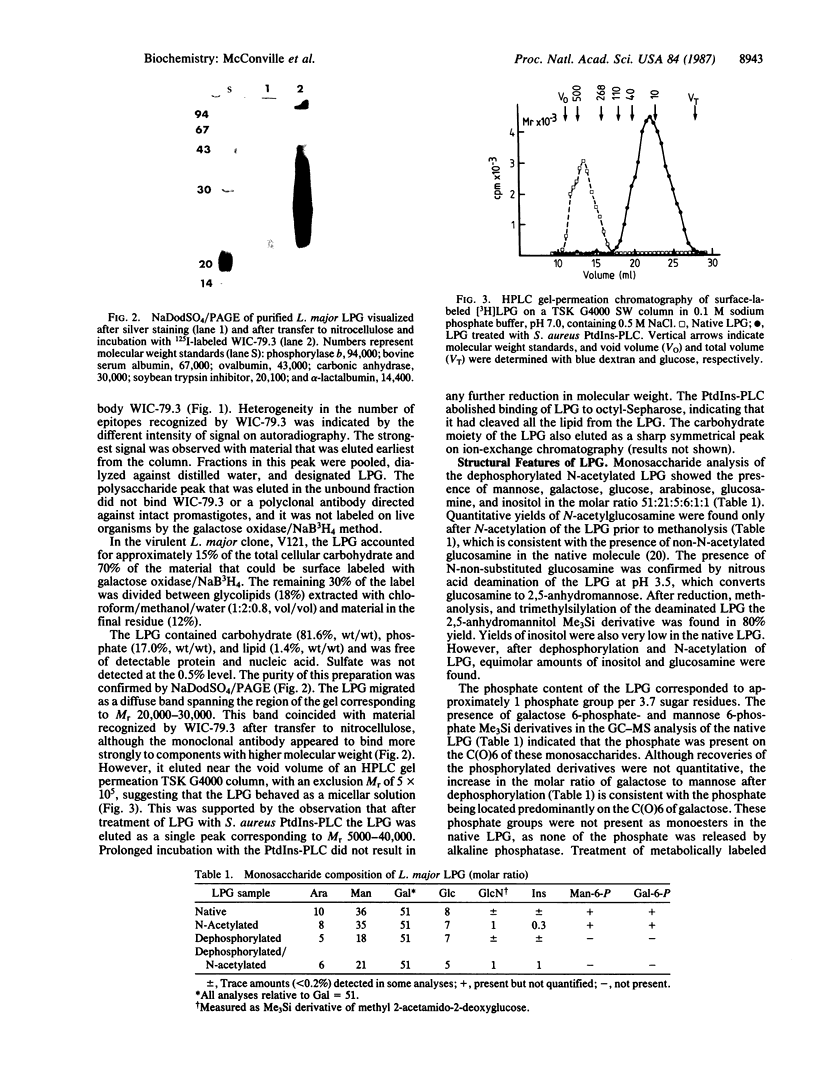
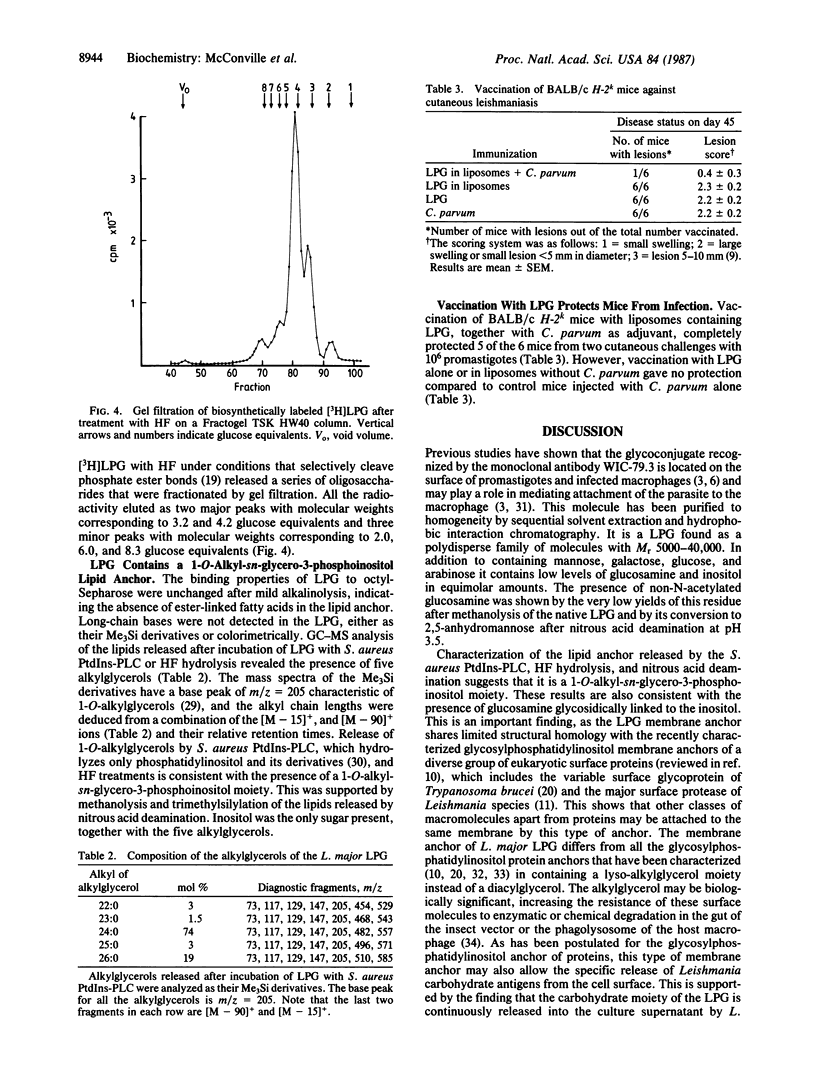

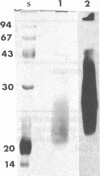
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Itasaka O., Hori T. Studies on glycosphingolipids of fresh-water bivalves. V. The structure of a novel ceramide octasaccharide containing mannose-6-phosphate found in the bivalve, Corbicula sandai. J Biochem. 1979 Jun;85(6):1469–1481. doi: 10.1093/oxfordjournals.jbchem.a132475. [DOI] [PubMed] [Google Scholar]
- Kaneshiro E. S., Jayasimhulu K., Lester R. L. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res. 1986 Dec;27(12):1294–1303. [PubMed] [Google Scholar]
- King D. L., Chang Y. D., Turco S. J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987 May;24(1):47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Dearborn D. G., Wright P. L. Lipophosphonoglycan of the plasma membrance of Acanthamoeba castellanii. Isolation from whole amoebae and identification of the water-soluble products of acid hydrolysis. J Biol Chem. 1974 Jun 10;249(11):3335–3341. [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Futerman A. H., Ackermann K. E., Sherman W. R., Silman I. Removal of covalently bound inositol from Torpedo acetylcholinesterase and mammalian alkaline phosphatases by deamination with nitrous acid. Evidence for a common membrane-anchoring structure. Biochem J. 1987 Jan 15;241(2):615–619. doi: 10.1042/bj2410615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Myher J. J., Marai L., Kuksis A. Identification of monoacyl- and monoalkylglycerols by gas--liquid chromatography--mass spectrometry using polar siloxane liquid phases. J Lipid Res. 1974 Nov;15(6):586–592. [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Shaw N., Stead A. The reaction of phosphoglycolipids and other lipids with hydrofluoric acid. Biochem J. 1974 Nov;143(2):461–464. doi: 10.1042/bj1430461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Trager W. Some aspects of intracellular parasitism. Science. 1974 Jan 25;183(4122):269–273. doi: 10.1126/science.183.4122.269. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Wilkerson M. A., Clawson D. R. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984 Mar 25;259(6):3883–3889. [PubMed] [Google Scholar]
- Zinbo M., Sherman W. R. Gas chromatography and mass spectrometry of trimethylsilyl sugar phosphates. J Am Chem Soc. 1970 Apr 8;92(7):2105–2114. doi: 10.1021/ja00710a052. [DOI] [PubMed] [Google Scholar]