Abstract
Bisphenol A (BPA) is a highly prevalent constituent of plastics that has been associated with diabetes, cardiovascular disease, and an increased risk of miscarriages in humans. In mice, BPA exposure disrupts the process of meiosis; however, analysis of the affected molecular pathways is lagging and has been particularly challenging. Here we show that exposure of the nematode Caenorhabditis elegans to BPA, at internal concentrations consistent with mammalian models, causes increased sterility and embryonic lethality. BPA exposure results in impaired chromosome synapsis and disruption of meiotic double-strand break repair (DSBR) progression. BPA carries an anti-estrogenic activity in the germline and results in germline-specific down-regulation of DSBR genes, thereby impairing maintenance of genomic integrity during meiosis. C. elegans therefore constitutes a model of remarkable relevance to mammals with which to assess how our chemical landscape affects germ cells and meiosis.
Keywords: toxicology, oogenesis
Meiosis is the cell division program by which haploid sperm and eggs are generated from diploid germ cells and is therefore essential for both sexual reproduction and generating genetic diversity. Errors in chromosome segregation during meiosis can result in aneuploidy that significantly contributes to infertility, miscarriages, and birth defects in humans (1). In addition to natural variations in human fertility, there may also be a contribution of environmental exposures to the etiology of human aneuploidies (2, 3).
Despite the devastating outcomes stemming from impaired meiosis, there is currently no alternative to the slow, costly, and technically challenging use of mammalian species for in vivo studies of environmental disruption of meiosis in multicellular organisms. Furthermore, there is tremendous need for developing reliable systems that are both relevant to mammals and suitable for high-throughput screening strategies to test potential toxicants impacting meiosis (4).
We explored the use of the genetically tractable nematode Caenorhabditis elegans to examine the effect of Bisphenol A (BPA) on the germline. BPA ranks among the highest production volume chemicals with a global annual production scale of ≈4 million metric tons. It is commonly used in the manufacture of several polymers, including polycarbonate and epoxy resins (5). Thus, BPA is found in a variety of items such as plastic bottles, the lining of both food and beverage cans, and dental sealants (5). Consistent with its widespread presence, urinary BPA is detected in >90% of the population in the United States (5–7). Higher levels of urinary BPA have been correlated with cardiovascular diseases and diabetes (7) and may be associated with an increased risk for miscarriages with abnormal embryonic karyotype (8). Rodent models of BPA exposure have revealed multiple levels of reproductive impairments, including chromosome synapsis defects, elevated recombination levels, and errors in chromosome segregation during meiosis following in utero exposure (9). However, although BPA severely impacts the mammalian meiotic program, we still lag in our understanding of the affected genes and pathways, as well as its mechanism of action in the germline.
To investigate how BPA exerts its effect on the germline, we have taken advantage of numerous features that make C. elegans an extremely amenable system for the study of meiosis. These features include a short reproduction cycle, a large number of germline nuclei, and the well-characterized spatial–temporal gradient in which these nuclei are positioned along the germline, allowing for easy access and identification of all stages of prophase I (10). Importantly, C. elegans shares a remarkable degree of gene conservation with humans (11, 12), which has proved invaluable for the identification of genes relevant to mammalian meiosis (reviewed in refs. 10 and 13).
Here we identify the mechanisms by which BPA affects germline maintenance in C. elegans. We show that BPA exposure significantly impairs oogenesis, resulting in elevated levels of sterility and embryonic lethality. BPA exposure results in impaired chromosome synapsis, altered meiotic DNA double-strand break (DSB) repair progression, subsequent activation of the ATL-1 and CHK-1 DNA damage checkpoint kinases, and increased CEP-1/p53-dependent germ cell apoptosis. The impaired chromosome remodeling observed in late prophase I, coupled with the appearance of chromosome fragments in both pachytene and diakinesis nuclei, further suggests a defect in meiotic DSB repair. Importantly, the expression of several DSB repair genes is down-regulated specifically in the germline following BPA exposure. Finally, the effects of BPA on germline nuclei can be rescued by coexposure to estrogen, suggesting an anti-estrogenic action of BPA on the germline. We therefore propose that BPA acts by altering the germline-specific expression of DNA DSB repair machinery components, leading to failed maintenance of genomic integrity during meiosis.
Results
BPA Exposure Results in Decreased Brood Size and Increased Embryonic Lethality in C. elegans.
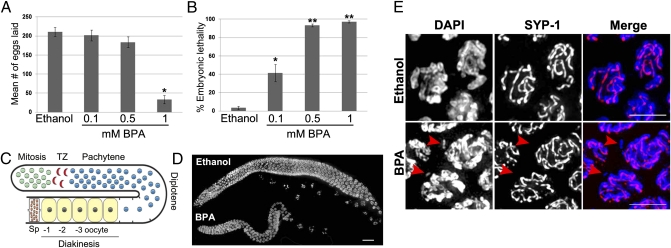
Exposure to BPA has been associated with a variety of reproductive impairments in several animal species, including decreased quality and production of both sperm and oocytes (9, 14–16), increased embryonic lethality, and increased recurrence of miscarriages in humans (8, 17). To address whether BPA also affects C. elegans reproduction, we exposed worms to various concentrations of BPA under several culture conditions. Four parameters were tested: BPA concentration (100 μM, 500 μM, or 1 mM), vehicle (DMSO or ethanol), cholesterol concentration (5 μg/mL, 0.5 μg/mL, or none), and culture method (liquid or plate). We determined that BPA dissolves more easily in ethanol and results in a higher penetrance of the observed phenotypes when diluted in this vehicle. Cholesterol is thought to mask the effect of endocrine-disrupting chemicals in C. elegans and was therefore omitted from the medium (18, 19). Exposing worms to BPA dissolved in 100% ethanol on plates without cholesterol, at a final concentration of 1 mM for 4 d (from eggs to adulthood), gave the most consistent results with low overall toxicity, as judged by both growth and behavior of the worms. Using these conditions, we observed a sixfold reduction in the mean number of eggs laid (increased sterility) and a dramatic increase in embryonic lethality (97.3%; n = 333) in worms exposed to 1 mM BPA compared with vehicle (P < 0.0001) (Fig. 1 A and B). Furthermore, none of the rare larvae observed either reached adulthood or survived after 3 d in culture (100% larval lethality). Taken together, these phenotypes indicate that BPA impairs C. elegans reproduction and are suggestive of errors in chromosome segregation.
Fig. 1.
BPA exposure results in sterility, increased embryonic lethality, reduced gonad size, impaired chromosome synapsis, and chromosome integrity in C. elegans. (A) Mean number of eggs laid by worms exposed either to vehicle (0.1% ethanol) or to the indicated doses of BPA. Error bars represent SEM. The entire brood sizes of 10 singled hermaphrodites were scored for each condition (n = 10); *P < 0.0001. (B) Embryonic lethality observed among the progeny of hermaphrodites exposed either to vehicle or to the indicated doses of BPA. Error bars represent SEM. n = 10; *P < 0.001 and **P < 0.0001. (C) Schematic representation of the progression of nuclei throughout the C. elegans germline. Nuclei enter meiosis at the transition zone (TZ). The oocyte closest to the spermatheca (Sp) is referred to as the −1 oocyte. (D) Low magnification images of DAPI-stained whole-mount gonads from age-matched hermaphrodites exposed to either ethanol or BPA. (Scale bar, 20 μm.) (E) At pachytene, incomplete synapsis and chromosome fragmentation are observed in 10% (n = 84) of nuclei (arrowheads) as revealed by DAPI (blue) and SYP-1 (red) immunostaining.
Uptake of BPA by C. elegans.
The exposure of C. elegans to BPA could in principle differ significantly from the concentration added to the plates, depending on levels of uptake and the ability of the worm cuticle to act as a partial barrier (20). We therefore measured the internal concentration of free BPA by liquid chromatography–tandem mass spectrometry analysis (Axys Analytical Services). We found that an external dose of 1 mM BPA corresponds to an uptake level of 2 μg/g (2 ppm) of worm extract, a value within the range of internal concentrations of BPA detected in several rodent exposure models (21–23) and of serum levels in occupational exposure cases (24). It is also an order of magnitude higher than the levels of BPA found in human placental tissues (23, 25). These results therefore indicate that BPA impairs C. elegans reproduction at internal levels consistent with mammalian models of high exposure.
Exposure to BPA Perturbs Both the Synaptonemal Complex and Chromosome Integrity During Pachytene.
The increased sterility and embryonic lethality caused by BPA exposure may be due in part to errors in chromosome segregation stemming from earlier defects taking place during meiosis. Analysis of DAPI-stained gonads revealed smaller gonads in BPA-exposed worms compared with vehicle, which do not stem from a general developmental delay but rather likely reflect a discrete effect of BPA on the worm gonad (Fig. 1 C and D and see below). We took advantage of the ability to visualize intact germline nuclei in C. elegans to monitor the progression through meiosis. We first examined the localization of HTP-3 and SYP-1, respectively axial and central region components of the synaptonemal complex (SC), a proteinaceous scaffold that normally assembles between pairs of homologous chromosomes (10). As nuclei exit mitosis and progress into meiosis, HTP-3 and SYP-1 are observed both as foci and as short chromosome-associated patches that increase in length through the transition zone (leptotene/zygotene stages) and extend along the full length of the homologous chromosomes by pachytene. Following exposure to BPA, neither the kinetics nor the extent of HTP-3 and SYP-1 localization on chromosomes were altered at transition zone compared with vehicle (Fig. S1, n = 10 gonads). In contrast, analysis of pachytene nuclei in BPA-treated worms revealed defects in chromosome synapsis as evidenced by the presence of DAPI-stained chromatin tracks that are devoid of SYP-1 staining (Fig. 1E). These asynapsed regions often corresponded to chromosome fragments detectable in ≈10% of all pachytene nuclei analyzed (n = 84) exclusively in BPA-exposed germlines. Whereas synapsis is impaired by BPA exposure, pairing of the homologs was normal as visualized by immunolocalization of the X chromosome pairing center end component HIM-8 (Fig. S2, 250 nuclei scored per treatment; n = 7 gonads). Taken together, these results indicate that following exposure to BPA, although the kinetics of establishment of synapsis appear normal, the integrity of both the SC and chromosomes is impaired.
BPA Exposure Results in Altered Meiotic DSB Repair Progression, DNA Damage Checkpoint Activation, and Altered Expression of DSB Repair Genes in the Germline.
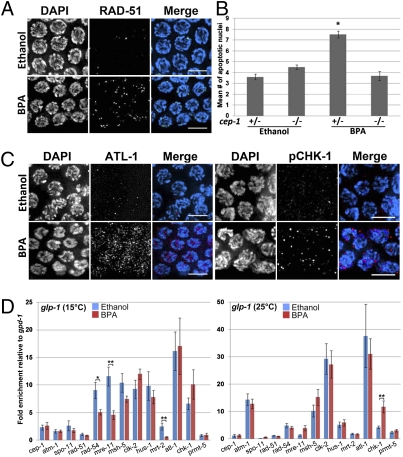
In a wide range of species, chromosome synapsis and recombination are tightly linked events as it is only in the context of a fully assembled SC that crossover recombination can be completed (10). In the context of mammalian exposure to BPA, both events are affected with incomplete synapsis and elevated levels of recombination being observed (9). We examined the progression of double-strand break repair (DSBR) by comparing the levels of RAD-51 foci in the germ-lines of BPA and vehicle-exposed worms. RAD-51 is a protein involved in strand invasion/exchange during DSBR (26). During mitosis RAD-51 foci are rare and then increase during early to midpachytene and dramatically decrease by late pachytene (zones 6 and 7) as meiotic DSB repair progresses. In contrast, BPA-exposed germlines display an elevated number of RAD-51 foci in late pachytene, indicating that BPA exposure alters the progression of DSBR during meiosis (Fig. 2A and Fig. S3). As unrepaired recombination intermediates can trigger a DNA damage checkpoint leading to germ cell apoptosis during late pachytene (27), we measured the number of apoptotic nuclei following BPA exposure by acridine orange staining (28). We observed a twofold increase in the number of apoptotic nuclei in BPA-exposed gonads compared with vehicle (P < 0.0001). This increase was not observed in homozygous p53/cep-1 mutants, indicating that BPA-induced germ cell apoptosis is p53/CEP-1 dependent and results from the activation of a DNA damage checkpoint (Fig. 2B). Consistent with these findings, we observed increased levels of two checkpoint kinases that cooperate in germ cell DNA damage sensing: ATL-1, the C. elegans homolog of mammalian ATR, and phosphorylated CHK-1 (Fig. 2C, n = 11 and n = 7 gonads, respectively) (29, 30).
Fig. 2.
Meiotic DSBR is impaired and germline-specific expression of DSB repair genes is altered following BPA exposure. (A) Late pachytene nuclei from BPA-exposed germlines show elevated levels of RAD-51 (green) foci compared with control. (B) Quantitation of germ cell apoptosis. Germ cell corpses were scored in ethanol- and BPA-exposed p53/cep-1 heterozygous or homozygous worms. n = 20–23 gonad arms scored per genotype. Error bars represent SEM. *P < 0.0001. (C) Immunostaining for ATL-1 (red) and phospho CHK-1 (red) on midpachytene nuclei from dissected gonads of ethanol- and BPA-exposed worms. Between 7 and 15 gonads were analyzed for each condition. (Scale bars, 5 μm.) (D) Expression levels of a panel of genes implicated in DSB repair and DNA damage checkpoints were assayed by quantitative RT-PCR. Error bars represent SEM for three biological replicates each performed in duplicate. *P = 0.017 and **P < 0.01.
To determine whether the defect in DSBR progression might stem from altered expression of DSBR genes, we measured the relative expression levels of a panel of key DNA repair genes by quantitative RT-PCR (Fig. 2D, Table S1). We used a glp-1(bn18) temperature-sensitive mutant (31) that lacks a germline at the restrictive temperature (25 °C), but is essentially wild type at the permissive temperature (15 °C), thus allowing us to distinguish whether changes in expression occurred in either the germline or the soma. Three genes were significantly down-regulated specifically in the germline: rad-54 and mre-11 (P = 0.017 and P < 0.01, respectively), which encode for DNA repair machinery components (32, 33), and mrt-2 (P < 0.01), a checkpoint gene involved in telomere replication and repair as well as DNA damage-induced apoptosis (34). In addition, chk-1 expression was increased in both the germline and the soma, albeit only significantly in the soma (P < 0.01) (Fig. 2D). These results suggest that the germline-specific altered expression of DSBR genes may account for the disruption in DSBR that ultimately leads to chromosome fragmentation and the activation of the DNA damage checkpoint in the germline.
BPA Causes Severe Chromosomal Abnormalities and a Delay in Mature Bivalent Formation During Late Prophase I.
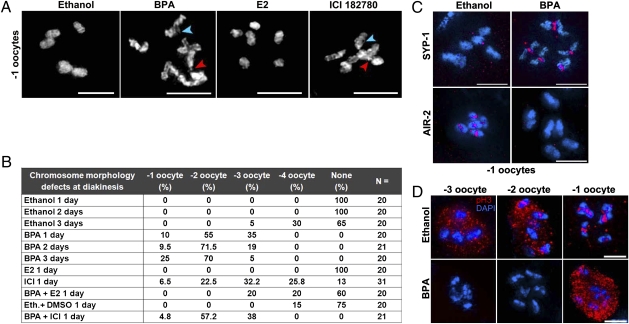
To assess whether the altered progression of meiotic DSBR results in chromosome morphology defects later during prophase I, we examined the diakinesis oocyte that is most proximal to the spermatheca (−1 oocyte) (Fig. 1C) following either vehicle or BPA treatment. In ethanol-treated gonads, we observed six intact DAPI-stained bodies representing the six pairs of homologous chromosomes held together by chiasmata. In contrast, we observed several chromosomal abnormalities such as chromosome fragments, chromosomes with a frayed appearance, and the formation of aggregates in 23% (n = 63) of the −1 oocytes following BPA exposure (Fig. 3A). These chromosome morphology defects were also observed in the −2 and −3 oocytes (Fig. 3B). Moreover, we observed a delay in the formation of six mature bivalents in late diakinesis (−1 to −4 oocytes) in BPA-treated gonads (Fig. 3B). This result was not due to a general delay in maturation of the gonad as the worms being examined both formed and laid eggs and also did not show an improvement in bivalent differentiation in subsequent days after initial analysis (17%, n = 30, of −1 oocytes showed chromosomal abnormalities 1 d post-L4 compared with 24%, n = 21, and 28%, n = 18, at 2 and 3 d post-L4, respectively).
Fig. 3.
BPA exerts anti-estrogenic effects on the C. elegans germline and causes severe defects at diakinesis. (A) Six intact bivalents are observed in control (ethanol)- and 17β-estradiol (E2)-exposed −1 oocytes. In contrast, chromosomes have a frayed appearance and aggregates, fragments (red arrowheads), and chromatin bridges (blue arrowheads) are observed in −1 oocytes in BPA and ICI 182780 exposed germlines. (B) Quantification of the chromosome morphology defects observed in bivalents at diakinesis. Worms were examined at 1, 2, or 3 d post-L4. Ethanol served as a control for all experiments except for BPA + ICI where a mixture of ethanol (0.1%) and DMSO (1%) was used. (C) Immunolocalization of SYP-1 (red) and AIR-2 (red) on DAPI-stained (blue) −1 oocytes reveals a delay in bivalent differentiation. (D) Histone H3 phosphorylation (pH3, red) is impaired at diakinesis in BPA-exposed worms. (Scale bars, 5 μm.)
Accurate chromosome segregation at meiosis requires the regulated disassembly of the SC (35, 36) and the subsequent Aurora-B kinase/AIR-2-dependent loss of cohesion between homologs (36–38). Accordingly, as in wild type, a weak SYP-1 signal is associated with the midsection of bivalents in 6% (n = 1/16) of the −2 oocytes in ethanol-treated gonads, and this signal is absent in all −1 oocytes (Fig. 3C). In contrast, SYP-1 staining was detectable on 80% (n = 12/15) of all −2 oocytes and 13% (n = 2/15) of the −1 oocytes in BPA-treated gonads. Further confirming the defect in bivalent maturation, we failed to detect AIR-2 on the bivalents of 100% of the gonads treated with BPA compared with 10% of ethanol-treated controls (Fig. 3C, n = 15 each), and phosphorylated histone H3, the canonical substrate of AIR-2, was detected in only 23% of all BPA treated −1 oocytes (Fig. 3D, n = 21). Taken together, these results suggest that the timing of SC disassembly and mature bivalent formation is delayed following BPA exposure compared with ethanol control.
BPA Exerts Anti-Estrogenic Effects on the C. elegans Germline.
BPA possesses endocrine disrupting activity in many developmental contexts including gonadal, brain, and mammary gland development. BPA has an affinity for multiple members of the estrogen receptor family, as exemplified by its binding to the human estrogen receptors α (ERα) and ERβ as well as the estrogen-related receptor γ (ERR-γ) (39). Surprisingly, in the context of mouse oogenesis, evidence suggests that BPA acts as an estrogen antagonist as the defects observed at the pachytene stage in BPA-treated animals also occurred at comparable frequencies in ERβ−/− mice and BPA exposure did not lead to increased chromosomal aberrations in these knockout mice (9). To investigate whether the effects of BPA on the C. elegans gonad are also mediated by its ability to modulate estrogen activity, we compared chromosome morphology at diakinesis following exposure to BPA, the canonical estrogen estradiol (E2), and the high-affinity estrogen antagonist ICI 182780 (40). Whereas 1 mM E2 exposure did not cause any observable chromosome morphology defects at diakinesis, 0.5 mM ICI 182780 treatment led to defects including frayed chromosome appearance and chromatin bridges in most oocytes at diakinesis, including 6.5% (n = 31) of the −1 oocytes (Fig. 3A). Similar types of defects were also observed in diakinesis oocytes when worms were exposed to the selective estrogen receptor modulator 4-hydroxytamoxifen (0.15 mM 4-OHT) (Fig. S4). The similar outcomes of ICI and BPA treatment on chromosome morphology suggest that these compounds may exert their effect on the germline through an anti-estrogenic activity. To test this hypothesis, we exposed worms to ethanol, BPA, and either a combination of BPA and E2 or BPA and ICI 182780. Coexposure to BPA and E2 largely rescued the chromosome morphology defects observed at diakinesis (Fig. 3B). Conversely, coexposure to ICI 182780 did not worsen the diakinesis defects observed following BPA treatment. In C. elegans, BPA can bind to the nuclear hormone receptor NHR-14, which also binds estrogen (41). Analysis of nhr-14 mutants, however, did not reveal germline defects and the mutants did not show increased sensitivity to BPA (Fig. S5). Thus, these results strongly imply that BPA alters chromosome morphogenesis via its anti-estrogenic activity and suggest an action of BPA on a yet to be identified nuclear hormone receptor.
BPA Exposure Results in Impaired Chromosome Segregation During Early Embryogenesis.
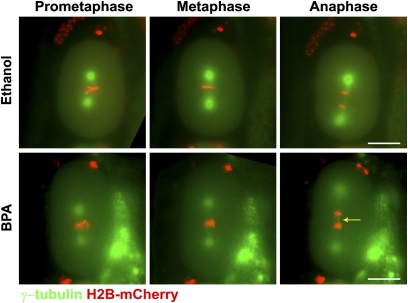
Considering the extent of the defects observed during late diakinesis, we predicted that the early steps of embryogenesis might be impaired in BPA-treated worms. We monitored the first mitotic division in ethanol- and BPA-exposed H2B::mCherry; γ-tubulin::GFP transgenic worms by live imaging (n = 60 each). Whereas pronuclear fusion, chromosome alignment at the metaphase plate, and chromosome segregation at anaphase were normal in ethanol controls, the first division of BPA-exposed embryos was highly abnormal. Specifically, chromosomes failed to align at the metaphase plate and/or segregate properly in 15% (n = 9/60) of one-cell embryos (Fig. 4, Movies S1 and S2) and the majority (5/9) of these embryos failed to undergo cytokinesis and either arrested or underwent formation of multiple spindle poles (Movie S3). These results, coupled with the elevated levels of embryonic lethality, indicate that BPA exposure results in impaired chromosome segregation during early embryogenesis in C. elegans.
Fig. 4.
BPA exposure results in chromosome segregation defects during early embryonic cell division. Time-lapse analysis is shown of the first embryonic division in ethanol- and BPA-exposed H2B::mCherry; γ-tubulin::GFP embryos (n = 60 per condition). Chromosomes (red) fail to properly align along the metaphase plate in the BPA-exposed embryo. The arrow indicates a chromatin bridge observed in the same embryo during anaphase. (Scale bars, 20 μm.)
Discussion
We investigated the effects of the highly prevalent chemical Bisphenol A on the germline of C. elegans. We found that BPA exerts anti-estrogenic properties that severely disrupt the progression of meiotic recombination and chromosome synapsis in C. elegans, leading to sterility and embryonic lethality.
While providing an analysis of the effects of BPA on the germ-line of C. elegans, our results also recapitulate some of the defects observed in mice (9). Similar to findings reported in mice, we observe instances of asynapsis and failure to maintain chromosome integrity at pachytene. We also observe altered meiotic recombination as highlighted by increased RAD-51, pCHK-1, and ATL-1 levels in late pachytene. In both species, early embryonic chromosome defects are observed and BPA effects are mediated by an intrinsic anti-estrogenic activity. To our knowledge, however, the identification of a germline-specific down-regulation of the expression of the DNA repair genes rad-54 and mre-11, as well as the checkpoint gene mrt-2 in BPA-exposed animals, has not been previously described. BPA exposure recapitulates some of the phenotypes observed in each mutant of these DNA repair genes. Notably, budding yeast Rad54 functions in the ATP-dependent displacement of Rad51 from DNA heteroduplexes, which are central homologous recombination intermediates (42). This result is congruent with the elevated levels of RAD-51 foci observed in late pachytene in BPA-exposed worms. We also observed DNA fragmentation at pachytene and diakinesis, highly reminiscent of the fragments observed in the mre-11 mutant following DNA damage (32). Finally, the mutation of C. elegans mrt-2 leads to end-to-end chromosome fusions (34) that may be analogous to the chromosome associations described by Hunt and colleagues in the mouse model of BPA exposure (9) and could account for the chromosome aggregation we observed at diakinesis following BPA exposure. Notably, we did not observe elevated RAD-51, ATL-1, or pCHK1 staining in the mitotic germline nuclei, suggesting that BPA exposure may elicit a specific stress on the meiotic DSB repair process. However, the down-regulated DSB repair genes operate in both mitotic and meiotic repair. Therefore, the exact molecular target that confers meiotic specificity to the DSB repair defects caused by BPA remains to be identified. Altogether, our analysis revealed a potential mechanism of function through which BPA can impact the germline that was not previously understood.
Interestingly, although the progression of meiotic recombination was clearly impaired in BPA-exposed worms, we did not observe an increased number of crossover events either as judged by ZHP-3::GFP foci, a marker of crossover or crossover precursor sites in late prophase (Fig. S6) (43), or by cytological analysis of chromosome morphology in diakinesis oocytes. This difference might stem from the tight regulation of crossover interference observed in C. elegans. In mammalian species, up to three crossover events per homolog pair are detected; however, in C. elegans, crossover interference strictly limits the number of exchanges to one (44). Therefore, the BPA-induced perturbation of meiotic recombination may not be able to override the strong control exerted on crossover number in C. elegans.
Exposure Levels and Activity Reveal That C. elegans Is a Relevant Model System to Examine the Effect of Environmental Toxicants in Meiosis.
We examined the internal levels of BPA following our exposure protocol and found that worms contained on average 2 μg/g of unconjugated BPA. These levels were within the range or lower than the internal free BPA levels detected in maternal kidneys, liver, and uterus, as well as in fetal liver and total fetal homogenate of pregnant rats perfused with a single dose of BPA at 10 mg/kg (22). Data on nonblood tissue levels of BPA both in rodent models and in humans are scarce and intraorgan concentrations in the BPA study on mouse meiosis (9) were not measured, making direct exposure comparison difficult. However, it is likely that both our results and those of Susiarjo and colleagues (9) represent the reproductive outcome following elevated BPA exposure. Therefore, our studies bear relevance to occupational exposure studies in humans (24, 45), and particularly to fetal and neonate exposure levels, as suggested by the up to 10 times higher levels of BPA detected in premature infants in neonatal intensive care units (46).
BPA Specifically Alters the Germline Expression of DSB Repair Components.
Analysis of both in vivo and in vitro exposure models has suggested that BPA forms DNA adducts (47, 48) leading to the formation of DSBs and aneuploidy in mitotically dividing cells (49, 50). Although these results might constitute a tempting explanation for the defects seen following BPA exposure, we did not find evidence of DNA damage in the mitotic zone, as determined by the absence of elevated levels of chromosome-associated ATL-1, pCHK-1, and RAD-51 in the mitotic zone. Furthermore, BPA exposure did not rescue the lack of chiasmata (12 univalents) observed in the meiotic DSB-deficient spo-11 mutants (Fig. S7), suggesting that BPA does not directly or indirectly induce DSBs. Finally, although the levels of RAD-51 foci were elevated in late pachytene nuclei of BPA-exposed germlines, we did not observe a concomitant increase in the total number of RAD-51 foci earlier in prophase (zones 3 and 4), also suggesting that BPA exposure does not lead to an increase in the number of DSBs at least in early prophase. Instead, BPA appears to specifically disrupt the germline DSB repair program, which was confirmed by our analysis of the expression of DNA repair genes in the context of the glp-1 mutants grown at either permissive or restrictive temperatures. This result is reminiscent of the role uncovered for estrogen receptor signaling in regulating meiotic progression (9). Indeed, ERβ−/− mice and BPA-exposed mice display very similar perturbations of chromosome synapsis and recombination (9). Further strengthening the link between ER signaling and players of the DNA repair machinery, estrogen via ERα has been shown to inhibit the activation of ATR and phosphorylation of Chk1 in breast cancer cell lines, which delays the formation of Rad51 foci and DNA repair following DNA damage (51). Moreover, normal estrogen levels are also required for high p53 signaling whereas either low estrogen levels or down-regulation of ERα leads to lower p53 levels and improper DNA damage response (52). These findings therefore support a connection between hormonal signaling and DNA repair and, together with our data, suggest an anti-estrogenic effect of BPA on the germline. However, until the nuclear hormone receptor that is sensitive to BPA is identified, the possibility of a non-ER–mediated effect of BPA on DNA repair cannot be formally excluded.
C. elegans Is a Relevant Model for the Study of Environmental Disruption of Meiosis.
At present, mammalian models are used for the study of environmental disruption of the process of meiosis. However, from inception to completion, mammalian female meiosis can span from several months in mice to several decades in humans. Therefore, there is a great need for cost-effective and highly tractable multicellular systems that can be used as an alternative to mammalian models for such studies. Such systems are of significant importance given that meiosis is essential for sexual reproduction and the effects of our chemical landscape on this process are poorly understood. The use of a genetically tractable model with such well-characterized developmental patterns as those of C. elegans opens up a number of experimental possibilities including the use of this system for large-scale screens and follow-up analysis to determine the mechanisms of action of potential toxicants on the process of meiosis. The prompt assessments made in this system will help design targeted follow-up studies in mammals and tremendously advance our understanding of the impact of environmental toxicants on human reproductive health.
Materials and Methods
C. elegans Genetics, Growth Conditions, and Drug Exposures.
C. elegans strains were cultured according to ref. 53 at 20 °C on NGM plates without cholesterol. The N2 Bristol strain was used as the wild-type background. The following mutations and chromosome rearrangements were used in this study: LGI, cep-1(lg12501), hT2 [bli-4(e937) qIs48] (I;III); LGIII, glp-1(bn18); LGIV, spo-11(ok79); and LGX, nhr-14(tm1473) (41, 54–56). The ZHP-3::GFP strain was kindly provided by V. Jantsch (43). BPA, 17β-estradiol, and 4-hydroxy-tamoxifen (Sigma-Aldrich) were dissolved in 100% ethanol and added to the medium before pouring plates for a final ethanol concentration of 0.1%. ICI 182780 (Tocris Bioscience) was dissolved in DMSO and added to the medium for a final DMSO concentration of 1%. Exposure was carried out from embryogenesis to adulthood by plating sodium hypochloride-treated eggs onto drug and control plates followed by incubation at 20 °C for 4 d.
Additional procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank A. Nottke for discussions and C. Tabin and K. Meyer for critical reading of the manuscript. We thank the Caenorhabditis Genetics Center and K. Blackwell (Harvard Medical School, Boston) for strains. We also thank A. La Volpe (Institute of Genetics and Biophysics Adriano Buzzati-Traverso, Napoli) for the RAD-51 antibody, M. Zetka (McGill University, Montreal) for the HTP-3 antibody, A. Dernburg (University of California Berkeley, Berkeley, CA) for the HIM-8 antibody, and S. Boulton (London Research Institute, South Mimms) for the ATL-1 antibody. This work was supported by National Institutes of Health Grant R01GM072551 (to M.P.C.) and by a Fonds de la Recherche en Santé du Québec postdoctoral fellowship (to P.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010386107/-/DCSupplemental.
References
- 1.Hunt PA, Hassold TJ. Human female meiosis: What makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Christianson RE, Sherman SL, Torfs CP. Maternal meiosis II nondisjunction in trisomy 21 is associated with maternal low socioeconomic status. Genet Med. 2004;6:487–494. doi: 10.1097/01.gim.0000144017.39690.4e. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PA. Meiosis in mammals: Recombination, non-disjunction and the environment. Biochem Soc Trans. 2006;34:574–577. doi: 10.1042/BST0340574. [DOI] [PubMed] [Google Scholar]
- 4.Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319:906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Toxicology Program NTP-CERHR monograph on the potential human reproductive and developmental effects of Bisphenol A. NTP CERHR MON. 2008;(22):i–III1. [PubMed] [Google Scholar]
- 6.Calafat AM, et al. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang IA, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 9.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colaiácovo MP. The many facets of SC function during C. elegans meiosis. Chromosoma. 2006;115:195–211. doi: 10.1007/s00412-006-0061-9. [DOI] [PubMed] [Google Scholar]
- 11.Leung MC, et al. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 13.Zetka M. Homologue pairing, recombination and segregation in Caenorhabditis elegans. Genome Dyn. 2009;5:43–55. doi: 10.1159/000166618. [DOI] [PubMed] [Google Scholar]
- 14.Hunt PA, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, et al. Effects of bisphenol A given neonatally on reproductive functions of male rats. Reprod Toxicol. 2006;22:20–29. doi: 10.1016/j.reprotox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Mok-Lin E, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi H, Kamata Y, Uemura T. Effects of 17beta-estradiol, bisphenol A and tributyltin chloride on germ cells of Caenorhabditis elegans. J Vet Med Sci. 2003;65:881–885. doi: 10.1292/jvms.65.881. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein P. Nuclear aberrations and loss of synaptonemal complexes in response to diethylstilbestrol (DES) in Caenorhabditis elegans hermaphrodites. Mutat Res. 1986;174:99–107. doi: 10.1016/0165-7992(86)90098-9. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Mitani N, Ishii N, Miki K. A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat Res. 2005;570:71–80. doi: 10.1016/j.mrfmmm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93:31–35. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 22.Moors S, Diel P, Degen GH. Toxicokinetics of bisphenol A in pregnant DA/Han rats after single i.v. application. Arch Toxicol. 2006;80:647–655. doi: 10.1007/s00204-006-0097-x. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.He Y, et al. Occupational exposure levels of bisphenol A among Chinese workers. J Occup Health. 2009;51:432–436. doi: 10.1539/joh.o9006. [DOI] [PubMed] [Google Scholar]
- 25.Schönfelder G, et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinaldo C, Bazzicalupo P, Ederle S, Hilliard M, La Volpe A. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics. 2002;160:471–479. doi: 10.1093/genetics/160.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage—induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- 28.Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics. 2000;156:617–630. doi: 10.1093/genetics/156.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Muse T, Boulton SJ. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 2005;24:4345–4355. doi: 10.1038/sj.emboj.7600896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser SC, et al. Functional dissection of Caenorhabditis elegans CLK-2/TEL2 cell cycle defects during embryogenesis and germline development. PLoS Genet. 2009;5:e1000451. doi: 10.1371/journal.pgen.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin J, Kimble J. glp-1 is required in the germline for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 32.Chin GM, Villeneuve AM. C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev. 2001;15:522–534. doi: 10.1101/gad.864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohara M, et al. Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics. 1997;147:1545–1556. doi: 10.1093/genetics/147.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S, Hodgkin J. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature. 2000;403:159–164. doi: 10.1038/35003120. [DOI] [PubMed] [Google Scholar]
- 35.Nabeshima K, Villeneuve AM, Colaiácovo MP. Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC. J Cell Biol. 2005;168:683–689. doi: 10.1083/jcb.200410144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Carvalho CE, et al. LAB-1 antagonizes the Aurora B kinase in C. elegans. Genes Dev. 2008;22:2869–2885. doi: 10.1101/gad.1691208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- 38.Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol. 2002;157:219–229. doi: 10.1083/jcb.200110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushima A, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007;142:517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- 40.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 41.Mimoto A, et al. Identification of an estrogenic hormone receptor in Caenorhabditis elegans. Biochem Biophys Res Commun. 2007;364:883–888. doi: 10.1016/j.bbrc.2007.10.089. [DOI] [PubMed] [Google Scholar]
- 42.Li X, et al. Rad51 and Rad54 ATPase activities are both required to modulate Rad51-dsDNA filament dynamics. Nucleic Acids Res. 2007;35:4124–4140. doi: 10.1093/nar/gkm412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhalla N, Wynne DJ, Jantsch V, Dernburg AF. ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet. 2008;4:e1000235. doi: 10.1371/journal.pgen.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillers KJ, Villeneuve AM. Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol. 2003;13:1641–1647. doi: 10.1016/j.cub.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Li D, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25:519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 46.Calafat AM, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson A, Roy D. In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s) Biochem Biophys Res Commun. 1995;210:424–433. doi: 10.1006/bbrc.1995.1678. [DOI] [PubMed] [Google Scholar]
- 48.Izzotti A, Kanitz S, D'Agostini F, Camoirano A, De Flora S. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat Res. 2009;679:28–32. doi: 10.1016/j.mrgentox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Iso T, Watanabe T, Iwamoto T, Shimamoto A, Furuichi Y. DNA damage caused by bisphenol A and estradiol through estrogenic activity. Biol Pharm Bull. 2006;29:206–210. doi: 10.1248/bpb.29.206. [DOI] [PubMed] [Google Scholar]
- 50.Tsutsui T, et al. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer. 1998;75:290–294. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009;20:3374–3389. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Cuesta L, Anaganti S, Hainaut P, Olivier M. Estrogen levels act as a rheostat on p53 levels and modulate p53-dependent responses in breast cancer cell lines. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0819-x. in press. [DOI] [PubMed] [Google Scholar]
- 53.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodoyianni V, Maine EM, Kimble J. Molecular basis of loss-of-function mutations in the glp-1 gene of Caenorhabditis elegans. Mol Biol Cell. 1992;3:1199–1213. doi: 10.1091/mbc.3.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed S. Uncoupling of pathways that promote postmitotic life span and apoptosis from replicative immortality of Caenorhabditis elegans germ cells. Aging Cell. 2006;5:559–563. doi: 10.1111/j.1474-9726.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- 56.Dernburg AF, et al. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell. 1998;94:387–398. doi: 10.1016/s0092-8674(00)81481-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.