Abstract
A hallmark of diabetes is an absolute or relative reduction in the number of functional β cells. Therapies that could increase the number of endogenous β cells under diabetic conditions would be desirable. Prevalent gene targeting mouse models for assessing β-cell proliferation and diabetes pathogenesis only address whether deletion of a gene prevents the development of diabetes. Models testing whether acute excision of a single gene can ameliorate or reverse preexisting hyperglycemia in established diabetes remain to be explored, which could directly validate the effect of gene excision on treating diabetes. Here, we report that acute and temporally controlled excision of the Men1 gene, which encodes menin, ameliorated preexisting hyperglycemia in streptozotocin-treated mice. Moreover, Men1 excision also improved the preexisting hyperglycemia and glucose intolerance in genetic db/db diabetic mice. Furthermore, acute Men1 excision reversed preexisting glucose intolerance in high-fat diet-fed mice. Men1 excision improved glucose metabolism at least partly through increasing proliferation of endogenous β cells and islet size. Acute Men1 excision up-regulated a group of proproliferative genes in pancreatic islets. Together, these findings demonstrate that established hyperglycemia can be reversed through repression of a single gene, Men1, in diabetic conditions, and suggest that menin is a vital regulator in pathogenesis of diabetes.
Keywords: cell proliferation, db/db, high-fat diet, type 2 diabetes
Both type 1 and type 2 diabetes ultimately result from an insufficient number of functional β cells in islets (1). Therefore, approaches that promote β-cell regeneration or proliferation and increase the number of endogenous β cells under diabetic conditions would be desirable. Thus far, many factors including multiple cell cycle regulators have been tested in mouse models, and their roles in β-cell proliferation and diabetes development have been determined (2). For instance, cyclin D1, cyclin D2, and cyclin-dependent kinase 4 (Cdk4) are crucial for β cell proliferation and preventing the development of hyperglycemia (2). Deletion of p27cip1/kip1 (p27 hereafter), a cyclin-dependent kinase inhibitor, prevents development of diabetes in db/db mice, partly by increasing the number of β cells (3). Deletion of Lkb1, a tumor suppressor involved in AMP kinase activation, promotes β-cell proliferation and ameliorates glucose intolerance (4). However, no report has shown that acute deletion of a single gene reverses preexisting glucose intolerance or hyperglycemia in mouse models. Such a study would be desirable and is closely related to treating diabetes, because it directly evaluates the impact of manipulating a single gene on treating preexisting diabetes.
Menin is a nuclear protein encoded by the Men1 gene that is mutated in patients with familial multiple endocrine neoplasia type 1 (MEN1) syndrome (5). Menin preferentially represses proliferation of endocrine cells including β cells (6, 7). Although Men1 excision after a long period promotes β-cell proliferation and increases blood insulin levels under normal conditions (6, 8, 9), little is known as to whether acute Men1 excision can correct preexisting abnormal glucose homeostasis in diabetic mice. Stressed endogenous β cells under diabetic conditions may respond to Men1 excision differently from normal β cells. Thus, it is important to determine whether Men1 excision actually ameliorates or reverses hyperglycemia in diabetic mice.
How menin regulates β-cell proliferation is not well understood. Although menin has been shown to be crucial for expression of cyclin-dependent kinase inhibitor p18ink4c (p18 hereafter) and p27 in islets (10, 11) and liver cells (12), Men1 excision does not affect liver cell proliferation (7). These findings raise the possibility that menin may also regulate β cell proliferation through effectors other than p18 and p27 (12). Menin up-regulates gene transcription through histone H3 modifications, such as H3K4 methylation (13, 14). However, it is unclear whether menin represses transcription of endogenous genes, especially proproliferative cell cycle genes in β cells.
In this study, we found that acute Men1 excision ameliorated preexisting hyperglycemia in streptozotocin (STZ)-treated mice. Moreover, acute Men1 excision also corrected preexisting glucose intolerance or hyperglycemia in genetic db/db or high-fat diet-fed diabetic mice. Acute Men1 ablation promoted β-cell proliferation and increased β-cell number partly by coordinately up-regulating multiple proproliferative cell cycle genes. Our findings suggest that menin actively regulates the process of diabetes and could be manipulated to treat diabetes.
Results
Insulin Secretion by Islets and Peripheral Insulin Sensitivity Are Not Affected by Men1 Ablation.
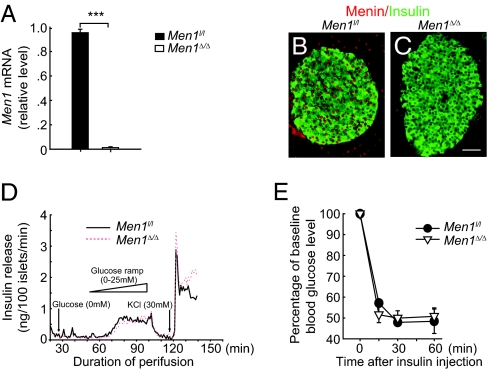
Conventional mouse knockout models have been used to determine whether gene ablation can prevent development of diabetes (2). To further determine the effect of gene ablation on reversing established abnormal glucose homeostasis, we used a conditional and inducible Men1 knockout model and determined whether acute Men1 excision ameliorated preexisting hyperglycemia in diabetic mice. Men1l/l;Cre-ER mice were generated by crossing Men1l/l mice to mice expressing the Ubc9 promoter-driven Cre-ERT2 transgene (6, 15). Men1l/l;Cre-ER and control Men1l/l mice were fed tamoxifen (TAM), and Men1 excision in pancreatic islets was determined by quantitative real-time PCR (qRT-PCR) and immunostaining, 30 d after TAM treatment. Menin expression was markedly reduced in Men1l/l;Cre-ER islets as compared with control islets (Fig. 1 A–C), indicating effective Men1 excision.
Fig. 1.
Men1 excision does not affect insulin secretion by islets and peripheral insulin sensitivity. Men1l/l or Men1l/l;Cre-ER mice at the age of 12 wk were fed tamoxifen (TAM) at 200 mg/kg of body weight per day. (A) Men1 mRNA levels in islets isolated from Men1l/l and Men1l/l;Cre-ER mice 30 d after TAM treatment (n = 10 mice). (B and C) Immunostaining for menin and insulin in islets from Men1l/l (B) and Men1l/l;Cre-ER mice (C) 30 d after TAM treatment (n = 6 mice). (Scale bar: 25 μm.) (D) Insulin release from islets isolated from Men1l/l and Men1l/l;Cre-ER mice 30 d after TAM treatment. One hundred size-matched islets of each genotype were hand-picked and cultured for 3 d. Insulin secretion stimulated by glucose (0–25 mM) and potassium chloride (KCl, 30 mM) were measured as described in SI Appendix. (E) Insulin tolerance test (ITT; insulin at 0.75 U/kg of body weight, i.p.) was performed in Men1l/l and Men1l/l;Cre-ER (n = 4–5 mice) 4 mo after TAM treatment. Test results were expressed as the percentage of baseline nonfasting blood glucose levels. ***P < 0.001.
To determine whether Men1 excision affects insulin secretion by pancreatic β cells in response to glucose, islet perifusion studies were performed by using islets isolated from mice, 30 d after TAM feeding. Size-matched islets isolated from Men1-excised and control mice were perifused with glucose or potassium chloride (4). The first-phase, second-phase, or total insulin secretion was similar between the Men1-excised and control islets (Fig. 1D), suggesting that Men1 excision does not affect insulin secretion by an individual β cell in response to glucose. To further determine whether Men1 excision affects overall insulin sensitivity in peripheral tissues other than pancreatic β cells, insulin tolerance tests (ITTs) were performed in Men1l/l;Cre-ER and control mice 4 mo after TAM feeding. There was no difference in insulin sensitivity between the control and Men1-excised mice (Fig. 1E).
Acute Men1 Ablation Ameliorates Preexisting STZ-Induced Hyperglycemia.
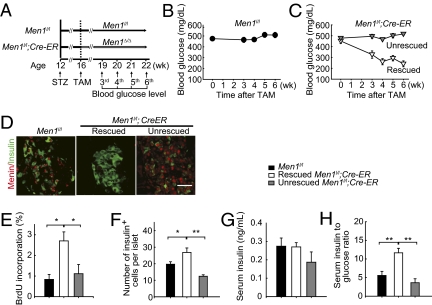
Next, we sought to determine the impact of acute Men1 excision on preexisting hyperglycemia in mice that were treated with STZ, a β-cell cytotoxic agent that preferentially damages β cells (16). Both Men1l/l;Cre-ER and control mice developed hyperglycemia 3 wk after STZ treatment (SI Appendix, Fig. 1A). We then examined whether Men1 excision could affect the preexisting hyperglycemia in the diabetic mice (Fig. 2A). No improvement in hyperglycemia was observed in the Men1l/l control mice after TAM treatment over 6 wk of observation (Fig. 2B). In contrast, blood glucose levels started to decrease in 55% of Men1l/l;Cre-ER mice as early as 3 wk after TAM feeding (Fig. 2C, rescued). However, 45% of the Men1l/l;Cre-ER mice remained hyperglycemic after TAM treatment (Fig. 2C, unrescued). Further investigation showed that Men1 was effectively excised in islets in the rescued mice after TAM treatment (Fig. 2D, Center), whereas Men1 excision was inefficient in the unrescued mice (Fig. 2D, Right). The inefficient Men1 excision might be partly attributable to STZ-induced alkylation of genomic DNA in β cells (16). These results strongly indicate that Men1 excision ameliorates hyperglycemia in mice with preexisting diabetes.
Fig. 2.
Acute Men1 excision ameliorates preexisting hyperglycemia in STZ-induced diabetes. (A) A schematic of experimental design. Control Men1l/l or Men1l/l;Cre-ER mice (n = 16 mice) at the age of 12 wk were i.p. injected with STZ at 40 mg/kg of body weight per day. Diabetic Men1l/l (n = 8 mice) or Men1l/l;Cre-ER (n = 11 mice) mice whose blood glucose levels were >250 mg/dL for two consecutive weeks after STZ injections were fed TAM. Blood glucose levels were monitored before and until 6 wk after the last dose of TAM treatment. (B) Average blood glucose levels in the control mice. (C) Average blood glucose levels in the rescued and unrescued Men1 l/l;Cre-ER mice. Blood glucose levels <250 mg/dL for two consecutive weeks after TAM treatment were defined as the rescued phenotype. (D) Immunostaining for menin and insulin in islets from the control Men1l/l, rescued Men1l/l;Cre-ER, and unrescued Men1l/l;Cre-ER mice. (Scale bar: 25 μm.) (E) Quantitation of BrdU incorporation by pancreatic β cells from the control, rescued, and unrescued Men1l/l; Cre-ER mice. (F) Quantitation of the number of insulin-positive cells in islets. (G) Nonfasting serum insulin levels 6 wk after TAM treatment. (H) Ratio of serum insulin (ng/mL) to blood glucose (mg/dL) levels, multiplied by 10,000. *P < 0.05; **P < 0.01.
Moreover, BrdU incorporation by β cells and the number of insulin-positive cells were higher in the rescued mice than those in the unrescued and control mice (Fig. 2 E and F and SI Appendix, Fig. 1 B and C). Random serum insulin levels were not significantly different among the three groups (Fig. 2G); however, Men1 ablation increased the ratio of serum insulin to blood glucose levels in the rescued mice, consistent with improved islet function and/or total insulin secretion from islets in response to blood glucose stimulation (Fig. 2H). As the Cre-ER transgene did not affect islet size (SI Appendix, Fig. 2 A and B), β cell proliferation (SI Appendix, Fig. 2C), and number of glucagon-producing α cells (SI Appendix, Fig. 2D) in normal and STZ-treated mice (SI Appendix, Fig. 2), we conclude that Men1 excision rescues preexisting STZ-induced hyperglycemia at least partly through increasing β cell proliferation and the number of β cells.
Acute Men1 Ablation Ameliorates Preexisting Hyperglycemia and Glucose Intolerance in Genetic db/db Diabetic Mice.
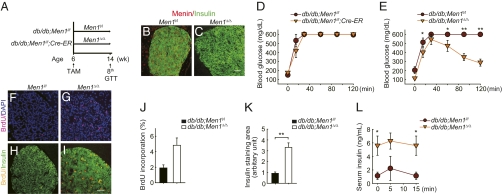
To further confirm our observation that acute Men1 excision reverses hyperglycemia in established diabetes, we chose db/db mice, a genetically defined type 2 diabetes mouse model with mutations in the leptin receptor gene (17, 18) and evaluated the impact of acute Men1 excision on glucose homeostasis, as described in a schema in Fig. 3A. Both db/db;Men1l/l;Cre-ER and control db/db;Men1l/l mice developed glucose intolerance at 6 wk of age, and they were fed TAM at this age (Fig. 3 A and D). Two months after TAM feeding, Men1 was completely excised in the pancreas in the db/db;Men1l/l;Cre-ER mice, but not the control db/db;Men1l/l mice (Fig. 3 B and C). Acute Men1 excision in db/db;Men1l/l;Cre-ER mice significantly improved glucose intolerance (Fig. 3 D and E), and lowered fasting blood glucose levels in mice, 8 wk after TAM treatment (0 min; Fig. 3E). Consistently, Men1 excision increased BrdU incorporation by β cells (Fig. 3 F–J) and islet size (Fig. 3K).
Fig. 3.
Acute Men1 excision ameliorates hyperglycemia in db/db mice. (A) A schematic of experimental design. Control db/db;Men1l/l and db/db;Men1l/l;Cre-ER mice (n = 5–7 mice) were fed TAM at the age of 6 wk. Mice were killed 8 wk after TAM feeding. (B and C) Immunostaining for menin and insulin in islets from control mice (B) and db/db;Men1l/l;Cre-ER mice (C) 8 wk after TAM treatment. (Scale bar, 25 μm.) (D and E) Glucose tolerance test (GTT, glucose at 2 g/kg of body weight, i.p.) before (D) and 8 wk after (E) TAM treatment. (F–I) BrdU incorporation by β cells. (F and G) Immunostaining for BrdU and DAPI. (H and I) Immunostaining for BrdU and insulin. (Scale bar: 25 μm.) (J) Quantitation of BrdU incorporation by β cells. (K) Quantitation of insulin-staining area. (L) Glucose-stimulated insulin secretion (GSIS, glucose at 3 g/kg of body weight, i.p.), measured 8 wk after TAM treatment. *P < 0.05; **P < 0.01.
We also found that, at age of 14 wk or 8 wk after TAM treatment, the fasting insulin level in the control db/db mice was 1.4 ng/mL (Fig. 3L, 0 min), consistent with the fact that compensatory high insulin levels (hyperinsulinemia) in db/db mice dropped because of deterioration of β cells at ≈14 wk of age (19). Notably, the fasting insulin level in the Men1-excised db/db mice was much higher than the control Men1-expressing mice (5.3 ng/mL; Fig. 3L, 0 min), indicating that Men1 excision is crucial for maintaining a sufficient number of functional β cells (Fig. 3 J and K) and compensatory hyperinsulinemia in db/db mice. Because Men1 excision did not change insulin secretion by size-match islets (Fig. 1D), body weight (SI Appendix, Fig. 3A), or peripheral insulin sensitivity (SI Appendix, Fig. 3B), our results strongly suggest that acute Men1 excision improves preexisting glucose intolerance and hyperglycemia in db/db diabetic mice through promoting β-cell proliferation and increasing the number of functional β cells.
Acute Men1 Ablation Reverses High-Fat Diet-Induced Preexisting Glucose Intolerance.
To determine whether acute Men1 excision reverses diet-related abnormal glucose homeostasis, we extended our study to a high-fat diet-induced type 2 diabetes mouse model. High-fat diet increases body weight and induces metabolic syndrome and diabetes in mice (20, 21). As menin expression is down-regulated in islets in obese Ay mice (22), we examined the level of menin expression in C57BL6J mice on a high-fat diet for 13 wk. Although the high-fat diet significantly increased body weight (SI Appendix, Fig. 4A) and induced glucose intolerance in C57BL6J mice (SI Appendix, Fig. 4B), it did not repress menin expression levels in islets, as compared with the mice on chow diet (SI Appendix, Fig. 4 C and D). Therefore, acute Men1 excision might be able to promote proliferation of endogenous β cells in high-fat diet-fed mice because menin is not repressed in the islets of these mice.
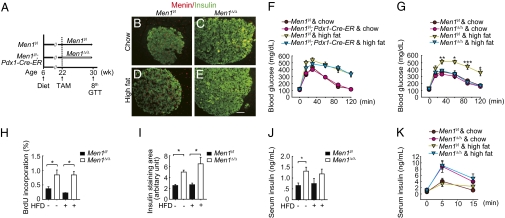
To detect the effect of acute Men1 excision in islets on high-fat diet-induced glucose intolerance, we chose to generate mice in which Men1 could only be excised in pancreatic islets. To this end, we generated Men1l/l;Pdx1-Cre-ER and control Men1l/l mice (23). Pdx1 expression is restricted to pancreatic β cells in adult mice, and Men1 was excised only in β cells, but not in the adjacent acinar cells in the Men1l/l;Pdx1-Cre-ER mice after TAM feeding (SI Appendix, Fig. 5 B and C). Men1l/l;Pdx1-Cre-ER and control Men1l/l mice were fed with either chow diet or high-fat diet for 16 wk (Fig. 4A). Compared with mice on chow diet, the control and Men1l/l;Pdx1-Cre-ER mice on high-fat diet for 16 wk gained substantially more body weight and developed glucose intolerance (SI Appendix, Fig. 5A and Fig. 4F). Eight weeks after TAM feeding, Men1 was efficiently excised in islets of Men1l/l;Pdx1-Cre-ER mice but not in the control mice (Fig. 4 B–E). Notably, high-fat diet-induced glucose intolerance was normalized 8 wk after Men1 excision (Fig. 4 F and G), demonstrating that acute Men1 excision corrected high-fat diet-induced glucose intolerance.
Fig. 4.
Acute Men1 excision reverses preexisting high-fat diet (HFD)-induced glucose intolerance. (A) A schematic of experimental design. Control Men1l/l and Men1l/l;Pdx1-Cre-ER mice (n = 10–16 mice) were fed high-fat diet or chow diet at the age of 6 wk for 16 wk and followed by TAM treatment. Mice were killed 8 wk after TAM feeding. (B–E) Immunostaining for menin and insulin in islets. (B and C) Mice fed chow diet. (D and E) Mice fed high-fat diet. (Scale bar: 25 μm.) (F and G) GTT (n = 8–15 mice) before (F) and 8 wk after (G) TAM treatment. (H) Quantitation of BrdU incorporation by β cells. (I) Quantitation of insulin-staining area. (J) Fasting insulin levels, 8 wk after TAM treatment. (K) GSIS (n = 8–15 mice), 8 wk after TAM treatment. *P < 0.05; **P < 0.01; ***P < 0.001.
Men1 excision increased BrdU incorporation by β cells and insulin staining areas in mice on either a high-fat diet or chow diet (SI Appendix, Fig. 5 D–G and Fig. 4 H and I). Men1 excision also elevated fasting circulating insulin levels and glucose-stimulated insulin secretion in mice on either chow diet or high fat diet (Fig. 4 J and K). These results demonstrate that Men1 excision increases β-cell proliferation, β-cell number, and circulating insulin concentrations and, subsequently, reverses glucose intolerance in high-fat diet-fed mice. Although Pdx1-Cre-ER induced Men1 excision in β cells, but not in the adjacent acinar cells in mice (SI Appendix, Fig. 5 B and C), we cannot completely rule out the possibility that Pdx1-Cre-ER-induced Men1 excision in tissues beyond β cells might also contribute to amelioration of glucose intolerance in mice.
Menin Represses Multiple Proproliferative Genes in Pancreatic β Cells.
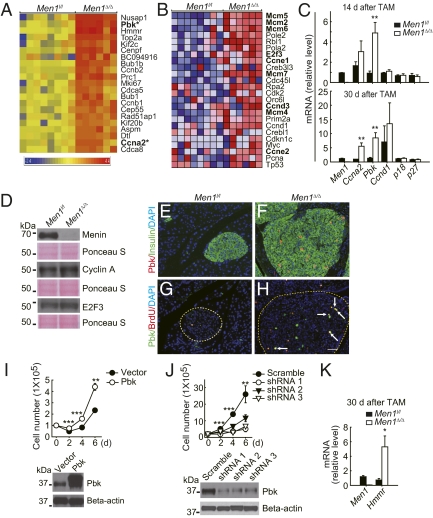
To understand how Men1 excision promotes β-cell proliferation, and to identify early changes in gene expression profiles after acute Men1 excision, cDNA microarray analysis was performed on Men1-excised and control islets isolated from mice 14 d after TAM feeding. Sixty-seven genes were significantly up-regulated (Fig. 5A and SI Appendix, Fig. 6 and SI AppendixTable 1). Twenty-two genes were moderately down-regulated in Men1-excised islets, as compared with control islets (SI Appendix, Table 2). Gene Ontology analysis (24) showed that most of the up-regulated genes were involved in controlling cell cycle, DNA synthesis, and mitosis (SI Appendix, Table 3). Gene Set Enrichment Analysis (GSEA) (25) further revealed that several groups of genes involved in G0/G1 to S phase transition and DNA replication were up-regulated in Men1-excised islets (Fig. 5B and SI Appendix, Table 4). Interestingly, many of the genes repressed by menin, including Ccne1, Ccne2, and Ccnd3 (Fig. 5B), are also target genes of the cell cycle-regulating transcription factor E2F3 (SI Appendix, Fig. 7) (25–27), and E2F3 itself was mildly up-regulated by Men1 excision (Fig. 5 B and D).
Fig. 5.
Proproliferative genes are up-regulated in Men1-excised islets. (A) A profile of genes up-regulated by >5-fold in Men1-excised islets. Pancreatic islets were isolated from control Men1l/l (n = 8 mice) or Men1l/l;Cre-ER mice (n = 6 mice) 14 d after TAM feeding and processed for RNA isolation and cDNA microarray analysis, as described in Materials and Methods and SI Appendix. Genes labeled in bold and with an asterisk were further investigated. (B) Genes involved in G0/G1 to S phase transition were enriched in Men1-excised islets. Gene expression profiles of control Men1l/l and Men1-excised islets were analyzed against c2 of Molecular Signatures Database with Gene Set Enrichment Analysis (GSEA). (C) Gene expression profiles were verified by qRT-PCR. mRNA levels in islets from control Men1l/l or Men1l/l;Cre-ER mice 14 or 30 d after TAM feeding (n = 6–10 mice), using TaqMan primers and probes with Gapdh levels as internal controls. (D) Protein levels in islets isolated from control Men1l/l or Men1 l/l;Cre-ER mice, 8 wk after TAM treatment (n = 4–5 mice). Cyclin A protein levels in islets were quantitated by measuring signal density and area of cyclin A2 bands in Western blotting (1.65-fold, Men1-excised vs. control islets). (E–H) Immunostaining for Pbk and BrdU in Men1l/l and Men1l/l;Pdx1-Cre-ER mice (n = 10–11 mice) fed with TAM at the age of 12 wk and killed 8 wk after TAM treatment. (E and F) Immunostaining for Pbk and insulin. (G and H) Immunostaining for Pbk and BrdU. Arrow denotes cells stained positively for Pbk and BrdU. (Scale bar: 25 μm.) (I and J) Growth curves for mouse Pancreatic Islet-derived Men1 Excisable cells (PIME1 cells), as described in SI Appendix. (I) Growth curves of control and Pbk overexpressing PIME1 cells. (Upper) Cell numbers after seeding. (Lower) Protein levels of Pbk in control and Pbk overexpressing cells. (J) Growth curves of control and Pbk knockdown PIME1 cells. (Upper) Cell numbers after seeding. (Lower) Pbk protein level in control and Pbk knockdown cells. (K) Hmmr mRNA levels in islets from control Men1l/l or Men1l/l;Cre-ER mice 30 d after TAM feeding, as described in Fig. 5C (n = 6 mice). *P < 0.05; **P < 0.01; ***P < 0.001.
Pbk, a PDZ-binding kinase regulating mitosis (28) and cyclin A were cell cycle genes up-regulated in Men1-excised islets (Fig. 5A). Cyclin A forms a complex with Cdk2 and promotes G1 to S phase and S phase progression (29). The effect of Men1 excision on regulating expression of cyclin A and Pbk was confirmed with qRT-PCR, Western blotting, or immnunofluorescent staining (Fig. 5 C and D; Fig. 5 E–H for Pbk immunostaining). Most BrdU-positive β cells in islets were also stained positive for Pbk (Fig. 5 G and H), suggesting that the Pbk expression level was increased in proliferating cells. Consistent with a positive role for Pbk in regulating β-cell proliferation, ectopic expression of Pbk in a β-cell line led to an increased cell number (Fig. 5I) and also the percentage of cells in S phase (SI Appendix, Fig. 8). Conversely, Pbk knockdown in β cells resulted in a reduction in the number of β cells (Fig. 5J), indicating that the Pbk expression level is correlated with increased β-cell proliferation in Men1-excised β cells. Moreover, Hmmr, which encodes the receptor of hyaluronic acid mediated motility (Rhamm), was up-regulated in Men1-excised islets (Fig. 5K).
Consistent with a previous report by Scacheri et al. (12), expression of Cdc20 (SI Appendix, Table 1), another menin-regulated cell cycle regulator, was also increased by Men1 excision. However, we did not observe a reduction in mRNA levels of either p18 or p27 in Men1-excised islets (Fig. 5C).
As acute Men1 excision in islets up-regulated genes that promote each phase of the cell cycle (Fig. 5 A and B and SI Appendix, Fig. 6), collectively, these results suggest that menin is a crucial player in repressing multiple genes governing G0/G1 to S transition, S-phase progression, and mitosis in islets, and Men1 excision may coordinately derepress these genes to promote β-cell proliferation.
Discussion
We used a temporally controlled Men1 excision approach and determined the direct impact of acute Men1 excision on preexisting glucose intolerance or hyperglycemia in three distinct diabetes mouse models. Conventional gene targeting mouse models, such as the knockout models for D-cyclins and p27, were used to evaluate the impact of gene ablation on the prevention of diabetes (2, 3). Furthermore, in those models, gene ablation is executed during embryonic development or before development of diabetes, and the observed effect of prevention could also be attributed to developmental or compensational changes in multiple organs including pancreatic islets. In contrast, we used a different experimental approach and determined the effect of acute Men1 ablation in diabetic mice after they have developed glucose intolerance or hyperglycemia. This method directly evaluates whether manipulation of a single gene can be used for treating preexisting diabetes. We found that Men1 excision in pancreatic β cells ameliorated preexisting STZ-induced hyperglycemia in diabetic mice. Acute Men1 excision corrected preexisting hyperglycemia in db/db diabetic mice and reversed preexisting glucose intolerance in high-fat diet-fed mice (21). To our knowledge, this is the first study to show that acute and temporally controlled excision of a single gene reverses preexisting hyperglycemia or glucose intolerance in diabetic mice.
Our results showed that Men1 excision ameliorated preexisting hyperglycemia or glucose intolerance mainly through increasing the number of functional endogenous β cells. Men1 excision consistently increased BrdU incorporation, a marker of cell proliferation, in three diabetic models, suggesting that regeneration or replication is increased due to Men1 excision in stressed β cells. Moreover, the number of β cells, islet size, and circulating insulin levels were significantly increased in Men1-excised diabetic mice. These findings suggest that Men1 excision reversed preexisting hyperglycemia in diabetic mice, at least partly through increased β cell proliferation and the number of functional β cells. However, we cannot rule out that Men1 excision in β cells or in other tissues also improves β-cell function, insulin secretion, or peripheral insulin sensitivity under diabetic conditions, contributing to the improvement of abnormal glucose metabolism in diabetic mice. It is also possible that non-β cells in the pancreas can reprogram into insulin-secreting β cells in diabetic conditions, because Men1-excised glucagon-secreting α cells can transdifferentiate into β cells and insulinomas (30).
Our molecular analysis using an acute Men1 excision system revealed that menin controls expression of multiple cell cycle genes, and Men1 excision may derepress these genes and promote β-cell proliferation. For instance, expression levels of Ccna2 and multiple Mcms, genes important for S phase progression or DNA replication, were increased in Men1-excised islets (Fig. 5 A and B). Pbk, Ccnb1, and Ccnb2, a group of genes involved in G2/M transition and mitosis, were also up-regulated by Men1 ablation (Fig. 5A). Because ectopic Pbk expression and Pbk knockdown significantly affected β-cell proliferation (Fig. 5 I and J; SI Appendix, Fig. 8), and Pbk is mainly expressed in β cells but not in acinar cells (Fig. 5 E–H), Pbk may act as one of tissue-specific effector of menin in controlling β cell proliferation. However, further studies are necessary to determine whether Pbk is essential for Men1 excision-induced β-cell proliferation.
It has been reported that p18 and p27 are down-regulated in islets in which the Men1 gene had been lost for a prolonged period (10, 11). Gene profiling analysis on islets from mice with floxed Men1;Rip-Cre shows that p18, but not p27, is down-regulated in pancreatic islets 15 wk after Men1 excision (12). Although both Scacheri et al. and our group found that cell cycle regulator Cdc20 was up-regulated in Men1-excised islets (12) (SI Appendix, Table 1), we did not observe a reduction in p18 or p27 mRNA levels in islets 14 d after Men1 excision. Different Cre systems (Ubc9-Cre-ER vs. Rip-Cre) used for the studies and/or different times when Men1 was excised (14 d vs. 15 wk) could partly account for the discrepancy (12). We previously found that mRNA levels of p18 and p27 were reduced in total pancreatic tissues from Men1l/l; Ubc9-Cre-ER mice that were fed TAM (31). Thus, we cannot rule out the possibility that the expression of p18 and p27 is reduced in β cells a long period after Men1 excision and that Men1 excision influences expression of p18 and p27 in nonendocrine cells in the pancreas.
We found that Men1 excision rapidly ameliorated hyperglycemia within 3 wk after Men1 excision. Moreover, Men1 expression has been reported to be physiologically suppressed in islets during and after pregnancy in mice by the prolactin signaling pathway to prevent gestational diabetes (22). These findings suggest that menin inhibition might be a valuable means of promoting β-cell proliferation and treating diabetes. However, great caution must be exercised because β-cell hyperplasia, insulinomas, and hyperinsulinemia have been detected in Men1 knockout mouse models and in MEN1 patients (5, 6, 8, 31). This finding raises a substantial concern that permanent menin inhibition as a therapeutic approach may eventually lead to insulinomas (6, 32). If the aforementioned situation is the case, targeting menin itself as a means to treat diabetes would not be viable.
However, because menin has multiple functions including regulation of β-cell proliferation and DNA repair (33), it might be possible to modulate the function of menin in regulating β-cell proliferation, yet retain its other functions, such as DNA repair, thus reducing the possibility of tumor formation. In this regard, menin has been reported to repress gene expression through interaction with repressive histone deacetylases (HDACs) (34) and inhibit cell proliferation (35). It is possible that menin represses expression of proproliferative genes, such as Ccna2 and Pbk, through repressive histone modifications via HDACs or perhaps other hitherto unidentified histone modifiers. Therefore, targeting or repressing interaction between menin and repressive histone modifiers could lead to increased expression of proproliferative genes and β-cell proliferation. These approaches could reduce the concern over directly targeting menin and thereby shedding light on improving diabetes therapy.
Materials and Methods
Mice.
Men1l/l;Cre-ER mice were generated by crossing floxed Men1 mice (Men1l/l, FVB/129Sv, from Francis Collins) to Cre-ERT2 mice (129Sv/C57BL6J) (6, 15). db/db; Men1l/l;Cre-ER mice were generated by crossing Men1l/l;Cre-ER mice to db/+ mice (BKS.Cg-Dock7m+/+Leprdb/J; The Jackson Laboratory) (17). Men1l/l;Pdx1-Cre-ER mice were generated by crossing floxed Men1 mice to Pdx1-Cre-ER mice (23). Only male mice were used for the following experiments. Genotyping of mice was performed by PCR on mouse tail DNA. All mouse studies were approved by the University Laboratory Animal Resource and the University of Pennsylvania committee on animal care; the animal care was in accordance with institutional guidelines.
Excision of the Floxed Men1 Locus.
Men1l/l;Cre-ER, db/db;Men1l/l;Cre-ER, Men1l/l;Pdx1-Cre-ER mice and their littermate controls were fed TAM (MP Biomedicals) at 200 mg/kg of body weight per day for two consecutive days, followed by 1 d off and then for another two consecutive days (31).
STZ-Induced Hyperglycemia.
Multiple low doses of STZ (Sigma) were injected (i.p.) at 40 mg/kg of body weight per day for five consecutive days (36). Mice were diagnosed as diabetic when their nonfasting blood glucose levels were >250 mg/dL for two consecutive weeks.
High-Fat Diet Feeding.
C57BL6J, control Men1l/l, and Men1l/l;Pdx1-Cre-ER mice at the age of 6 wk were fed either high-fat diet (51 kcal% fat; Research Diets) or chow diet (Harlan) for 13–16 wk.
Physiological Measurements.
Blood glucose levels were assayed from tail vein blood. Serum insulin levels were measured by ELISA using a mouse insulin kit (Crystal Chem). Glucose tolerance test (GTT; glucose at 2 g/kg of body weight, i.p.) and glucose-stimulated insulin secretion (GSIS; glucose at 3 g/kg of body weight, i.p.) were performed on mice fasting overnight. Insulin tolerance test (ITT, insulin at 0.75 U/kg of body weight, i.p.) was performed on nonfasting mice.
Methods on islets perifusion, immunohistochemistry, cDNA microarray analysis, qRT-PCR, Gene Ontology, GSEA, and Western blotting were described in SI Appendix.
Statistical Analysis.
Results are expressed as mean ± SEM. For two-group comparison, unpaired Student's t test or rank sum test was used. For multiple-group comparison, one-way ANOVA or ANOVA on ranks was used. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Jizhou Yan and Wei Qin for technical assistance and Drs. Gary Koretzky, Steven Reiner, Mitchell A. Lazar, Morris Birnbaum, and Klaus Kaestner for stimulating discussions. This work was supported in part by National Institutes of Health Grants R01-CA-113962, R56-DK08512, and R01-DK085121 (to X.H.), American Diabetes Association Grant 7-07-RA-60 (to X.H.), and National Institutes of Health Grant R01 DK068157 (to D.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012257107/-/DCSupplemental.
References
- 1.Butler AE, et al. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic β-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 3.Uchida T, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med. 2005;11:175–182. doi: 10.1038/nm1187. [DOI] [PubMed] [Google Scholar]
- 4.Fu A, et al. Loss of Lkb1 in adult β cells increases β cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree JS, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23:6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scacheri PC, et al. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mamm Genome. 2004;15:872–877. doi: 10.1007/s00335-004-2395-z. [DOI] [PubMed] [Google Scholar]
- 8.Bertolino P, et al. Pancreatic β-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–4841. [PubMed] [Google Scholar]
- 9.Biondi CA, et al. Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol Cell Biol. 2004;24:3125–3131. doi: 10.1128/MCB.24.8.3125-3131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karnik SK, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci USA. 2005;102:14659–14664. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milne TA, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci USA. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scacheri PC, et al. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis. PLoS Genet. 2006;2:e51. doi: 10.1371/journal.pgen.0020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes CM, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama A, et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 16.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 18.Coleman DL, Hummel KP. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia. 1974;10(Suppl):607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, et al. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 20.Biddinger SB, et al. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 21.Winzell MS, Ahrén B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 22.Karnik SK, et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 23.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 24.Dennis G, Jr., et al. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 25.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bild AH, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 27.Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: Unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gaudet S, Branton D, Lue RA. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci USA. 2000;97:5167–5172. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg AR, et al. Overexpression of human cyclin A advances entry into S phase. Oncogene. 1995;10:1501–1509. [PubMed] [Google Scholar]
- 30.Lu J, et al. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138:1954–1965. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 31.Schnepp RW, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res. 2006;66:5707–5715. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harding B, et al. Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr Relat Cancer. 2009;16:1313–1327. doi: 10.1677/ERC-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busygina V, Kottemann MC, Scott KL, Plon SE, Bale AE. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–8403. doi: 10.1158/0008-5472.CAN-06-0061. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63:6135–6139. [PubMed] [Google Scholar]
- 35.Wu T, Zhang X, Huang X, Yang Y, Hua X. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. J Biol Chem. 2010;285:18291–18300. doi: 10.1074/jbc.M110.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liadis N, et al. Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25:3620–3629. doi: 10.1128/MCB.25.9.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.