Abstract
On the morning of July 16, 1945, the first atomic bomb was exploded in New Mexico on the White Sands Proving Ground. The device was a plutonium implosion device similar to the device that destroyed Nagasaki, Japan, on August 9 of that same year. Recently, with the enactment of US public law 111-140, the “Nuclear Forensics and Attribution Act,” scientists in the government and academia have been able, in earnest, to consider what type of forensic-style information may be obtained after a nuclear detonation. To conduct a robust attribution process for an exploded device placed by a nonstate actor, forensic analysis must yield information about not only the nuclear material in the device but about other materials that went into its construction. We have performed an investigation of glassed ground debris from the first nuclear test showing correlations among multiple analytical techniques. Surprisingly, there is strong evidence, obtainable only through microanalysis, that secondary materials used in the device can be identified and positively associated with the nuclear material.
Keywords: secondary ion mass spectrometry, autoradiography, electron probe microanalysis, plutonium, trinitite
“What will happen when the explosions come—when a part of New York or Cairo or Adelaide has been hollowed out by a device in the kiloton range? Since even a so called fizzle yield could kill a number of thousands of people, how many nuclear detonations can the world tolerate?”
—John McPhee, The Curve of Binding Energy (Farrar, Straus and Giroux, New York, 1974).
The critical role of nuclear forensics has been detailed and brought to the attention of the public and scientific communities in recent reports issued by the National Research Council (1) and jointly by the American Association for the Advancement of Science and the American Physical Society (2). Nuclear forensic analysis will be the only route to attribution when a device, delivered not by a military attack from an established nation, but by a transnational group, destroys part of a city.
Trinitite, the glassed ground debris from the first nuclear test [the Trinity test (3)], can be readily obtained from mineral collectors and is an easy starting point to explore the forensic information that can be obtained from this type of material. There have been numerous studies of nuclear bomb debris since those fateful days of 1945; however, none have ever been concerned with forensic-style questions that may lead to information for attribution. There are few publications on Trinitite, more on material from Hiroshima and Nagasaki, but nearly all have been concerned with residual radioactivity, or, in the case of Trinitite, radiological studies of the substance itself. In 1948, Ross published his petrographic observations of the glass (4). His was the only publication to report on anything other than the radiological properties of the material. Considerations of heterogeneity were unimportant to radiological investigators, and, for the most part, Trinitite and other material were effectively viewed as homogeneous objects. Although speculation exists that Trinitite was produced by the ingestion of soil into the blast–fireball that subsequently rained down on the desert floor, no published studies exist that attempt to verify or discredit this or any other hypotheses on the formation of Trinitite.
Considerable effort was expended by scientists during the Cold War and the period of buildup of the nuclear arsenals of the world to characterize products from the explosions, debris, and fallout for the purposes of checking the yield or efficiency of a device or for detecting undeclared tests by other nations. No one went back to his or her test site and asked the question, “Had we not known what had happened here, what could we tell about the device that produced this event?”
The device exploded during the Trinity test (the gadget) is known to have been a plutonium implosion device (3). The mass of the fissile material was approximately 6 kg. Nearly all of the Pu was in the form of 239Pu. A critical property of the Pu composition is that the amount of 240Pu be low enough so as not to produce preignition of the fission reaction and cause the bomb to “fizzle,” having a reduced yield. Parekh et al. (5) measured the 240Pu/239Pu value to be 1.28% mole/mole, which is slightly higher but consistent with what is suspected about Pu that came from the reactors at Hanford at the time and well below the 7% limit (6) of the 240Pu concentration considered to be weapons-grade material.
The Pu core was surrounded by a tamper fashioned of tubealloy, which is uranium metal of natural isotopic composition. The explosives used were “composition B,” made of 59.5% RDX (1,3,5-trinitroperhydro-1,3,5-triazine), 39.5% TNT (2-methyl-1,3,5-trinitrobenzene), with 1% paraffin wax as a binder, and Baratrol, which consisted of 25% to 33% TNT, barium nitrate, and 1% paraffin. Other components such as aluminum shells and framing were used, and the “gadget” was exploded atop a 30-m-tall steel tower. These are all materials that could possibly contribute to forensic evidence and provide information about the design.
Trinitite is expected to have incorporated components from the local natural materials. Geologically the site is in the northern Tularosa Basin, composed of alluvial, aeolian, and volcanic materials dominantly related to past marine and mountain-building episodes and subsequent erosion, leaching, and alteration. Minerals present in the local rocks and sediments include arkosic sand, quartz, feldspars (partially sericitized microcline and albite plagioclase), carbonates (e.g., calcite, trona, aragonite, and dolomite), sulfates (e.g., gypsum, selenite, bassanite, mirabilite, hexahydrite, thenardite, bloedite, and anhydrite), chlorides (halite, sylvite, and bischofite), hornblende, olivine, and clays (kaolinite and illite), magnetite, ilmenite, augite, and both meteoritic and volcanic dust (4, 7–9). Although the general area southward at the White Sands National Monument is known for its gypsum dunes, these tend to grade into higher quartz content northward (7). Likewise the chloride content increases northward. As will be seen in the results, some of the calcium- and silicon-bearing minerals are of special interest.
The incorporation of natural materials such as local sediments into bomb debris complicates the interpretation of bomb-specific clues and is therefore important to consider. An example is barite, which, if present, confounds the forensic analyses of barium because of possible mixing with Ba(NO3)2 used in the explosive lens (10) and possible residual  . Bulk analytical methods are unable to adequately tease apart populations of end-member components derived from distinct starting materials, whereas microscopic methods can detect lower levels of materials due to localized microconcentrations and spatial relationships. This additional information that microscale methods can provide on discrete source materials thereby multiplies evidential information.
. Bulk analytical methods are unable to adequately tease apart populations of end-member components derived from distinct starting materials, whereas microscopic methods can detect lower levels of materials due to localized microconcentrations and spatial relationships. This additional information that microscale methods can provide on discrete source materials thereby multiplies evidential information.
If a nonstate actor were to produce an improvised, one-of-a-kind nuclear weapon and detonate it in a population center, would we be able to glean information from the debris and differentiate it from other debris that would be produced from the interaction of the blast with the surrounding urban material?
Here we present results of the study of a single piece of Trinitite, purchased from a mineral collector. The button-shaped, oblate piece had one lustrous convex glassed side that was green in color. It weighed approximately 7.5 g and was about 3 cm in diameter and 0.5 cm thick. It was littered with vesicles of sizes extending to the thickness of the sample. A polished thin section was made along a plane parallel to the short axis of the sample. Measurements were made of the alpha particle energy spectra on the thin section and gamma-ray energy spectra were acquired from a remaining piece weighing about 7 g. The thin section was coated with carbon to reduce charging prior to electron microprobe (e-probe) and scanning electron microscope (SEM) analyses. Light microscopy (LM) was used to characterize the minerals and glasses in the section, and the entire thin section was mapped via microfocusing X-ray florescence (XRF). An autoradiograph was made including alpha and beta radioactivity discrimination to map the location of radiation sources in the thin section. Secondary ion mass spectrometry (SIMS) was performed to determine trace element concentrations and isotopic abundances.
Results
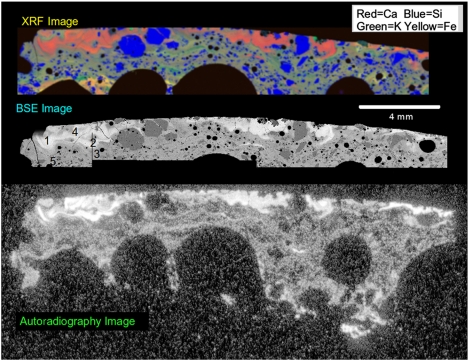
The analyses of the Trinitite thin section were performed with instruments that are commonly (except for autoradiography) employed for geochemical measurements. The first measurements were made in the SEM followed by the e-probe. Although Ross (4) had described in his petrographic observations that it appeared the glass near the surface had undergone flow, it was surprising to see the clear compositional differences in backscattered electron images (BSE) and in X-ray images that revealed the large-scale flow reaching 2 mm to 3 mm in from the surface (Fig. 1).
Fig. 1.
Images of the Trinitite thin section showing major elements from XRF, the backscattered electron signal representing the average atomic number of the matter and the autoradiograph showing the position of alpha decays in the sample. This figure is approximately 20 mm across. Numbers on the BSE image indicate the regions where quantitative analysis was performed. Region 6 in Table 1 is outside the region shown. The areas were chosen to provide a representative set of compositions given in Table 1.
Upon discovery of the compositional heterogeneity of the Trinitite, an autoradiograph was made, additional e-probe X-ray data and images were collected, and an X-ray compositional map was acquired of the entire thin section by XRF. Data from each of these methods are displayed in Fig. 1. The autoradiograph, shown at the bottom of Fig. 1, largely shows the location of alpha decays in the thin section. There is also a measurable background of 40K decay that is common in many materials, including glass slides such as the one used for the thin section mount. The intense alpha activity at the top surface of the Trinitite is associated with high Ca concentrations that form the swirling shapes reminiscent of turbulent eddies. Silicon-rich areas near the surface can be seen embedded and in direct contact with the Ca-rich eddies. These are sand grains that were heated above their glass transition temperatures but not long enough to significantly lose their original shapes, several of them showing residual euhedral features. Observations by LM also reveal these eddied areas of the glass by a difference in refractive index produced by compositional differences frozen into the glass before it had reached equilibrium (Fig. 2). Table 1 gives representative X-ray compositional data of the selected areas indicated by numbers in Fig. 1.
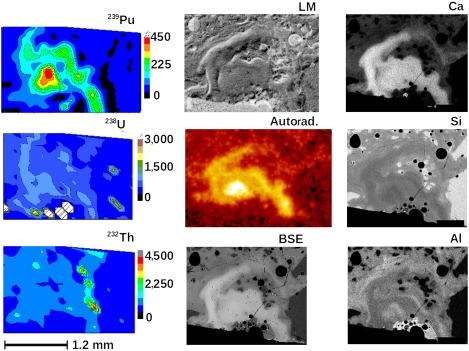
Fig. 2.
Images from each method employed to analyze the Trinitite thin section. The three images on the left of 239Pu, 238U, and 232Th were acquired by SIMS. The images shown in the center column are from LM, showing differences in index of refraction, autoradiography, showing mainly the α-activity, and the BSE image from the e-probe. The images on the right are X-ray images from the e-probe. Each image was cropped and scaled to show approximately the same 2-mm region of the section as closely as possible.
Table 1.
X-ray microanalyses of glass at selected locations
| No. | O (stoich) | Na | Mg | Al | Si | K | Ca | Ti | Fe |
| 1 | 44.0 | 0.77 ± 0.01 | 0.70 ± 0.01 | 8.45 ± 0.01 | 24.0 ± 0.02 | 1.23 ± 0.01 | 18.8 ± 0.02 | 0.29 ± 0.01 | 1.78 ± 0.01 |
| 2 | 45.1 | 0.75 ± 0.01 | 0.86 ± 0.01 | 7.96 ± 0.01 | 26.2 ± 0.02 | 1.10 ± 0.01 | 15.6 ± 0.02 | 0.33 ± 0.01 | 2.13 ± 0.02 |
| 3 | 46.4 | 0.99 ± 0.01 | 0.66 ± 0.01 | 5.97 ± 0.01 | 30.3 ± 0.02 | 1.77 ± 0.01 | 11.8 ± 0.02 | 0.26 ± 0.01 | 1.87 ± 0.01 |
| 4 | 44.4 | 0.71 ± 0.01 | 0.96 ± 0.01 | 9.73 ± 0.01 | 23.7 ± 0.02 | 0.72 ± 0.01 | 17.0 ± 0.02 | 0.30 ± 0.01 | 2.46 ± 0.02 |
| 5 | 46.3 | 1.27 ± 0.01 | 0.97 ± 0.01 | 6.55 ± 0.01 | 29.8 ± 0.02 | 2.30 ± 0.01 | 10.1 ± 0.02 | 0.35 ± 0.01 | 2.47 ± 0.02 |
| 6 | 53.2 | N.D. | < 0.02 | < 0.05 | 46.67 ± 0.02 | ND | < 0.01 | < 0.01 | < 0.01 |
All values are in weight percentage; oxygen calculated by assumed stoichiometry; integrated spectrum 0.1 keV to 20 keV: ∼5 × 106 counts. The measurement statistic reported is 1σ based upon the intensity ratio of unknown/standard. ND, not determined.
LM micrographs reveal all sizes of vesicles (submicrometer to more than 5 mm), some in the glass clinging to partially melted silicate grains. In addition, regions of eddied glass near the surface show distinctive differences in refractive index corresponding to the regions of high Ca concentration. In Fig. 2, this can be seen in the panel labeled LM. The LM method used, differential interference contrast, reveals optical path differences that are caused by refractive index variations in the glass related to the compositional differences (4) as can be seen from the backscattered electron image of the same area (BSE panel in Fig. 2).
The larger portion of the sample after sectioning, about 7 g, was assayed for 75 h with a low-background Ge gamma-ray spectrometer (relative efficiency 37%, resolution 1.70 keV FWHM at 1,333 keV) (11) Numerous gamma rays were detected above background (Table 2). Because of the irregular sample geometry, the large uncertainty in counting efficiency determination and self-absorption corrections permitted only semiquantitative analysis of the gamma spectrum; the apparent activity in Table 2 is likely to be an underestimate. The presence of more than 40 Bq of fission product 137Cs as the most conspicuous peak in the spectrum is unquestionable evidence of the event. One characteristic peak of 239Pu was detected, and 241Am is surely present. Several long-lived neutron capture products (60Co, 133Ba, and 152,154Eu) were identified by multiple gamma rays, along with decay products of thorium and radon. The results of alpha spectrometry (Table 3) show the highest source of activity as 239Pu, leading to the identification of the intense areas on the autoradiograph as being indicative of the presence of 239Pu in the eddied Ca-rich areas identified from XRF imaging (Fig. 1).
Table 2.
Gamma-ray counting (background subtracted)
| Nuclide | Origin | Half-life, a | Gamma energies, keV | Apparent activity, Bq | Counting precision, 1σ |
| 40K | Natural | 1.25 × 109 | 1,461 | 2.5 | 3% |
| 60Co | Activation | 5.3 | 1,173, 1,333 | 0.03 | 15% |
| 133Ba | Activation | 10.5 | 81, 303, 356, 384 | 0.9 | 2% |
| 137Cs | Fission | 30.2 | 662 | 40 | 0.1% |
| 152Eu | Activation | 13.2 | (122), 245, 344, 779, 964, 1,086, 1,112, 1,408 | 5.5 | 1% |
| 154Eu* | Activation | 8.6 | (123), 723, 873, 996, 1,005, 1,275 | 0.16 | 10% |
| 208Tl | Th daughter | 1.4 × 1010 | 583, 2615 | 0.02 | 10% |
| 212Pb | Th daughter | 1.4 × 1010 | 238 | 0.12 | 9% |
| 214Pb | U-Ra daughter | 1.6 × 103 | 295, 352 | 0.18 | 15% |
| 228Ac | Th daughter | 1.4 × 1010 | 339, 911, 969 | 0.08 | 15% |
| 239Pu | Transuranic | 2.4 × 104 | 129 | 1000 | 8% |
| 241Am | Pu daughter | 432 | 59 | 13 | 1% |
Overlapping low-energy lines of 152Eu and 154Eu were excluded from the activity calculation.
Table 3.
Alpha-spectrometry results of Trinitite thin section
| Nuclide | Energy, MeV | Counts, 64 h | Activity, Bq | Combined uncertainty, 1σ |
| 239+240Pu | 5.15, 5.16 | 930 | .012 | 21% |
| 241Am + 238Pu | 5.49, 5.50 | 64 | .0008 | 24% |
| 237Np + 234U + 235U + 236U | 4.4 to 4.8* | 45 | .0006 | 100% |
| 233U + 229Th + 242Pu | 4.82 to 4.90* | 50 | .0006 | 100% |
*Within low-energy absorption tail of main peak, which considerably elevates the Type B uncertainty for these radionuclides.
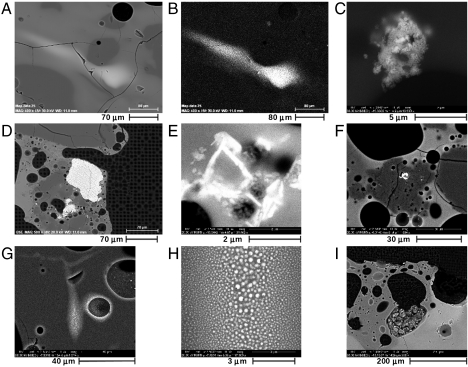
Unusual small structures were discovered throughout the section that appear to be the result of nonequilibrium processes (Fig. 3). Zr-rich areas that form wisps appear to have been once molten and carried with the flow of the molten surface (Fig. 3 A and B). Another Zr-rich region on the wall of a vesicle exhibits dendritic morphology (Fig. 3C). An inclusion of what appears to be titaniferous magnetite can be found far from the top surface (Fig. 3D). Ti-rich laths appear near a vesicle far from the surface (Fig. 3E). Ba-rich inclusions exist in at least two places in the section (Fig. 3F). A region of Fe-rich spheres with sizes ranging down to approximately 3 nm can be found near a vesicle relatively deeply placed below the surface (Fig. 3 G and H). The structure of this feature of Fe-rich spheres is indicative of insolubility of the Fe in the surrounding melt matrix during formation. Iron-rich spheres also decorate the interior of a nearby vesicle that touches the Fe-rich-sphere region in the melt. Some vesicles near the surface are filled with unmelted particles (Fig. 3I). Some of these vesicles are completely closed. Information that may assist in attribution through further study of inclusions such as these is best obtained by microanalysis and would be easily missed with bulk analysis, alone.
Fig. 3.
Images acquired with the SEM and e-probe. Descriptions of each image are given in the text. Each of the images is (A) BSE, (B) Zr X-ray, (C) BSE, (D) BSE, (E) BSE, (F) BSE, (G) BSE, (H) BSE, and (I) BSE.
SIMS was used to explore the isotopic compositions and trace element abundances of several of the prominent features observed by other methods in the section. One of the most striking correlations is shown in Fig. 2, where the abundance of 239Pu is clearly correlated with a portion of the high intensity region in the autoradiograph that also correlates with the Ca concentration and refractive index variations in the material. The Pu concentration is estimated to be present in the eddied regions near the surface at approximately 200 ng/g to 400 ng/g and U appears in this sample, judging from the SIMS signals, at the level of approximately five times that of the Pu signal. These estimates were based on comparison of the SIMS signals of Pu and U to Ca and the average Ca concentration computed from the e-probe measurements. The uncertainty in this estimate is large (perhaps a factor of 2) because the concentration of each of these elements varies considerably and there are no standards containing known amounts of Pu and U with a major element composition similar to the glass. The isotopic composition of U, Pu, and Pb measured in single spots by SIMS in the most Pu-rich region is given in Tables 4 and 5. The Pu isotopic composition shows that 240Pu/239Pu is approximately 0.016 mole/mole. This is consistent with previous measurements (10) and below the 7% limit considered weapons grade. Fig. 2 also shows that the 239Pu signal is correlated with the 238U signal except for some particles near the surface and small regions adjacent to or in a neighboring silicate grain. A hydride correction was made for the 239Pu by measuring the 235UH signal and was approximately 10-3 of the U signal. Thorium is present in the Pu- and U-rich regions (as detected by SIMS) but is present at higher concentrations outside of the Pu-rich region. Additionally, the Pb isotopic composition, measured by SIMS in individual spots in the material, differs between regions (Table 5). The Pb isotopic composition in the Pu-rich region has a radiogenic signature, as would be expected if the tubealloy or natural uranium tamper were chemically refined to U metal from ore and then made into the tamper for the gadget with, perhaps, some mixing with common Pb. The Pb isotopic composition far from the Pu-rich surface region is distinctly that of common Pb (12).
Table 4.
Uranium and plutonium isotopic ratios by SIMS and typical natural uranium isotopic ratios
|
234U/238U |
235U/238U |
240Pu/239Pu |
||||
| 〈x〉 | 1σ | 〈x〉 | 1σ | 〈x〉 | 1σ | |
| Trinitite | 0.000074 | 0.000013 | 0.00721 | 0.00015 | 0.0176 | 0.0006 |
| Typical natural | 0.000055 | 0.00725 | ||||
Table 5.
Lead isotopic ratios in Trinitite and “common” lead ratios for reference
|
204Pb/208Pb |
206Pb/208Pb |
207Pb/208Pb |
||||
| 〈x〉 | 1σ | 〈x〉 | 1σ | 〈x〉 | 1σ | |
| Pu-rich region | 0.01 | 0.01 | 0.62 | 0.11 | 0.72 | 0.13 |
| Glass far from surface | 0.026 | 0.005 | 0.47 | 0.03 | 0.44 | 0.03 |
| Common Pb | 0.027 | 0.460 | 0.422 | |||
Conclusions
The most significant feature of the SIMS data in combination with the autoradiography and e-probe data is the correlation of the U abundance with the Pu abundance in the vitrified Ca-rich regions that have the most intense alpha activity. This is a definitive demonstration that at least the tamper material is associated with the nuclear material, in this case Pu, and can be identified as coming from a part of the nuclear weapon. The difference in Pb isotopic abundances is also a key indicator that the U in the Pu-Ca-rich region is from the tamper and not from environmental U-bearing minerals that will also contain Th, likely in higher abundance than U. Geographically different isotopic compositions, which are well known, in elements such as U and Pb can be used to indicate potential sources of material. If a tamper were made of Pb in an improvised device, instead of U, its isotopic composition would be part of the information that could lead to attribution.
The visible heterogeneity of the sample near the surface indicates that the material was not completely mixed, forming a homogeneous glass, but rather specific signatures have been preserved in the heterogeneity of the glassed material. This is a promising observation for those who may attempt to deconvolve bomb material from debris and hope to attribute the construction of a weapon to a particular organization. Bulk analysis of this piece of Trintite, if it were treated as a homogeneous object, would reveal the presence of Pu, U, and Pb but would not produce the compelling evidence that spatial correlation between the Pu and U does. In addition, performing only elemental analysis, without isotopic signatures, would not reveal the differing isotopic compositions of Pb between the Pu-bearing region and the subsurface material. It is only through the information obtained by microanalytical methods, spatially resolved radiological, elemental, and isotopic compositions, that a convincing association can be made.
There are a plethora of features in the unequilibrated glass from the Trinity test. Some, as yet unexplored features, may hold information about the construction of the device that can be obtained only through further spatially resolved microanalytical methods. It is clear that components from the device, especially in an inefficient one such as the gadget or a terrorist’s likely nuclear weapon, will produce identifiable signatures surrounding the blast that should be readily associated with the nuclear material driving the explosion.
Discussion
It is a matter of debate as to whether a nonstate actor, intent on producing an improvised nuclear weapon that may be delivered in a shipping container from aboard a container ship and hauled into New York, Washington, London, or Tel Aviv, will choose Pu or U as the fissile material. It is likely that a nonstate actor will not have a choice and will use whatever is available, what can be purchased on the black market, or stolen, or donated by a state wishing to sponsor terrorists. Certainly the composition of the nuclear fuel will provide some clues as to the origin of the device. However, the construction will rely on locally obtained materials. For example, a tamper made of lead, rather than uranium, would work almost as well. Ted Taylor, the prominent nuclear weapons designer (The Curve of Binding Energy is largely an account of Taylor’s ideas) suggests an aluminum tamper would work (13). However, these associations cannot be confidently made through bulk analysis of the debris, but by microanalysis only. The results presented here show that it is possible to glean some information about the materials that went into constructing the weapon. Investigators, however, must look in the right place, and with the right set of tools. In doing so, it may be possible to trace the inferred composition of the components to their origin.
Only further microanalytical investigation of not only Trinitite but the ground debris from nuclear test sites in the United States and around the world will teach us more about the forensic information that can be coaxed from surrounding debris. Blast debris from the only places where a nuclear weapon was detonated in a population center, Hiroshima and Nagasaki, will likely yield key information as to which types of materials will provide the most useful information about the composition of the device and the materials that went into it. Performing these types of studies may be the only route to attribution “…when the explosions come….”
Methods
The data for this multidisciplinary study were obtained largely with commercial instruments some of which are common. The analysis methods used for each technique are generally well known. The autoradiographs were acquired with a Fuji BAS-5000* scanner with the thin section placed directly on a Model TR2025 imaging plate. Multiple exposures were made over periods that varied from hours to several days. Gamma-ray spectra were acquired with a well-characterized and shielded Ge detector (11) over approximately 3 d on an approximately 7-g piece of Trinitite. Alpha spectrometry was performed on the thin section with a Canberra Alpha Analyst, PIPS series A. Two different field-emission electron-beam instruments were employed for this study. An FEI Quanta 200F equipped with a silicon drift detector (SDD) was used during the initial survey of the thin section. Later a JEOL 8500F with a Bruker AXS SDD detector was utilized to survey the entire thin section and produce compositional X-ray images. An EDAX Eagle-III microfocusing XRF surveyed the section in an attempt to find signatures of the actinides anywhere in the sample. An Olympus AX70 microscope and a Leica Confocal microscope were used to perform light microscopy of the thin section. Isotopic and trace element abundances were determined with the Cameca ims-1280 secondary ion mass spectrometer at National Institute of Standards and Technology. Positive secondary ions were collected from the sample that was sputtered with O- primary ions at an impact energy of 23 keV. Numerous U-bearing materials of known isotopic composition were used as working standards to check the veracity of the hydride correction made for the Pu isotopic measurements.
Acknowledgments.
This work has been approved through the National Institute of Standards and Technology Washington Editorial Review Board, ERB Control Number: G2010-1126.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Disclaimer: Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.National Research Council of the National Academies. 2010. Committee on Nuclear Forensics, Nuclear Forensics, A capability at Risk, ISBN: 0-309-15875-3 ( http://www.nap.edu)
- 2.Joint Working Group of the American Physical Society and the American Association for the Advancement of Science. Nuclear Forensics: Role, State of the Art, and Program Needs. 2010. ISBN: 978-0-87168-720-3 ( http://cstsp.aaas.org/files/Complete.pdf)
- 3.Los Alamos Historical Document Retrieval and Assessment Project. Chapter 10: The Trinity Test http://www.lahdra.org/pubs/pubs.htm.
- 4.Ross CS. Optical properties of glass from Alamogordo, New Mexico. Am Mineral. 1948;33:360–362. [Google Scholar]
- 5.Parekh PP, et al. Radioactivity in Trinitite six decades later. J Environ Radioactiv. 2006;85:103–120. doi: 10.1016/j.jenvrad.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Bragin V, et al. Proliferation-resistance and safeguardability of innovative nuclear fuel cycles. IAEA-SM-367/15/07.
- 7.Hoidale GB, Smith SM, Blanco AJ, Barber TL. A study of atmospheric dust. Atmospheric Sciences Laboratory, White Sands Misisle Range, New Mexico, United States Army Electronics Command. 1967;ECOM-5067:1–140. [Google Scholar]
- 8.Eby N. Trinitite—the atomic rock. Geol Soc Am Abstracts with Programs. 2010;42:77. [Google Scholar]
- 9.Love DW, Allen BD, Myers RG. Gypsum crystal morphologies and diverse accumulations of gypsum and other evaporites in the Tularosa basin. New Mexico Geology. 2008;30:120–121. [Google Scholar]
- 10.Schlauf D, et al. David Atkatz, Christopher Bragg., editors. Trinitite redux: Comment on “Determining the yield of the Trinity nuclear device via gamma-ray spectroscopy.” [Am. J. Phys. 63, 411–413 (1995)] Am J Phys. 1997;65:1110–1112. [Google Scholar]
- 11.Lindstrom RM, Lindstrom DJ, Slaback LA, Langland JK. A low-background gamma ray assay laboratory for activation analysis. Nucl Instrum Methods. 1990;A299:425–429. [Google Scholar]
- 12.Fahey AJ, Ritchie NWM, Newbury DE, Small JA. The use of lead isotopic abundances in trace uranium samples for nuclear forensics analysis. J Radioanal Nucl Ch. 2010;284(3):575–581. [Google Scholar]
- 13.McPhee J. The Curve of Binding Energy. New York: Farrar, Straus and Giroux; 1974. [Google Scholar]