Abstract
Sister chromatids are held together, from the time they are made during S phase until they are pulled apart just before cell division, by a protein complex called cohesin. The mechanistic details by which sister chromatid cohesion is established and maintained have remained elusive, particularly in vertebrate systems. Sororin, a protein that interacts with the cohesin complex, is essential for cohesion in vertebrates, but how it participates in the process is unknown. Here we demonstrate that sororin recruitment depends on active DNA replication and that sororin loading onto chromosomes depends upon another essential cohesion factor, the acetyltransferase Eco2. We find that Eco2, like sororin, is a substrate of the anaphase-promoting complex (APC), which ensures that protein levels remain low before S phase. These findings demonstrate that sororin and Eco2 work together to form a unique regulatory module that limits cohesion to cells with replicated chromatin and support a model in which cohesion in vertebrates is not fully established until the G2 phase of the cell cycle.
Keywords: anaphase-promoting complex, cohesion establishment, acetylation
The events that result in sister chromatid cohesion are conceptually divisible into three discrete steps: cohesin loading, cohesion establishment, and cohesion maintenance.
Cohesion establishment, thought of as the conversion of DNA-bound cohesin complexes to a state in which they hold two sister chromatids together, is poorly understood at the molecular level. Several lines of evidence suggest that establishment occurs during DNA replication (1). An emerging model is that cohesin loads on the chromatin before replication (2, 3) and the Smc3 subunit of the complex is then modified by members of the Eco family of acetyltransferase proteins (Eco1/Ctf7 in yeast) (4–7). Eco-dependent acetylation makes cohesin refractory to the activity of the Wapl protein, which destabilizes cohesin/chromatin interactions in G2 (5, 8). Eco1/Ctf7 interacts directly with replication fork components, perhaps explaining how cohesion establishment is integrated with DNA replication (9–11). In the absence of both Eco1 and Wapl/Rad61, cohesion is still established (5). This suggests that cohesion establishment is in fact a default state that in the absence of Wapl/Rad61 need not be integrated with DNA replication by Eco1.
It is not clear whether cohesion maintenance is an active process, or simply the result of stable associations that develop during DNA replication. In support of the latter hypothesis, in vertebrates a pool of cohesin that associates stably with the chromatin develops during DNA replication and persists into G2 (12). It has been proposed that this stable pool of cohesin represents complexes that are actively engaged in cohesion. Just what this stability represents at a molecular level is not known. To date, the only factors exclusively involved in maintenance have their most obvious impact in metaphase where they influence the ability to resist removal of cohesin from the chromosomes in mitosis (13–15).
In a screen for cell cycle-regulated proteins, we identified a protein we named sororin and showed that this protein interacts with the cohesin complex and is required for cohesion (16). Sororin is a substrate of the anaphase-promoting complex (APC), a ubiquitin ligase that controls the degradation of a number of cell cycle regulators. Thus, sororin is degraded as cells exit mitosis and begins to accumulate during S phase. Sororin is required for development of the stable pool of cohesin in G2 cells (17) and thus is involved in either cohesion establishment or maintenance. To date there is no clear structural or functional ortholog of sororin outside of the chordates.
To better understand how sororin controls cohesion, we have used the Xenopus egg extract system to analyze how sororin recruitment is regulated by DNA replication, cell cycle progression, and the presence of other cohesion factors. Here we show that, unlike the cohesin complex, sororin association with the chromatin requires DNA replication. We also show that the sororin-binding site accumulates during DNA replication and requires the presence of the cohesin complex. Sororin added to extracts after replication is complete is still able to bind chromatin, indicating that it recognizes a site that persists in the G2 phase of the cell cycle. Recruitment of sororin requires the Eco2 acetyltransferase, which we demonstrate also to be regulated by cell cycle-dependent ubiquitylation. Finally, we show that Eco2 must be present during DNA replication to allow sororin recruitment. We propose that sororin and Eco2 cooperate to ensure that cohesion only occurs in cells with replicated chromatin.
Results
Sororin Recruitment to Chromatin Requires the Cohesin Complex.
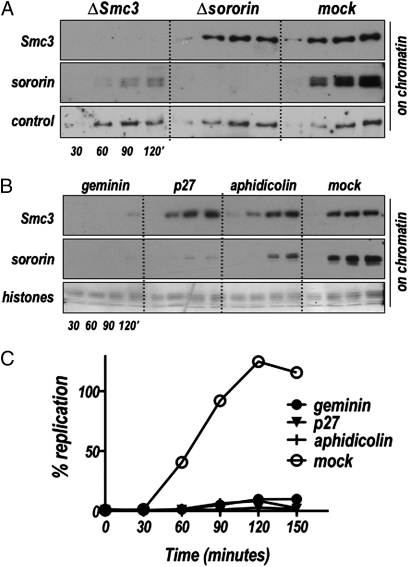
Demembranated sperm nuclei added to interphase Xenopus egg extract decondense, assemble a nuclear envelope and chromatin, undergo one round of DNA replication, and establish sister chromatid cohesion all through recruitment of factors from the extract. In previous work we showed that sororin overexpression increases the amount of cohesin associated with mitotic chromosomes assembled in egg extracts (18). This suggested a model in which sororin is required to recruit cohesin to chromatin. To test this hypothesis directly, we immunodepleted sororin from egg extracts and then assessed levels of cohesin associated with chromatin over the course of nuclear assembly and DNA replication. Sperm nuclei were added to sororin-depleted extracts, samples were collected at various times, and chromatin-associated cohesin and sororin were assayed by immunoblot. The levels of chromatin-associated cohesin are unaffected in extracts depleted of sororin (Fig. 1A). In contrast, when cohesin is depleted from extracts with anti-Smc3 antibody before addition of nuclei, chromatin-associated sororin levels are greatly reduced (Fig. 1A; ΔSmc3). Although sororin is able to interact with the cohesin complex, depletion of cohesin did not lead to loss of sororin from the extract, nor did depletion of sororin lead to a reduction in cohesin levels (Fig. S1). This suggests that the interaction between sororin and cohesin is not constitutive in egg extracts and is consistent with earlier results showing that the affinity between sororin and cohesin is low in solution and unlikely to survive most fractionation techniques (17, 18). Thus sororin recruitment to the chromatin depends on cohesin, but cohesin binds efficiently in the absence of sororin.
Fig. 1.
Regulation of sororin recruitment to chromatin by the cohesin complex and DNA replication. (A) Sororin depends on cohesin for association with chromatin. Nuclear assembly reactions were set up in extract that had been depleted of cohesin (ΔSmc3), sororin (Δsororin), or mock-depleted (mock), and chromatin was isolated at the indicated time points and assayed for cohesin (Smc3) and sororin by immunoblot. Control: background band used as loading control. (B) Sororin recruitment requires DNA replication. Sperm nuclei were added to interphase extract supplemented with geminin, p27, aphidicolin, or a buffer control (mock). At the indicated times, chromatin was isolated and analyzed by immunoblot for the presence of cohesin (Smc3) and sororin. (C) DNA replication assay of the same samples shown in B. All three inhibitors effectively blocked DNA replication. Coomassie stained histones were used as a loading control (Lower).
Cohesin is recruited to chromatin in telophase coincident with replication licensing (2, 3). When replication licensing is blocked, cohesin no longer associates with the chromatin. We tested whether replication licensing might similarly regulate sororin recruitment. Geminin is a cell cycle-controlled protein that prevents recruitment of the essential licensing factor Cdt1 to origins of replication (19–21). Consistent with our observation that cohesin binding to chromatin is important for sororin recruitment, the addition of geminin to egg extract to inhibit licensing blocked both cohesin and sororin binding to the chromatin (Fig. 1B).
Sororin Recruitment to Chromatin Requires DNA Replication.
Following the replication-licensing step, prereplication complexes (pre-RCs) are formed. This leads to the recruitment of a complex of several proteins including the Cdc7 kinase, its activating subunit Dbf1, and the cohesin loading proteins Scc2 and Scc4, to the chromatin (22), which in turn leads to cohesin loading on the chromatin. We were interested in determining whether sororin was recruited to the chromatin at a similar step. Addition of the cyclin-dependent kinase (cdk) inhibitor protein p27 is sufficient to block the onset of DNA replication by preventing origin firing, which requires phosphorylation of replication proteins. The binding of Cdc7/Drf1/Scc2/Scc4 complex, and thus cohesin, is unaffected by inhibition of Cdks. To test whether sororin loading is affected by Cdk activity, we added p27 to extracts and then assayed recruitment of cohesin and sororin to the chromatin. Consistent with previously published results (3), cohesin recruitment was unaffected by the presence of p27 protein. In contrast, sororin binding to the chromatin was greatly reduced (Fig. 1B). This indicates that cohesin binding to chromatin is necessary but not sufficient for sororin binding.
There are several possible models for how p27 addition might uncouple the chromatin loading of cohesin and sororin. The first is that Cdk activity—either phosphorylation of sororin itself or of some other chromatin-associated protein(s)—is required for sororin to bind chromatin. Another possibility is that sororin recruitment requires replication elongation, which is also blocked when Cdk activity is inhibited. To distinguish between these two models we determined whether sororin recruitment was affected by aphidicolin, an inhibitor of Polα DNA polymerase. Aphidicolin inhibited sororin binding to the chromatin (Fig. 1B). Together with the p27 result, our data indicate that full sororin recruitment to chromatin requires not just replication licensing and pre-RC formation, but replication elongation.
Sororin Can Bind Efficiently After DNA Replication Is Complete.
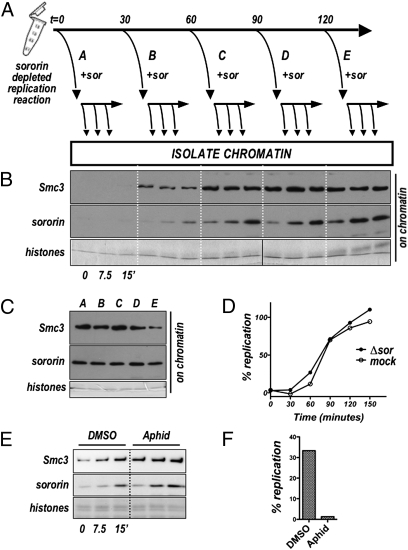
We next designed and performed an experiment to determine whether sororin is recruited only during DNA replication or whether the sororin-binding site persists after replication is complete (Fig. 2). We depleted sororin from interphase extract, added sperm nuclei, and at various times after initiation of the reaction removed part of the reaction and added in vitro-translated sororin to it. Aliquots of these subreactions were collected at the indicated times, and the levels of chromatin-associated sororin and cohesin were assessed by immunoblot. Sororin added to sororin-depleted extracts before DNA replication (t = 0) did not efficiently bind to chromatin, consistent with normal kinetics of both cohesin and sororin binding in untreated extract. However, as time progressed, sororin added to the reaction was recruited to chromatin with increasing efficiency. Maximal sororin binding was achieved on nuclei that had been in the reaction for ≈90 min, which is when DNA replication is largely complete (Fig. 2C). Interestingly, the levels of sororin associated with the chromatin at the end of the experiment (t = 150′) were comparable in all samples, regardless of when sororin was added (Fig. 2D). These results indicate that sororin binding sites on chromatin accumulate during DNA replication and remain available after DNA replication is complete.
Fig. 2.
The sororin-binding site remains after DNA replication is complete. (A) Experimental scheme to test whether sororin binds chromatin only during DNA replication or after replication is complete. Aliquots of a nuclear assembly reaction in sororin-depleted extract were supplemented with in vitro-translated sororin at indicated times after the start of the reaction (t = 0, 30, 60, 90, or 120′, Upper). Following sororin addition, chromatin fractions were isolated immediately (0′) and at 7.5 and 15′ and assayed by immunoblot for cohesin (Smc3) and sororin (B). (C) DNA replication was assessed in extract depleted for sororin (as used in B) and mock-depleted extract. (D) Chromatin was collected from all reactions shown in (B) at 150′ after the start of the experiment and analyzed for cohesin (Smc3) and sororin by immunoblot. Ongoing replication is not required for sororin recruitment. (E) Nuclei were added to a sororin-depleted extract and the reaction was allowed to proceed for 60′. Aphidicolin (or DMSO control) was added. After 10 min, in vitro-translated sororin was added to both extracts and the levels of chromatin-associated Smc3 and sororin were assessed by immunoblot. (F) DNA replication levels over a 30-min period from the time of sororin addition.
To confirm that sororin recruitment can occur when DNA replication is no longer active, we determined whether addition of aphidicolin would affect the kinetics of sororin binding to chromatin. Nuclei were added to sororin-depleted extract and the nuclear assembly and replication reaction was allowed to proceed for 80 min. Aphidicolin was added and after a brief incubation, in vitro-translated sororin was added. When compared with vehicle control (DMSO), addition of aphidicolin had no effect on the kinetics of sororin loading although DNA replication was greatly inhibited. (Fig. 2 E and F). We conclude that the sororin-binding sites developed during DNA replication remain available when further replication is inhibited. The requirement for the cohesin complex (Fig. 1), in conjunction with these data suggests that sororin binds chromatin-associated cohesin that has been modified during DNA replication.
Eco2 Is Necessary but Not Sufficient for Sororin Recruitment to Chromatin.
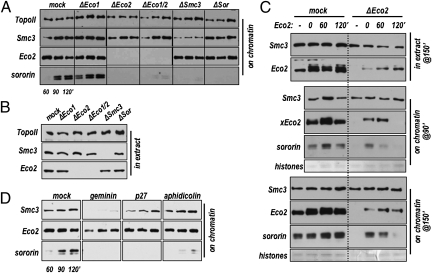
Because Eco-mediated acetylation of cohesin subunits underlies replication-dependent cohesion establishment we decided to test whether this modification also regulates sororin binding. There are two Eco protein family members in vertebrates. Antibodies were generated against both Xenopus proteins: xEco1 and xEco2 (Fig. S2). In extracts both xEco1 and xEco2 could be seen to associate with chromatin over the course of nuclear assembly and replication; by 90 min after initiation of the reaction, the levels of chromatin-associated Eco1 and Eco2 generally reached maximum levels. Although our antibody to xEco1 was reasonably effective in control immunoblots, the signal was weak in egg extracts (e.g., S2A, lane 1). This appears to be due to developmental regulation of Eco1, which in Xenopus is expressed only at low levels until the midblastula transition (MBT) (Fig. S2). Whereas Eco1 depletion had little to no effect on the sororin recruitment, depletion of Eco2 caused a notable reduction in the levels of sororin on chromatin (Fig. 3A). This reduction in sororin was not due to changes in the level of cohesin on chromatin, which was unaffected by Eco2 depletion (see mock vs. ΔEco2; Fig. 3A). Consistent with Fig. 1A, depletion of cohesin did not affect xEco2 loading, but did abolish loading of sororin onto chromatin.
Fig. 3.
Eco2 is required for recruitment of sororin to chromatin. (A) Nuclei were added to interphase extracts that were mock depleted (mock) or immunodepleted of Eco1 (ΔEco1), Eco2 (ΔEco2), Eco1 and Eco2 together (ΔEco1/2), cohesin (ΔSmc3), or sororin (ΔSor). At the indicated times, chromatin fractions were isolated and analyzed by immunoblot for the presence of topoisomerase (loading control), Smc3, Eco2, and sororin. (B) Protein levels in total extract. Depleted extracts used in A were analyzed by immunoblot for topoisomerase II, Smc3, or Eco2 (Eco1 was undetectable in this experiment, and sororin was obscured by background bands in total extract). (C) Rescue of sororin recruitment with recombinant Eco2 interphase extract was depleted of Eco2 and either left unsupplemented (−) or supplemented with recombinant 6His–Eco2 at 0, 60, or 120′ as indicated (Upper). Chromatin-bound fractions were collected at both 90 and 150′ after sperm addition and analyzed by immunoblot for the presence of Smc3, Eco2, and sororin. Also shown are immunoblots for Eco2 and Smc3 in total extracts (extract) (Upper). Coomassie-stained histones were used as loading controls. (D) Eco2 recruitment to chromatin independent of replication interphase extracts were supplemented with geminin, p27, or aphidicolin as in Fig. 1. Chromatin-associated proteins were analyzed at the indicated times after the addition of sperm nuclei.
The replication inhibitors aphidicolin and p27 had no detectable impact on Eco2 loading, although sororin levels on chromatin were greatly reduced, as above (Fig. 1B). Similarly, inhibition of licensing by geminin, which blocked the recruitment of both cohesin and sororin had only a partial effect on Eco2 recruitment (Fig. 3D). Together these data indicate that Eco2 is necessary but not sufficient for sororin recruitment to chromosomes. They also clearly indicate that Eco2 recruitment alone does not underlie the replication requirement for sororin binding.
Eco2 Must Be Present During DNA Replication to Recruit Sororin.
To rule out the possibility that an unidentified critical component was codepleted from the extract along with Eco2, we attempted to restore sororin loading by adding recombinant Eco2 to the extract. When recombinant protein was added to Eco2-depleted extracts, sororin recruitment to chromatin was restored (Fig. 3C, Lower, 0′ add back). Although in somatic cells Eco2-dependent acetylation affects the rate of DNA replication (23), we saw no measurable difference in replication rates between Eco2-depleted and control extracts (Fig. S3). Thus delayed DNA replication cannot explain the Eco2 requirement for sororin binding.
With the experiment in Fig. 3C we also addressed the question of whether Eco2 might underlie the replication requirement for sororin recruitment. We added recombinant Eco2 to Eco2-depleted extract at various times after initiation of DNA replication and tested for the recruitment of both Eco2 and sororin to chromatin. When added to the extract at the beginning of the nuclear assembly reaction (t = 0 min), recombinant Eco2 bound to chromatin and fully rescued sororin loading. However, although Eco2 added at later time points was able to bind chromatin to endogenous levels, sororin recruitment was greatly reduced (Fig. 3C, Middle and Bottom). These data suggest that the presence of Eco2 on chromatin during DNA replication allows development of the sororin binding site and that Eco2 added after replication cannot function in this capacity.
Sororin and Eco2 Are Coregulated by the Anaphase-Promoting Complex.
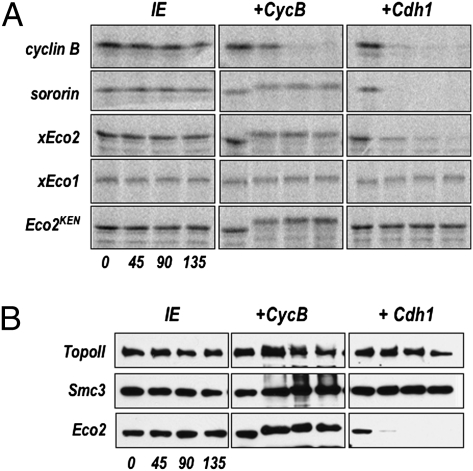
We noted the presence of a conserved KEN box in several Eco2 proteins (Fig. S4), which in many Cdh1-dependent APC substrates serves as a destruction signal (24). To test whether Eco2 is a substrate of the APC we incubated in vitro-translated radiolabeled xEco2 in interphase extracts that had been supplemented with either recombinant cyclin B or Cdh1. Cyclin B drives the extract into mitosis and results in activation both of mitotic kinases and of the APC by the endogenous Cdc20. Cdh1 activates the endogenous APC directly. Once the APC is activated, the extract contains the remaining factors for ubiquitin-dependent proteolysis, and substrates are rapidly degraded. When cyclin B was added to the extracts the electrophoretic mobility of the in vitro-translated xEco2 decreased, consistent with mitotic phosphorylation of the protein, although the level of the protein was unaffected. In contrast, xEco2 was rapidly degraded following addition of Cdh1 (Fig. 4A). Conversion of the KEN to an AAA sequence in xEco2 completely stabilized the protein over the course of the experiment (Fig. 4A, Lower Right).
Fig. 4.
Eco2 is a substrate of Cdh1-activated APC. (A) Radiolabeled in vitro-translated proteins (indicated at Left: cyclin B, sororin, xEco2, xEco1, and xEco2KEN) were incubated in interphase extract (IE), extract supplemented with cyclin B (+CycB), or supplemented with Cdh1 protein (+Cdh1). Aliquots were removed at the indicated times (t = 0, 45, 90, and 135′), separated by SDS/PAGE, and the gel was dried and exposed in a phosphor storage cassette. The phosphorimages are shown. Loading controls are shown in Fig. S6. (B) APC-dependent degradation of endogenous Eco2 protein interphase extract was analyzed by immunoblot for Smc3, Eco2, and sororin at the indicated times following the addition of buffer alone (IE), recombinant cyclin B (+CycB), or Cdh1 (+Cdh1).
When analyzed by immunoblot, endogenous xEco2 in egg extracts underwent similar APC-dependent regulation, shifting in the presence of cyclin B and being degraded following Cdh1 addition (Fig. 4B). Although most vertebrate orthologs of Eco1 also contain a KEN box (Fig. S5), xEco1 showed no evidence for APC-dependent degradation (Fig. 4A). These data are consistent with the somatic expression profiles, in which Eco2 levels decrease at mitotic exit, whereas Eco1 levels are relatively constant. There is an additional significant decrease in Eco2 levels in G2, suggesting that additional mechanisms control Eco2 levels in response to cell cycle progression (25). The data presented here indicate that Eco2 and sororin, which we have shown work together, are coregulated by APC-dependent ubiquitination.
Discussion
Our data are consistent with the model shown in Fig. 5, in which activities at the replication fork stimulate Eco2-dependent acetylation of cohesin, resulting in cohesion establishment. Sororin is then able to recognize and stabilize this “established” conformation. We have shown that Eco2 is not able to rescue sororin recruitment when it is added to the system after DNA replication, suggesting that the presence of Eco2 near the site of active replication is necessary for cohesion establishment. Mechanistically, there are two possible explanations for this. First, Eco2 catalytic activity might be stimulated by activities related to DNA replication. Alternatively, the cohesin complex itself might be somehow altered during passage of the replication fork in a way that makes it a proper substrate for Eco-dependent acetylation. In both models, Eco2-mediated acetylation followed by sororin binding would ensure cohesion establishment is replication dependent. The dependency of sororin recruitment on DNA replication raises the interesting possibility that cohesion establishment in vertebrates may not be completed until G2. Consistent with this notion, sororin is required for stable association of cohesin with chromatin in G2 cells (17). Although no molecular determinant for this stability has been identified, it is assumed that this stability represents cohesin complexes that are actively holding chromatids together.
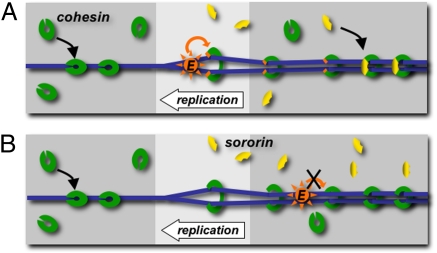
Fig. 5.
Models for regulation of cohesion by sororin and Eco2 cohesin (green clamps) associates with chromatin (blue) before DNA replication. DNA replication either promotes activation of the Eco2 acetyltransferase (orange) directly, causing it to modify the cohesin complex, or causes a change in the cohesin complex (indicated as opening of the complex), which makes it a good Eco2 substrate. Once the cohesin complex is modified by Eco2 it can be bound by sororin (yellow) (A). Eco2 added after DNA replication is able to bind chromatin, but unable to modify cohesin and generate the sororin-binding site (B).
It is not clear why there are two Eco proteins in vertebrates, whereas in other organisms there is only one. Depletion of either homolog from HeLa cells results in cohesion failure, suggesting nonredundant function. Interestingly, the single Eco protein in budding yeast, Eco1/Ctf7, has different cohesin subunit substrates, depending on the context in which it is used (26); it may be that these functions have diverged to two separate proteins in vertebrates. It is also possible that together sororin and Eco2 comprise a regulatory module that performs a function unique to chordates, perhaps to assure cohesion is retained throughout a relatively extended G2 period. The coregulation of sororin and Eco2 by the APCCdh1 may also help prevent ectopic (such as intrachromatid or interchromosomal) cohesion events particularly in G1; it ensures that these positive regulators are abundant only after there are in fact two sisters that can be tied together.
In vertebrates, a complex of Wapl and Pds5 destabilizes the interaction between cohesin and chromatin in G2 and prophase (8, 27). Sororin may function by inhibiting Wapl, either by direct binding, or perhaps by preventing Wapl's access to cohesin. Future experiments will elucidate the functional interaction between sororin, Eco2, and the Wapl–Pds5–SA1/2 cohesin stability network.
Materials and Methods
Antibodies.
Rabbit anti-sororin antibodies were generated by immunization with recombinant full-length X. laevis sororin A. Rabbit anti-Smc3 antibodies were prepared either by immunization with a C-terminal peptide (CEMAKDFVEDDTTHG) or with a recombinant fragment expressing the C-terminal 165 amino acids of human Smc3. Additional rabbit polyclonal antibodies against xRad21 and xSmc1 (28) were generously provided by Rebecca Heald (University of California Berkeley, Berkeley CA). Rabbit anti-xEco1 antibody was generated against a bacterially expressed fusion protein encoding amino acids 537–678 of the full-length protein. Rabbit anti-xEco2 antibody was made by immunizing with a bacterially expressed protein encoding amino acids 1–147 of the full-length protein. Anti-topoisomerase antibody (29) was used for a loading control on chromatin immunoblots. All antibodies, with the exception of the anti-topoisomerase antibody, were affinity purified.
Plasmids.
Xenopus sororin A plasmids were described previously (18). The sequence of sororin B was obtained from IMAGE clone 5511973 (Open Biosystems). Sororin B protein was transcribed and translated from the same clone using SP6 polymerase. Bacterial expression construct for geminin is described elsewhere (20). The pET30-derived expression construct for His6-tagged human p27 was a gift from Scott Plafker (University of Oklahoma Health Sciences Center, Oklahoma City).
Proteins.
In vitro translations were done using the TnT System (Promega) with either SP6 or T7 polymerase as appropriate to the vector. In vitro-translated sororin was added at 1:40 dilution to extract. Xenopus geminin and human p27 were expressed in bacteria, purified using standard molecular biology techniques, and stored in aliquots at −80 °C until use. Where used, geminin was added to a final concentration of 500 nM, and p27 was added to 200 nM. X. laevis Eco2 was expressed in Sf9 insect cells using vectors derived from pFastBac (Invitrogen), purified using standard techniques and stored at −80 °C in 10 mM Tris 7.7, 300 mM NaCl, 1 mM DTT, and 10% glycerol. Eco2 was added to extract to near endogenous levels, ∼40 nM.
Other Reagents.
Protease inhibitors included a mixture of leupeptin, pepstatin, and chymostatin, prepared (LPC; 10 mg/mL each, Sigma) and used as a 1,000× stock in extracts where specified. Aphidicolin (AG Scientific), prepared as a stock of 10 mg/mL, was dissolved in DMSO, stored in aliquots at −80 °C, and diluted just before use in ELB and added to extracts at 150 μm.
Interphase Extracts and Sperm Preparation.
Xenopus interphase extracts were prepared essentially as described (30, 31). Eggs were collected in MMR (5 mM Hepes 7.8, 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EDTA), dejellied in 2% cysteine in MMR, activated with ionophore (A23187, Fisher) in 0.2× MMR, washed several times with EB (50 mM Hepes, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 50 mM sucrose), packed (1,000 rpm for 1 min), then crushed (10,000 rpm for 10 min) in a Beckman JS13.1 rotor. Following the crushing spin the cytosolic layer was removed and supplemented with 0.15 volumes of EDBS (50 mM Hepes pH 7.6, 50 mM KCl, 2 mM DTT, 0.4 mM MgCl2, 0.4 mM EGTA, 10% sucrose), cyclohexamide (100 μm) cytochalasin (10 μg/mL), DTT (2 mM), and mixed well. The supplemented extracts were spun at 27,000 rpm in a Beckman Sw55 rotor for 20 min at 4 °C, and the supernatant, including the associated cloudy membrane layer, was mixed thoroughly and then snap frozen in 100- or 200-μL aliquots in liquid nitrogen and stored at −80 °C. Extracts were supplemented just before use with 50 μg/mL cyclohexamide, prepared as a 10-mg/mL stock and stored at −80 °C, and energy mix (5 μg/mL creatine phosphokinase, 20 mM phosphocreatine, 2 mM ATP, final) was prepared fresh from frozen components as a 35× stock.
Sperm preparation has been described previously (32). Briefly, isolated testes were minced and washed in buffer X (100 mM Hepes pH 7.5, 800 mM KCl, 150 mM NaCl, 50 mM MgCl2, 10 mM EDTA, 200 mM sucrose), vortexed and spun with mild centrifugation (10 s at 1,000 rpm), repeating until supernatant is clear. Combined supernatants are collected and centrifuged twice (50 s at 1,500 rpm followed by 10 min at 4,000 rpm at 4 °C) to isolate the sperm pellet that is then resuspended in buffer X and overlaid on a sucrose gradient before centrifugation (25 min at 33,000 rpm at 4 °C). The sperm pellet is then resuspended in buffer X and centrifuged (10 min at 5,000 rpm at 4 °C), resuspended in buffer X + 0.4% Triton X-100 + LPC and incubated at 4 °C rotation for 30 min. This solution is laid over a sucrose gradient and centrifuged (10 min at 2,100 rpm at room temperature), resuspended in buffer X + 3% BSA + LPC and centrifuged (10 min at 2,100 rpm at room temperature) twice, and resuspended in buffer X + 3% BSA + LPC + 1 mM DTT. Sperm concentration is determined by hemocytometer.
Immunodepletions.
Affinity-purified antibodies were used in the following amounts to deplete 100 μL extract: cohesin, 80 μg; sororin, 20 μg; Eco1, 30 μg; and Eco2, 80 μg. Mock depletions were done with comparable amounts of rabbit normal IgG (Jackson Immunoresearch). Antibodies were bound to protein A beads (Affiprep, Bio-Rad or Dynabeads, Invitrogen) in 100 mM phosphate buffer, pH 8.1. Beads were washed three times with phosphate buffer and three times with ELB (10 mM Hepes, 50 mM KCl, 2.5 mM MgCl2, 1 mM DTT, 250 mM sucrose) before being incubated with extract for 1 h on ice with occasional mixing at 4 °C. Affiprep and Dynabeads were removed from the extracts by centrifugation at 12,000 rpm for 2 min in a fixed angle rotor, or with a magnet for 1 min, respectively. Depletions were repeated a second time, and the depleted extract was used immediately.
Chromatin Spin-Down Assays.
All nuclear assembly reactions contained 3,400 sperm/μL and nocodazole (3.3 μg/mL) (Sigma Aldrich) unless otherwise stated. To assay for chromatin association, 10-μL aliquots of nuclear assembly reactions were collected, rapidly suspended in 60 μL ice cold ELB, and layered onto a cushion composed of ELB containing 0.5 M sucrose. Samples were spun for 25 s at 12,000 g in a horizontal rotor at 4 °C. The pellets were resuspended in 150 μL ELB containing 0.6% Triton X-100 and layered on a second cushion as above. Samples were spun for 1 min at 12,000 g, resuspended in sample buffer, boiled for 3 min, and loaded in their entirety. In all cases, the portion of the gel containing histones was removed and stained to confirm uniform loading of the chromatin.
DNA Replication Assays.
Replication reactions were supplemented with 32P-dATP (3,000 Ci/mmol, Perkin-Elmer) to a final concentration of 0.1 μCi/μL, and incubated for 150 min after addition of sperm nuclei. Ten-microliter aliquots of nuclear assembly reactions were collected every 30 min and rapidly mixed in 75 μL ice-cold stop buffer (0.5% SDS, 20 mM Tris, pH 8.0, 20 mM EDTA). Proteinase K (0.5 mg/mL) was added, and incubated for 1 h at 37 °C, followed by phenol extraction, and spotted onto filters for total and TCA precipitated samples. Determination of percent replication was done as described (33).
Degradation Assays.
Baculovirus-expressed human Cdh1 (450 nm final) or bacterially expressed cyclin B (250 nm final) were added to extract supplemented with 35S-radiolabeled xCyclin, xEco1, xEco2, or sororin A (for autoradiogram) or to unsupplemented extract (for immunoblot analysis). PhosphorImager analysis was performed on a Molecular Dynamics Storm 840 (GE Life Sciences).
Alignments.
Protein alignments were done using the ClustalW algorithm included with the Lasergene software suite (DNAStar).
Supplementary Material
Acknowledgments
We are grateful to R. Heald (University of California Berkeley, Berkeley, CA), T. McGarry (Northwestern University, Chicago), S. Plafker (University of Oklahoma Health Sciences Center, Oklahoma City), and A. Straight (Stanford University, Stanford, CA) for providing reagents; to X. Zhu and M. Rape for discussing unpublished results; and to D. Dawson, D. Obeso, and all members of the Rankin laboratory for critical review of the work. This work was supported by Grant HR06-175S from the Oklahoma Center for the Advancement of Science and Technology and by Grant RR016478 from the National Center for Research Resources, a component of the National Institutes of Health. S.R. is a Pew Scholar in the Biomedical Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. HQ435674).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011069107/-/DCSupplemental.
References
- 1.Skibbens RV. Establishment of sister chromatid cohesion. Curr Biol. 2009;19:R1126–R1132. doi: 10.1016/j.cub.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie PJ, Hirano T. Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr Biol. 2004;14:1598–1603. doi: 10.1016/j.cub.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi TS, Yiu P, Chou MF, Gygi S, Walter JC. Recruitment of Xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nat Cell Biol. 2004;6:991–996. doi: 10.1038/ncb1177. [DOI] [PubMed] [Google Scholar]
- 4.Rolef Ben-Shahar T, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science. 2008;321:563–566. doi: 10.1126/science.1157774. [DOI] [PubMed] [Google Scholar]
- 5.Rowland BD, et al. Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity. Mol Cell. 2009;33:763–774. doi: 10.1016/j.molcel.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Unal E, et al. A molecular determinant for the establishment of sister chromatid cohesion. Science. 2008;321:566–569. doi: 10.1126/science.1157880. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, et al. A handcuff model for the cohesin complex. J Cell Biol. 2008;183:1019–1031. doi: 10.1083/jcb.200801157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenna MA, Skibbens RV. Mechanical link between cohesion establishment and DNA replication: Ctf7p/Eco1p, a cohesion establishment factor, associates with three different replication factor C complexes. Mol Cell Biol. 2003;23:2999–3007. doi: 10.1128/MCB.23.8.2999-3007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moldovan GL, Pfander B, Jentsch S. PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell. 2006;23:723–732. doi: 10.1016/j.molcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Skibbens RV, Corson LB, Koshland D, Hieter P. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 1999;13:307–319. doi: 10.1101/gad.13.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlich D, Koch B, Dupeux F, Peters JM, Ellenberg J. Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr Biol. 2006;16:1571–1578. doi: 10.1016/j.cub.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 13.Dai J, Sullivan BA, Higgins JM. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell. 2006;11:741–750. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe Y, Kitajima TS. Shugoshin protects cohesin complexes at centromeres. Philos Trans R Soc Lond B Biol Sci. 2005;360:515–521, discussion 521. doi: 10.1098/rstb.2004.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankin S, Kirschner MW. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr Biol. 1997;7:451–454. doi: 10.1016/s0960-9822(06)00192-8. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz J, et al. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol. 2007;17:630–636. doi: 10.1016/j.cub.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18:185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Li A, Blow JJ. Cdt1 downregulation by proteolysis and geminin inhibition prevents DNA re-replication in Xenopus. EMBO J. 2005;24:395–404. doi: 10.1038/sj.emboj.7600520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 21.Wohlschlegel JA, et al. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi TS, Walter JC. Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication. Genes Dev. 2005;19:2295–2300. doi: 10.1101/gad.1339805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terret ME, Sherwood R, Rahman S, Qin J, Jallepalli PV. Cohesin acetylation speeds the replication fork. Nature. 2009;462:231–234. doi: 10.1038/nature08550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 25.Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell. 2005;16:3908–3918. doi: 10.1091/mbc.E04-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidinger-Pauli JM, Unal E, Koshland D. Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage. Mol Cell. 2009;34:311–321. doi: 10.1016/j.molcel.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kueng S, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Kenney RD, Heald R. Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts. J Cell Sci. 2006;119:5057–5066. doi: 10.1242/jcs.03277. [DOI] [PubMed] [Google Scholar]
- 29.Luke M, Bogenhagen DF. Quantitation of type II topoisomerase in oocytes and eggs of Xenopus laevis. Dev Biol. 1989;136:459–468. doi: 10.1016/0012-1606(89)90271-6. [DOI] [PubMed] [Google Scholar]
- 30.Blow JJ. Preventing re-replication of DNA in a single cell cycle: Evidence for a replication licensing factor. J Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 32.Tutter AV, Walter JC. Chromosomal DNA replication in a soluble cell-free system derived from Xenopus eggs. Methods Mol Biol. 2006;322:121–137. doi: 10.1007/978-1-59745-000-3_9. [DOI] [PubMed] [Google Scholar]
- 33.Blow JJ, Laskey RA. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.