Abstract
Ocean acidification (OA) refers to the ongoing decline in oceanic pH resulting from the uptake of atmospheric CO2. Mounting experimental evidence suggests that OA will have negative consequences for a variety of marine organisms. Whereas the effect of OA on the calcification of adult reef corals is increasingly well documented, effects on early life history stages are largely unknown. Coral recruitment, which necessitates successful fertilization, larval settlement, and postsettlement growth and survivorship, is critical to the persistence and resilience of coral reefs. To determine whether OA threatens successful sexual recruitment of reef-building corals, we tested fertilization, settlement, and postsettlement growth of Acropora palmata at pCO2 levels that represent average ambient conditions during coral spawning (∼400 μatm) and the range of pCO2 increases that are expected to occur in this century [∼560 μatm (mid-CO2) and ∼800 μatm (high-CO2)]. Fertilization, settlement, and growth were all negatively impacted by increasing pCO2, and impairment of fertilization was exacerbated at lower sperm concentrations. The cumulative impact of OA on fertilization and settlement success is an estimated 52% and 73% reduction in the number of larval settlers on the reef under pCO2 conditions projected for the middle and the end of this century, respectively. Additional declines of 39% (mid-CO2) and 50% (high-CO2) were observed in postsettlement linear extension rates relative to controls. These results suggest that OA has the potential to impact multiple, sequential early life history stages, thereby severely compromising sexual recruitment and the ability of coral reefs to recover from disturbance.
Keywords: carbon dioxide, climate change, pH, juvenile, elkhorn coral
The susceptibility of reef-building corals to increasing CO2 levels has been a central issue in the context of global climate change. Present-day atmospheric CO2 (pCO2) levels are estimated at 387 ppm, 30% higher than the natural range over the last 650,000 y (1). pCO2 is increasing at an annual rate of 0.5% (2), 200 times faster than any changes that occurred during the last eight glacial cycles (1). Approximately one-third of all CO2 emissions from the past 200 y has been absorbed by the oceans (3). On dissolution in seawater, CO2 reacts with H2O, triggering a series of chemical reactions that alter the seawater carbonate chemistry; [CO2]aq and [HCO3−] increase, and [CO32−], pH, and the carbonate saturation state (Ω) decrease, causing ocean surface waters to become more acidic. Increasing atmospheric CO2 concentrations have already depleted seawater carbonate concentrations by ∼30 μmol kg−1, simultaneously reducing the pH of the ocean's surface waters by 0.1 units relative to the preindustrial era (a 30% increase in [H+]) (2). Further reductions of 0.3–0.5 pH units are projected by the end of this century as the oceans continue to absorb anthropogenic CO2 (2, 3).
Mounting experimental evidence suggests that ocean acidification (OA) will have negative consequences for numerous marine organisms, primarily oceanic calcifiers that rely on the delicate balance of dissolved inorganic carbon species for the formation of their shells and skeletons (4–7). Although recent research efforts have aimed to constrain the mechanisms and effects (both physiological and ecological) of elevated pCO2 on adult corals, comparatively little attention has been given to the effect of OA on earlier life history stages.
The majority of reef-building corals rely on external fertilization and the development, survival, and settlement of lecithotrophic (i.e., energy-limited) planula larvae (8). Coral larvae spend hours to days developing in the water column before they are capable of settling on the reef. Larval settlement requires the recognition of water-soluble and substrate-bound chemical cues, physical attachment to the substrate, and subsequent metamorphosis. Recruitment (identification and inclusion in a population) necessitates survival and growth of the newly settled individual. Successful coral recruitment is determined by three sequential life history stages: (i) larval availability (including gamete production and successful fertilization), (ii) settlement ecology (related to larval and substrate condition), and (iii) postsettlement ecology (growth and survival) (9). Environmental factors that disrupt these various processes can result in compromised recruitment or recruitment failure and profoundly affect marine population dynamics (10–12).
The objective of the present study was to investigate the effect of pCO2 on the three sequential life history stages that are critical to successful sexual recruitment of broadcast-spawning reef corals. The experimental coral chosen for this study was the ecologically significant Caribbean elkhorn coral Acropora palmata (Lamark). This fast-growing, branching species has historically functioned as a primary framework builder on many Caribbean shallow reefs. However, Caribbean acroporid populations have experienced widespread decline over the last several decades due to hurricanes, disease, bleaching, and predation (13, 14). A drastic reduction in population size resulted in the designation of A. palmata as a threatened species under the US Endangered Species Act in 2006 (15). Recovery of A. palmata populations likely will require high rates of recruitment (13, 16, 17). Although this coral's primary reproductive mode is asexual fragmentation, sexual recruitment is critical for maintaining the genetic diversity of future populations. To determine the effects of OA on the sexual recruitment of this species, we tested fertilization, settlement, and postsettlement growth at three pCO2 levels, representing average conditions during the spawning season [400 μatm (control)] and the range of pCO2 increases expected during this century [560 μatm (mid-CO2) and 800 μatm (high-CO2)], as determined by the Intergovernmental Panel on Climate Change (2).
Results
Fertilization Assays.
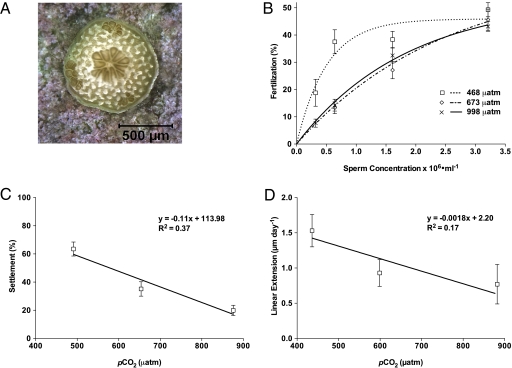
Fertilization success decreased with increasing CO2 concentration, with average reductions (averaged across all sperm concentrations) of 12% at the high-CO2 level and 13% at the mid-CO2 level. At the highest sperm concentration (3.21 × 106 sperm mL−1), fertilization was reduced by 7% at the mid-CO2 level and by 12% at the high-CO2 level. As sperm concentration declined, the reduction in fertilization success was exacerbated at elevated CO2 levels: fertilization was reduced by 29% at the mid-CO2 level and by 15% at the high-CO2 level at 1.61 × 106 sperm mL−1 and by 64% at the mid-CO2 level and by 63% at the high-CO2 level at 6.41 × 105 sperm mL−1. At the most dilute sperm concentration (3.20 × 105 sperm mL−1), fertilization was reduced by 59% at the high-CO2 level. Data from this sperm concentration were unreliable for the mid-CO2 level due to sperm contamination and thus were omitted from the regression analysis.
Nonlinear regressions were fit to percent fertilization at sperm concentration data (Fig. 1B). The model parameters, listed in Table 1, are valid for A. palmata fertilization under the laboratory conditions as described in Materials and Methods, using sperm concentrations ranging from 105 to 106 sperm mL−1. A comparison-of-fit test rejected the null hypothesis (H0) that one curve best fits all data sets (F4,103 = 13.69, P < 0.0001).
Fig. 1.
Results of fertilization, settlement, and growth experiments (mean ± 1 SEM). (A) A 26-d-old A. palmata juvenile reared under control conditions (ambient CO2). (B) Nonlinear regressions of fertilization data by CO2 treatment. Regressions were fit separately for each CO2 concentration. Parameter estimates are outlined in Table 1. Data from the 0.32 × 105 sperm mL−1 · 673 μatm treatment were omitted from the regression due to the unavailability of reliable data for this cell as a result of sperm contamination (Materials and Methods and Fig. S2B). (C) Percent settlement of A. palmata by treatment (11 dAS). Model parameters are for actual settlement data (n = 24 wells); P values are from linear regression analysis of arcsine-transformed percentage data. (D) Linear extension (μm day−1) of A. palmata juveniles by treatment over 50 d. Individuals exhibiting partial or full mortality were excluded from the analysis, resulting in fewer replicates in the mid- and high-CO2 treatment groups (control, n = 11; mid-CO2, n = 9; high-CO2, n = 5).
Table 1.
Parameter estimates for nonlinear regressions of fertilization data
| 468 μatm | 673 μatm | 998 μatm | |
| Best-fit values | |||
| Max % Fert | 45.93 | 62.21 | 53.07 |
| C | 1.98 | 0.40 | 0.54 |
| SE | |||
| Max % Fert | 3.30 | 17.97 | 6.02 |
| C | 0.46 | 0.20 | 0.12 |
| 95% confidence interval | |||
| Max % Fert | 39.25–52.62 | 25.39–99.01 | 40.86–65.28 |
| C | 1.06–2.90 | −0.0003–0.80 | 0.30–0.78 |
| Degrees of freedom | 38 | 28 | 37 |
| R2 | 0.40 | 0.56 | 0.84 |
Max % Fert is the asymptotic average maximum percentage of fertilization, and C is a rate coefficient that determines how quickly the maximum is attained.
Settlement Assays.
Compared with control, percent settlement was reduced by 45% at the mid-CO2 level and by 69% at the high-CO2 level. A significant linear relationship was seen between CO2 concentration and percent larval settlement (F1,70 = 41.79, P < 0.0001, linear regression analysis of arcsine-transformed data using least squares residuals) (Fig. 1C).
Postsettlement Growth.
Compared with control, there was a significant reduction in linear extension, of 39% at the mid-CO2 level and 50% at the high-CO2 level (F1,23 = 4.86, P < 0.05, linear regression analysis using least squares residuals) (Fig. 1D). The reduced sample sizes at the mid- and high-CO2 levels resulted from greater partial and full mortality at these levels.
Discussion
This study demonstrates that that OA will affect three sequential life history phases necessary for successful coral recruitment of A. palmata: (i) larval availability (by compromising fertilization), (ii) settlement ecology (by reducing settlement success), and (iii) postsettlement ecology (by impeding postsettlement growth). Our results indicate that OA has the potential to reduce fertilization by 12–13% (averaged across all sperm concentrations) and to decrease settlement success by 45–69% at pCO2 concentrations expected for the middle and the end of this century. The compounding effect of OA on these early life history stages translates into a 52–73% reduction in the number of larval settlers on the reef. The net impact on recruitment likely will be even greater, given that depressed postsettlement growth is likely to result in elevated rates of postsettlement mortality.
Sexual reproduction of many sessile marine invertebrates occurs through the release (spawning) of gametes into the water, followed by external fertilization. Consequently, water chemistry can greatly affect fertilization success. Elevated pCO2 has been shown to impair fertilization success of other marine invertebrates, including oysters (18) and sea urchins (19, 20). Reduced sperm flagellar motility, which has been shown to occur in both urchins (19) and at least one species of coral (21), is one possible explanation for the reductions in fertilization reported herein.
Our data suggest that as sperm concentration declines, the effect of OA on fertilization success intensifies (Fig. 1B). Sperm limitation (i.e., too few sperm to fertilize all eggs) is recognized as one of the key factors limiting the reproductive success of sessile, broadcast-spawning marine invertebrates (22, 23). Thus, it is likely that in situ fertilization occurs at dilute sperm concentrations, with which the effects of OA would be exacerbated. Reproductive failure due to sperm limitation is of increasing concern as hurricanes, disease, and other environmental and anthropogenic stressors continue to reduce coral population densities (i.e., Allee effects) (23, 24). Increasing OA threatens to diminish larval supply, a major determinant of recruit density (25, 26), and depressed recruitment will likely compromise coral reef resilience (the ability of reefs to recover after disturbance). Three-quarters of reef-building coral species spawn gametes and rely on external fertilization and planktonic development (8); thus, if A. palmata is representative of spawning species in general, then the fertilization and recruitment success of many spawning corals will likely be impaired as CO2-driven OA intensifies.
The results of our fertilization assays underscore the need for caution when designing and interpreting experiments testing the effects of CO2 on the fertilization of broadcast-spawning marine invertebrates. At our highest sperm concentration (approaching optimal concentrations but unlikely to occur in nature), no treatment effect of CO2 was observed; however, as sperm concentration decreased, a significant effect of CO2 emerged (Fig. 1B). In addition, comparison of confidence intervals for slope (Table 1) indicates a significantly higher fertilization rate in the control (468 μatm) compared with either of the elevated CO2 treatments. The two elevated CO2 treatments were not significantly different from one another, however. These results suggest that there may be a threshold response to elevated CO2 within the range of CO2 values and sperm concentrations that were tested for this coral species. Future studies should test CO2 effects across a broad range of OA scenarios and sperm concentrations to accurately assess the presence of a treatment effect.
The effect of pCO2 on larval settlement may indicate either a direct effect (physiological disruption of settlement and/or metamorphosis) or an indirect effect (interference with benthic habitat cues or behavior). Although these two mechanisms are not mutually exclusive, previous settlement experiments conducted with Porites astreoides larvae indicated that elevated pCO2 does not significantly affect the ability of larvae to successfully settle and metamorphose (6). It is important to note, however, that this previous study tested only the direct effect of pCO2 on larval settlement and did not address the potential for indirect effects. The presence of an effect in the present study suggests that an indirect mechanism is primarily responsible for the trends reported herein, whereby pCO2 alters the community composition of the substrata during the conditioning phase, thereby altering the biological and chemical cues responsible for settlement. Both positive settlement cues from crustose coralline algae (CCA) and settlement interference by turf algae have been documented (27–31). Visual inspection of the settlement substrata used in the present study confirms that the higher CO2 treatments resulted in greater colonization by filamentous algae. Visible differences in colonization by CCA that were apparent approximately 3 wk after settlement experiments are consistent with previously documented reductions in CCA colonization as a result of OA (32) and suggest that nonvisible differences were present at the time of settlement. Both the increase in filamentous algae and the decrease in CCA colonization may explain the depressed settlement observed on high-CO2–conditioned tiles.
The observed reductions in linear extension of A. palmata juveniles are consistent with the hypothesis that calcification and, ultimately, growth decline as pCO2 increases and saturation state decreases (4–7). Slowed juvenile growth likely will translate into increased juvenile mortality, given that the risk of mortality is inversely proportional to juvenile growth rate and colony size (26, 33, 34). In addition, for corals and other species that exhibit direct relationships among colony size, onset of sexual maturity (35), and fecundity (34), reduced growth will substantially diminish reproductive potential. Slowed growth will result in a longer time spent in juvenile, nonreproductive life stages, which, in combination with adult loss, would shift population structures toward dominance by smaller size classes, ultimately reducing effective population sizes, population fecundity, and the resilience of reef-building corals (36).
Sessile, broadcast-spawning organisms face several population bottlenecks during early life, including fertilization, settlement, and early postsettlement survivorship and growth (26). As a result, natural larval and early juvenile mortality of many marine invertebrates often exceeds 99% (37, 38). Stochastic events or chronic stressors that further reduce survivorship during these critical stages have the potential to significantly alter future population sizes (26, 37). Although OA is now recognized as a substantial threat to marine calcifiers and their ability to secrete calcium carbonate shells and/or skeletons, our findings demonstrate that increasing pCO2 has the potential to impact multiple life history stages of corals, including critical processes independent of calcification. The compounding nature of these impacts on successive life history stages suggests that the consequences of OA on coral populations and reef communities might be more severe than originally perceived.
Materials and Methods
The fertilization experiments described in this report were conducted in field laboratories in Key Largo, FL. Settlement and growth experiments were conducted at the Climate Change Laboratory at the Rosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, FL.
Spawn Collection.
Two stands of previously genotyped Acropora palmata in the Upper Florida Keys were monitored for spawning during August 2009. Gamete bundles were collected from two distinct genets at Elbow Reef and one at Sand Island. Corals spawned at ≈2200 hours. Gamete bundles disintegrated ≈30 min after release, whereupon eggs and sperm from each colony were separated via pipet. Eggs were washed between five and seven times with filtered seawater to remove residual sperm. Equal volumes of eggs from each genet were combined to create a “stock” egg batch for use in fertilization experiments. The same was done with the sperm.
Seawater Chemistry.
Seawater chemistry was manipulated via direct bubbling with CO2-enriched air to create three target conditions: 400 μatm (control), 560 μatm (mid-CO2), and 800 μatm (high-CO2). Actual chemical and physical conditions that persisted during each experiment are outlined in Table 2. The control was bubbled only with air and approximates the average ambient seawater chemistry that was observed at 0–4 h postspawning (2200–0200 hours, hours of maximum sperm activity and highest potential for fertilization) on Elbow Reef, Key Largo, FL (the site of gamete collection) as determined by hourly water sampling at 11 d postspawning (n = 4 hourly samples) (Table 2). Treatment water was filtered (0.2 μm) before use in experiments, to limit respiratory alterations of desired CO2 levels. Water samples were obtained and analyzed at the start of fertilization and settlement experiments to verify distinct treatments; samples were obtained weekly for growth experiments. Water samples were analyzed for TA and pH. TA was determined in duplicate using automated, open-cell Gran titration (SOP3b; ref. 39), and accuracy was checked against certified seawater reference material (from A. Dickson, Scripps Institute of Oceanography). pH on the total scale was determined using an Orion ROSS combination pH electrode (Thermo Scientific) calibrated at 25 °C against a seawater Tris buffer (SOP6a; ref. 39). Concentrations of CO32−, Ca2+, and Ωarag were computed from TA, pH, temperature, and salinity using the CO2SYS program (from E. Lewis, Brookhaven National Laboratory), dissociation constants for carbonate determined by Mehrbach et al. (40) as refit by Dickson and Millero (41), and the dissociation constant for boric acid determined by Dickson (42). pH is reported on the total scale, the scale on which K1 and K2 were determined in the Gran functions.
Table 2.
Physical and chemical conditions during the experimental periods (mean ± 1 SD)
| Salinity | T °C | TA μmol kg−1 | pHT | pCO2 μatm | HCO3− μmol kg−1 | CO32− μmol kg−1 | CO2 μmol kg−1 | TCO2 μmol kg−1 | bCa2+ mmol kg−1 | Ωarag | |
| Ambient seawater (n = 4 hourly samples) | |||||||||||
| Ambient seawater | 36.5 ± 0.1 | 30.054 ± 0.001 | 2,385 ± 3 | 8.00 ± 0.01 | 454 ± 12 | 1,793 ± 8 | 242 ± 4 | 11.0 ± 0.3 | 2,046 ± 4 | 10.7 ± 0.3 | 3.89 ± 0.07 |
| Fertilization (n = 1 initial) | |||||||||||
| Control | 35.1 ± 0.1 | 28.0 ± 0.1 | 2,310 ± 2 | 7.986 ± 0.004 | 468 ± 5 | 1,794 ± 3 | 210 ± 2 | 12.3 ± 0.1 | 2,016.7 ± 0.9 | 10.3 ± 0.3 | 3.38 ± 0.03 |
| Mid-CO2 | 34.4 ± 0.1 | 28.0 ± 0.1 | 2,293 ± 1 | 7.8538 ± 0.0008 | 673 ± 1 | 1,899.0 ± 0.4 | 161.3 ± 0.3 | 17.76 ± 0.03 | 2,078.0 ± 0.7 | 10.1 ± 0.3 | 2.608 ± 0.005 |
| High-CO2 | 34.5 ± 0.1 | 28.0 ± 0.1 | 2,352.4 ± 0.5 | 7.715 ± 0.003 | 998 ± 7 | 2,045 ± 1 | 126.4 ± 0.7 | 26.3 ± 0.2 | 2,197.6 ± 0.8 | 10.1 ± 0.3 | 2.04 ± 0.01 |
| Settlement (n = 1 initial) | |||||||||||
| Control | 34.5 ± 0.1 | 28.0 ± 0.1 | 2,339 ± 2 | 7.966 ± 0.002 | 491 ± 3 | 1,834.3 ± 0.4 | 207 ± 1 | 12.93 ± 0.07 | 2,054.2 ± 0.6 | 10.1 ± 0.3 | 3.34 ± 0.12 |
| Mid-CO2 | 34.8 ± 0.1 | 28.0 ± 0.1 | 2,388 ± 3 | 7.87 ± 0.01 | 654 ± 19 | 1,956 ± 6 | 178 ± 4 | 17.2 ± 0.5 | 2,151 ± 3 | 10.2 ± 0.3 | 2.87 ± 0.06 |
| High-CO2 | 34.9 ± 0.1 | 28.0 ± 0.1 | 2,336.2 ± 0.9 | 7.751 ± 0.003 | 876 ± 7 | 1,998 ± 1 | 139.6 ± 0.9 | 23.1 ± 0.2 | 2,159.7 ± 0.5 | 10.3 ± 0.3 | 2.23 ± 0.01 |
| Growth (n = 5 weekly samples) | |||||||||||
| Control | 34 ± 1 | 28.2 ± 0.1 | 2,482 ± 42 | 8.04 ± 0.03 | 436 ± 41 | 1,886 ± 53 | 247 ± 15 | 11 ± 1 | 2,144 ± 46 | 10.0 ± 0.3 | 4.0 ± 0.2 |
| Mid-CO2 | 34.2 ± 0.7 | 28.2 ± 0.1 | 2,471 ± 32 | 7.93 ± 0.04 | 599 ± 69 | 1,987 ± 55 | 200 ± 13 | 16 ± 2 | 2,203 ± 46 | 10.0 ± 0.3 | 3.3 ± 0.2 |
| High-CO2 | 35 ± 1 | 28.12 ± 0.09 | 2,479 ± 37 | 7.79 ± 0.05 | 882 ± 111 | 2,109 ± 52 | 154 ± 14 | 23 ± 3 | 2,286 ± 46 | 10.1 ± 0.3 | 2.5 ± 0.2 |
Averages for ambient seawater are based on hourly water sampling at 0–4 h postspawning (2200–0200 hours) on Elbow Reef (site of gamete collection), 11 d after A. palmata spawning in August 2009. All measurements are based on duplicate or triplicate analyses for each sampling period. pHT, pCO2, HCO3−, CO32−, CO2, TCO2, and Ωarag were calculated using CO2SYS with an input temperature of 25 °C and an output temperature according to the tank or ambient seawater as noted in the table. Calcium concentration was calculated based on 10.28 mmol kg−1 of Ca2+ at a salinity of 35.
Fertilization Assays.
Concentrated sperm (3.21 × 108 sperm mL−1), representing equal contributions from three parental genotypes, were diluted to 3.21 × 106, 1.61 × 106, 6.41 × 105, and 3.20 × 105 sperm mL−1 in filtered treatment water. Optimal sperm concentrations for other species of broadcast-spawning corals have been reported to range between 105 and 106 sperm mL−1 (43). After 1-mL subsamples of each dilution were fixed in 10% formalin for verification of sperm concentrations (eight replicate counts by hemocytometer), 10 mL of each sperm dilution–treatment combination was transferred via pipet into each of 10 replicate 15-mL glass vials. A 10-mL volume of sperm-free seawater was transferred into an extra vial as a negative (no-sperm) control. Then 100 μL of eggs (∼200–250 eggs) was immediately added to each vial, and the mixture was swirled. Fertilization experiments were initiated within 3 h of spawning and conducted at 28 ± 0.1 °C. Embryos were subsampled at 4 h and fixed in 10% formaldehyde. Subsequently, 100–200 undamaged eggs from each subsample were examined using a dissecting microscope and classified as either fertilized (showing normal cleavage patterns of cell division) or unfertilized (showing no signs of cleavage).
Nonlinear regressions (exponential rise to maximum, two parameter) were fit to percent fertilization at sperm concentration data. Regressions were fit separately for each CO2 concentration using least squares residuals. The data were fit to the following model:
where %Fert is the percent fertilization at sperm concentration (SC), max%Fert is the asymptotic average maximum percent fertilization, and C is a rate coefficient that determines how quickly (or slowly) the maximum is attained (i.e., the slope). Justification for the use of this model is provided in SI Text. A comparison-of-fit test was conducted on arcsine-transformed data to test the null hypothesis (H0) that one curve best fits all data sets.
Data from the 0.32 × 105 sperm mL−1 · 673 μatm treatment were omitted from the regression (“x” in Fig. S2B), resulting in 30 df at 673 μatm. Reliable data were not available for this cell due to sperm contamination. This notion is supported by the model results, which indicate an expected value much lower than what was found for that cell. In addition, one outlier was removed from the 998-μatm treatment, resulting in 39 df (“+” in Fig. S2C); this outlier was >2.5 SD away from the mean. It is important to note that omitting these points did not change the statistical outcome of the regression analyses, but did provide a better fit for the model.
Settlement Assays.
Gametes not used for fertilization experiments were fertilized, reared to competency in ambient seawater, and used in assays to test the effects of pCO2 on settlement success. Five-day-old larvae [5 days after spawning (dAS)] were introduced to settlement assays, where they were offered limestone settlement tiles that had been preconditioned for 40 d in recirculating aquaria corresponding to the three CO2 treatments. A single source of live rock was divided equally among the three treatment aquaria to provide a consistent source of crustose coralline algae and microfauna during the conditioning phase of the experiment. Settlement assays were conducted in six-well nontreated polystyrene tissue culture plates (BD Biosciences) using the same treatment water as used for substrate conditioning. One settlement tile, 10 mL of treatment water, and 10 larvae were randomly added to each well. Four 24-well plates were used per treatment. The plates were securely covered and submerged in treatment tanks to ensure temperature control (28 °C) and to prevent gas exchange (i.e., equilibration of mid- and high-CO2 levels with atmosphere). Water was exchanged by syringe every 48 h, with care taken to not disturb larvae.
Tiles were examined at 6, 8, and 11 dAS. Previous studies have shown that the first permanent larval settlement (i.e., metamorphosed and permanently settled juvenile polyps) of two acroporid species occurred at 5–6 dAS, and most larval settlement (85–97% of total) occurred within 9–10 dAS (44). The number of settled larvae on the top and bottom of each tile was counted using a dissecting microscope. Larvae were classified as “settled” when they had fully metamorphosed (i.e., a flat/disk-shaped appearance rather than pear-shaped), with little or no possibility of active detachment and further migration (11). Percentage data were arcsine-transformed and analyzed using regression analysis.
Linear Extension.
After settlement, juveniles from the settlement experiments were reared in recirculating treatment aquaria over the course of 50 d. Linear extension was routinely measured using an optical micrometer, capable of measuring linear extension rates to a precision of 0.5 μm. During each measurement period, juveniles were briefly removed from the treatment aquaria, fitted onto the stage of the micrometer, and advanced through the path of the optical micrometer. Each measurement was conducted in less than 3 min, thereby minimizing the time that spat were out of the water. Linear extension was calculated as the change in elevation over time. Individuals exhibiting partial or full mortality were excluded from the analysis. Data were analyzed by regression analysis.
Supplementary Material
Acknowledgments
We thank L. Johnston, A. Valdivia, D. Williams, and R. Wilborn for field and laboratory assistance; N. Ehrhardt for statistical advice; and D. Manzello and two anonymous reviewers for a constructive review of the manuscript. This project was funded in part by the Mote Marine Laboratory's “Protect Our Reefs” specialty license plate and by the National Oceanic and Atmospheric Administration's Coral Reef Conservation Program, with logistical support provided by the Florida Keys National Marine Sanctuary (FKNMS). Collection of coral spawn was conducted under Permit FKNMS-2007-009. Additional support was provided by the National Science Foundation (Grant OCE 0547169) and the Korein Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007273107/-/DCSupplemental.
References
- 1.Siegenthaler U, et al. Stable carbon cycle–climate relationship during the Late Pleistocene. Science. 2005;310:1313–1317. doi: 10.1126/science.1120130. [DOI] [PubMed] [Google Scholar]
- 2.Intergovernmental Panel on Climate Change . In: Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. New York: Cambridge Univ Press; 2007. [Google Scholar]
- 3.Sabine CL, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305:367–371. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- 4.Jokiel PL, et al. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs. 2008;27:473–483. [Google Scholar]
- 5.Marubini F, et al. Coral calcification responds to seawater acidification: A working hypothesis towards a physiological mechanism. Coral Reefs. 2008;27:491–499. [Google Scholar]
- 6.Albright R, Mason B, Langdon C. Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs. 2008;27:485–490. [Google Scholar]
- 7.Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA. Morphological and compositional changes in the skeletons of the new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst. 2009;10 10.1029/2009GC002411. [Google Scholar]
- 8.Veron JEN. Corals of the World. Vol. 3. Townsville, Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd.; 2000. [Google Scholar]
- 9.Ritson-Williams R, et al. New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci. 2009;38:437–457. [Google Scholar]
- 10.Gaines S, Roughgarden J. Larval settlement rate: A leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA. 1985;82:3707–3711. doi: 10.1073/pnas.82.11.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison PL, Wallace CC. In: Ecosystems of the World: Coral Reefs 25. Dubinsky Z, editor. Amsterdam: Elsevier; 1990. pp. 133–207. [Google Scholar]
- 12.Doherty P, Fowler T. An empirical test of recruitment limitation in a coral reef fish. Science. 1994;263:935–939. doi: 10.1126/science.263.5149.935. [DOI] [PubMed] [Google Scholar]
- 13.Bruckner AW. Proceedings of the Caribbean Acropora workshop: Potential application of the US Endangered Species Act as a conservation strategy. 2002 NOAA Technical Memorandum NMFS-OPR-24 (National Oceanic and Atmospheric Administration, Silver Spring, MD) [Google Scholar]
- 14.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 15.National Marine Fisheries Service Endangered and threatened species: Final listing determinations for Elkhorn coral and Staghorn coral. Fed Regist. 2006;71:26852–26872. [Google Scholar]
- 16.Szmant AM, Miller MW. Settlement preferences and post-settlement mortality of laboratory cultured and settled larvae of the Caribbean hermatypic corals Montastraea faveolata and Acropora palmata in the Florida Keys, USA. Proc 10th Int Coral Reef Symp. 2006;1:43–49. [Google Scholar]
- 17.Williams DE, Miller MW, Kramer KL. Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs. 2008;27:697–705. [Google Scholar]
- 18.Parker LM, Ross PM, O'Connor WA. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850) Glob Change Biol. 2009;15:2123–2136. [Google Scholar]
- 19.Havenhand JN, Buttler FR, Thorndyke MC, Williamson JE. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr Biol. 2008;18:R651–R652. doi: 10.1016/j.cub.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Kurihara H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser. 2008;373:275–284. [Google Scholar]
- 21.Morita M, et al. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote. 2010;18:103–107. doi: 10.1017/S0967199409990177. [DOI] [PubMed] [Google Scholar]
- 22.Levitan DR, Peterson C. Sperm limitation in the sea. Trends Ecol Evol. 1995;10:228–231. doi: 10.1016/S0169-5347(00)89071-0. [DOI] [PubMed] [Google Scholar]
- 23.Levitan DR, Sewell MA, Chia FS. How distribution and abundance influences fertilization success in the sea urchin Strongylocentrotus franciscanus. Ecology. 1992;73:248–254. [Google Scholar]
- 24.Baums IB, Miller MW, Hellberg ME. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol. 2005;14:1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes TP, et al. Supply-side ecology works both ways: The link between benthic adults, fecundity, and larval recruits. Ecology. 2000;81:2241–2249. [Google Scholar]
- 26.Vermeij MJA, Sandin SA. Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology. 2008;89:1994–2004. doi: 10.1890/07-1296.1. [DOI] [PubMed] [Google Scholar]
- 27.Birrell CL, McCook LJ, Willis BL. Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull. 2005;51:408–414. doi: 10.1016/j.marpolbul.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Kuffner IB, et al. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser. 2006;323:107–117. [Google Scholar]
- 29.Morse DE, Hooker N, Morse ANC, Jensen RA. Control of larval metamorphosis and recruitment in sympatric Agariciid corals. J Exp Mar Biol Ecol. 1988;116:193–217. [Google Scholar]
- 30.Webster NS, et al. Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl Environ Microbiol. 2004;70:1213–1221. doi: 10.1128/AEM.70.2.1213-1221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritson-Williams R, Paul VJ, Arnold SN, Steneck RS. Larval settlement preferences and post-settlement survival of the threatened Caribbean corals Acropora palmata and A. cervicornis. Coral Reefs. 2010;29:71–81. [Google Scholar]
- 32.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT. Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci. 2008;1:114–117. [Google Scholar]
- 33.Hughes TP, Jackson JBC. Population dynamics and life histories of foliaceous corals. Ecol Monogr. 1985;55:141–166. [Google Scholar]
- 34.Babcock RC. Comparative demography of three species of scleractinian corals using age- and size-dependent classifications. Ecol Monogr. 1991;61:225–244. [Google Scholar]
- 35.Szmant AM. Reproductive ecology of Caribbean reef corals. Coral Reefs. 1986;5:43–54. [Google Scholar]
- 36.Done TJ. Coral community adaptability to environmental change at the scales of regions, reefs and reef zones. Am Zool. 1999;39:66–79. [Google Scholar]
- 37.Gosselin LA, Qian PY. Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser. 1997;146:265–282. [Google Scholar]
- 38.Hunt HL, Scheibling RE. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar Ecol Prog Ser. 1997;155:269–301. [Google Scholar]
- 39.Dickson AG, Sabine CL, Christian JR. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3, North Pacific Marine Science Organization. 2007. Available at: http://cdiac.ornl.gov/oceans/Handbook_2007.html.
- 40.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- 41.Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. [Google Scholar]
- 42.Dickson AG. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 °K. Deep-Sea Res. 1990;37:755–766. [Google Scholar]
- 43.Oliver J, Babcock R. Aspects of the fertilization ecology of broadcast spawning corals: Sperm dilution effects and in situ measurements of fertilization. Biol Bull. 1992;183:409–417. doi: 10.2307/1542017. [DOI] [PubMed] [Google Scholar]
- 44.Nozawa Y, Harrison PL. Temporal patterns of larval settlement and survivorship of two broadcast-spawning acroporid corals. Mar Biol. 2008;155:347–351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.