Abstract
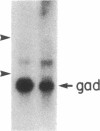
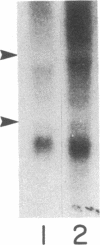
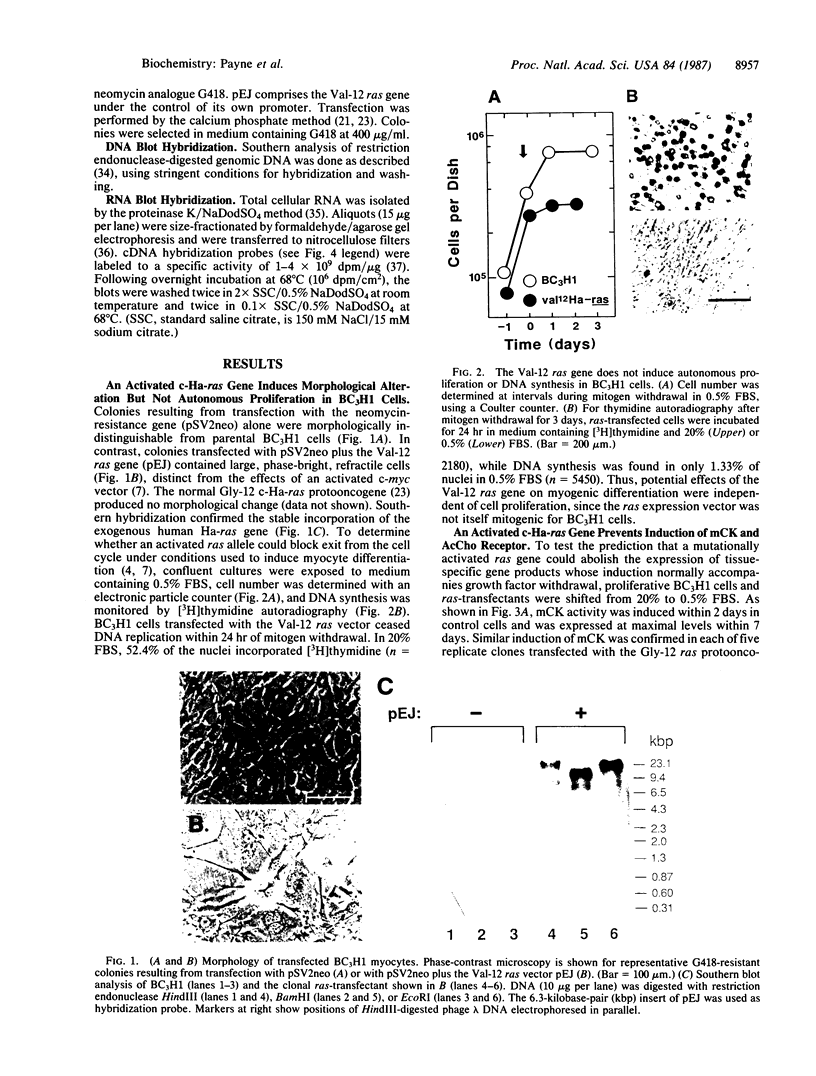
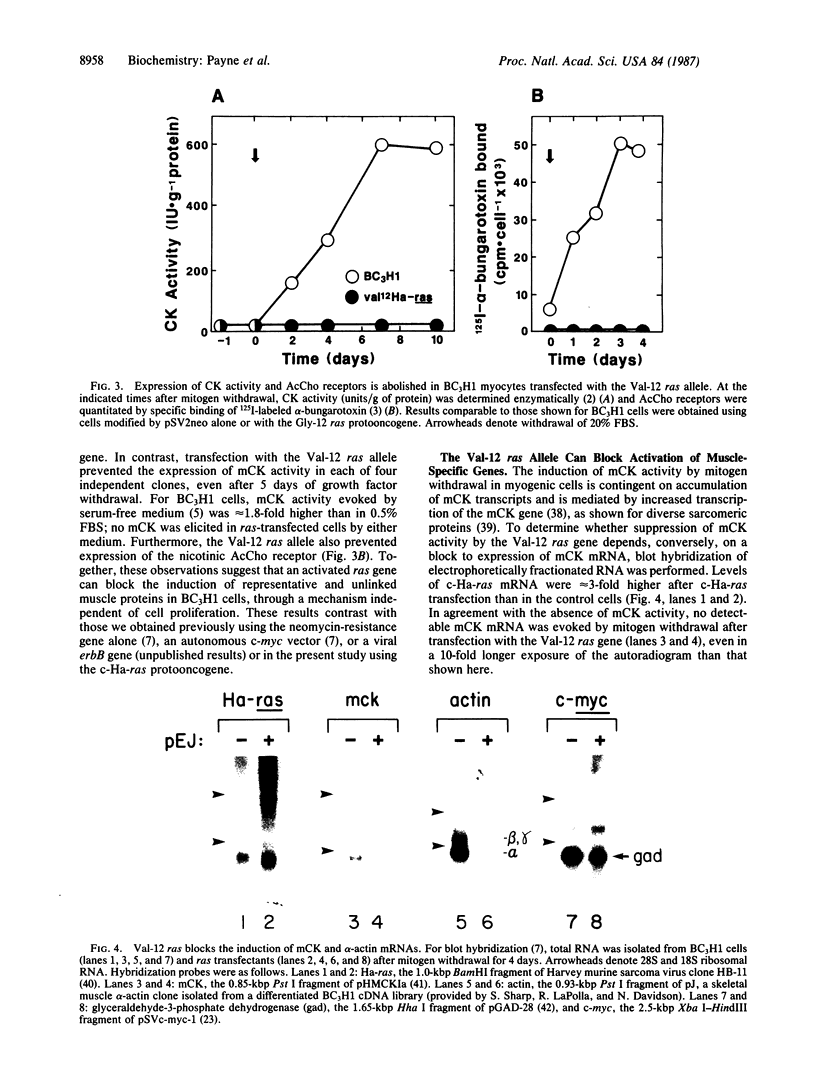
During myogenesis, induction of muscle-specific genes is subject to negative control by polypeptide mitogens and type-beta transforming growth factor. Since transduction of growth factor signals may require proteins encoded by cellular ras oncogenes, we have tested whether a mutationally altered Harvey ras expression vector, by itself, can prevent establishment of a differentiated phenotype in BC3H1 mouse myoblasts. Transfection with the valine-12 allele of the human Harvey ras gene, under the control of its own promoter, was sufficient to prevent the induction of both muscle creatine kinase activity and the nicotinic acetylcholine receptor following mitogen withdrawal but did not inhibit withdrawal from the cell cycle. The loss of creatine kinase activity resulted from a corresponding block to induction of muscle creatine kinase mRNA. Similarly, mitogen withdrawal elicited little or no alpha-actin mRNA in ras-transfected cells. These results suggest that an activated ras allele can inhibit myogenesis through a mechanism independent of cell proliferation and can preclude activation of genes whose up-regulation normally accompanies mitogen withdrawal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Barrieux A., Neeley W. E., Contreras P. Influence of thyroid hormone on the in vitro translational activity of specific mRNAs in the rat heart. J Biol Chem. 1983 Jun 25;258(12):7738–7745. [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Three types of muscle-specific gene expression in fusion-blocked rat skeletal muscle cells: translational control in EGTA-treated cells. Cell. 1987 May 22;49(4):515–526. doi: 10.1016/0092-8674(87)90454-5. [DOI] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone G., Tatò F., Alemà S. Distinctive effects of the viral oncogenes myc, erb, fps, and src on the differentiation program of quail myogenic cells. Proc Natl Acad Sci U S A. 1985 Jan;82(2):426–430. doi: 10.1073/pnas.82.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Gospodarowicz D., Weseman J., Moran J. S., Lindstrom J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J Cell Biol. 1976 Aug;70(2 Pt 1):395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lathrop B., Olson E., Glaser L. Control by fibroblast growth factor of differentiation in the BC3H1 muscle cell line. J Cell Biol. 1985 May;100(5):1540–1547. doi: 10.1083/jcb.100.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschka S. D. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struct. 1980;14(4):483–498. doi: 10.1002/jss.400140407. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Knepper J. E., Butel J. S., Smith J. R., Pereira-Smith O. M. Mitogenic effects of the proto-oncogene and oncogene forms of c-H-ras DNA in human diploid fibroblasts. Mol Cell Biol. 1986 Aug;6(8):2990–2993. doi: 10.1128/mcb.6.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Lindstrom J. Expression of acetylcholine receptor alpha-subunit mRNA during differentiation of the BC3H1 muscle cell line. J Biol Chem. 1984 Mar 10;259(5):3330–3336. [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Sebanne R., Lindstrom J. Regulation of surface expression of acetylcholine receptors in response to serum and cell growth in the BC3H1 muscle cell line. J Biol Chem. 1983 Nov 25;258(22):13946–13953. [PubMed] [Google Scholar]
- Olson E. N., Spizz G., Tainsky M. A. The oncogenic forms of N-ras or H-ras prevent skeletal myoblast differentiation. Mol Cell Biol. 1987 Jun;7(6):2104–2111. doi: 10.1128/mcb.7.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perryman M. B., Kerner S. A., Bohlmeyer T. J., Roberts R. Isolation and sequence analysis of a full-length cDNA for human M creatine kinase. Biochem Biophys Res Commun. 1986 Nov 14;140(3):981–989. doi: 10.1016/0006-291x(86)90732-1. [DOI] [PubMed] [Google Scholar]
- Schmid A., Renaud J. F., Fosset M., Meaux J. P., Lazdunski M. The nitrendipine-sensitive Ca2+ channel in chick muscle cells and its appearance during myogenesis in vitro and in vivo. J Biol Chem. 1984 Sep 25;259(18):11366–11372. [PubMed] [Google Scholar]
- Schneider M. D., Payne P. A., Ueno H., Perryman M. B., Roberts R. Dissociated expression of c-myc and a fos-related competence gene during cardiac myogenesis. Mol Cell Biol. 1986 Nov;6(11):4140–4143. doi: 10.1128/mcb.6.11.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. D., Perryman M. B., Payne P. A., Spizz G., Roberts R., Olson E. N. Autonomous expression of c-myc in BC3H1 cells partially inhibits but does not prevent myogenic differentiation. Mol Cell Biol. 1987 May;7(5):1973–1977. doi: 10.1128/mcb.7.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Sümegi J., Ringertz N. R. Density-dependent arrest of DNA replication is accompanied by decreased levels of c-myc mRNA in myogenic but not in differentiation-defective myoblasts. J Cell Physiol. 1985 Dec;125(3):465–470. doi: 10.1002/jcp.1041250315. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Stern D. F., Roberts A. B., Roche N. S., Sporn M. B., Weinberg R. A. Differential responsiveness of myc- and ras-transfected cells to growth factors: selective stimulation of myc-transfected cells by epidermal growth factor. Mol Cell Biol. 1986 Mar;6(3):870–877. doi: 10.1128/mcb.6.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch A. R., Offord J. D., Chalkley R., Rubenstein P. A. Characterization of actin mRNA levels during BC3H1 cell differentiation. J Biol Chem. 1986 Jan 15;261(2):849–855. [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]