Abstract
Phylogenetic threats such as spiders evoke our deepest primitive fears. When close or looming, such threats engage evolutionarily conserved monitoring systems and defense reactions that promote self-preservation. With the use of a modified behavioral approach task within functional MRI, we show that, as a tarantula was placed closer to a subject's foot, increased experiences of fear coincided with augmented activity in a cascade of fear-related brain networks including the periaqueductal gray, amygdala, and bed nucleus of the stria terminalis. Activity in the amygdala was also associated with underprediction of the tarantula's threat value and, in addition to the bed nucleus of the stria terminalis, with monitoring the tarantula's threat value as indexed by its direction of movement. Conversely, the orbitofrontal cortex was engaged as the tarantula grew more distant, suggesting that this region emits safety signals or expels fear. Our findings fractionate the neurobiological mechanisms associated with basic fear and potentially illuminate the perturbed reactions that characterize clinical phobias.
Keywords: expectancy errors, imminence, distance
Evolutionary pressures have selected for mechanisms that encourage the avoidance of close or looming threat (1–3). In humans, this innate capacity is particularly evident with phylogenetic threats such as spiders, which rate among the most ubiquitous of phobias (4). For example, in everyday life it is common to experience a primal surge of terror when a spider crawls within our personal space, yet we may observe a distant spider without trepidation. In the clinical setting, behavioral approach tasks are used to expose phobic patients to phobogenic stimuli (e.g., spiders or snakes) at varying distances to evaluate and treat their fear responses. Such approaching threats elicit precipitous increases in subjective fear and autonomic arousal (5, 6), which are characteristics of the exaggerated emotional responses observed in phobic subjects.
Research in rodents has shown that encountering a distant or close natural predator (e.g., a cat) evokes distinct defense reactions in the form of behavioral quiescence and vigorous flight, respectively (7). In humans, functional neuroimaging has begun to reveal the brain networks implicated in such situations of immediate danger. One study showed that the active avoidance of an artificial predator with the ability to chase, capture, and inflict pain resulted in brain activity switching from ventromedial prefrontal cortex to the midbrain, including the periaqueductal gray (PAG), as the threat became more imminent (8). Two recent studies have also shown that tracking the increasing proximity of a shock stimulus is associated with activity in the bed nucleus of the stria terminalis (BNST), a region highly connected to the amygdala (9), whereas courage associated with self-administered movement of a snake toward the subject's own head increases activating the ventromedial prefrontal cortex (10). These studies point to an anatomical network involved in the monitoring of threat, the instigation of defensive reactions, and overcoming fear (2, 11–13).
An outstanding issue not addressed in previous studies (e.g., ref. 9) is the extent to which different aspects of threat monitoring linked to this brain network can be fractionated, in particular, whether there is a critical distinction between threat proximity and whether the threat level is on an escalating or descending trajectory. For example, a close threat moving away from us is liable to elicit a different response to an equally close threat moving toward us. Two further unexplored questions include what neural substrates are involved in monitoring any mismatch between threat/fear expectations and actual threat/fear—a crucial mechanism calibrating how the system deals with anticipated aversive events to ensure that such predictions are accurate. Finally, to our knowledge, no studies using phylogenetic threats have examined habituation of the fear system over time and whether greater fear of spiders results in sustained activation in core fear areas such as the midbrain.
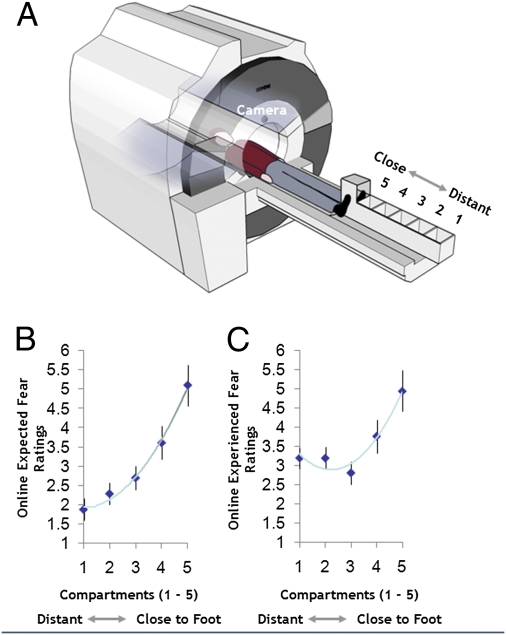
In the present study, we used functional MRI (fMRI) in conjunction with a modified behavioral approach task to examine brain activation associated with these two putative functions of the human fear network as healthy participants viewed a tarantula spider being placed at varying distances from their foot. When in the MRI scanner bore, participants placed their foot into a custom-built “imminence box” containing six compartments separated by sliding partitions (Fig. 1A). Via a video feed, participants watched a live and active tarantula (Lasiodora parahybana; Fig. S1A) placed randomly into five of these compartments at distances of 1 to 18 cm, 19 to 36 cm, 37 to 54 cm, 55 to 72 cm, and 73 to 90 cm from their foot (Fig. S1 B and C). Unbeknownst to participants, they were actually viewing prerecorded films of the spider in the different compartments, which were controlled for the movement of the spider (SI Experimental Procedures). Each experimental trial commenced with viewing a schematic representation of the imminence box indicating which compartment the tarantula would enter next. Participants then used a visual analog scale (VAS) to predict how afraid they would feel when subsequently viewing the tarantula in the indicated compartment (i.e., expected fear). The participants next saw the tarantula in the compartment and directly after, rated how afraid they felt (i.e., experienced fear; Fig. S2). A strength of this behavioral approach task is the fact that the absolute proximity of the phobogenic threat can be decoupled from its trajectory, i.e., whether it is coming closer or moving away, thus making this methodology an ideal test bed for exploring these different components of threat.
Fig. 1.
Experimental paradigm and key behavioral results. (A) Participants were placed supine on the MRI scanner bed and asked to position their foot with the shoe removed into the open-topped imminence box. Participants believed that, via a camera feed, they could observe the experimenter moving the tarantula closer or further away from their foot in real time. The subjects’ tasks were to predict how afraid they believed they would feel (i.e., expected fear) when the spider was placed in a compartment previously indicated to them and then how scared they actually felt (i.e., experienced fear) when viewing the tarantula in that compartment. Mean group ratings for expected fear (B) and experienced fear (C) as a function of tarantula proximity.
With the use of this paradigm, we pursued two primary hypotheses: first, increased proximity of the tarantula would increase subjective fear ratings and progressively invoke the brain's fear network, including the midbrain PAG, amygdala, BNST, striatum, and insula (8, 11), after controlling for whether the tarantula was approaching or retreating. Second, because the amygdala and BNST have been implicated in filtering and monitoring emotionally relevant information (9, 14), we hypothesized that, irrespective of absolute distance, activity in these regions would differentiate the tarantula's approach versus retreat.
A further potentially important function of the fear-based threat processing network is to index when events turn out to be more threatening or fear-inducing than anticipated, not only because such events may require the rapid deployment of additional processing resources, but because such mismatches would need to be registered to calibrate the threat prediction system over time. Inspired by the work of Gray and McNaughton (15), Rachman's match/mismatch theory (16), and attentional theories of amygdala function (17, 18), we took advantage of the current methodology to also explore whether subject-specific differences in the degree of expectancy errors (i.e., the underprediction of the fear response to the tarantula) would be mediated by any elements of the fear network, in particular the amygdala.
Results
How the Human Brain Responds to Absolute Proximity of the Tarantula.
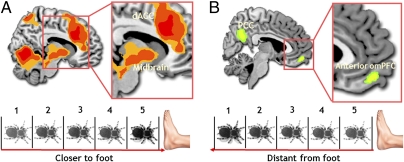
We first examined fear response as a function of the tarantula's absolute proximity, controlling for whether the tarantula was approaching or retreating. VAS-experienced fear ratings were progressively higher with increased proximity (Fig. 1C; repeated-measures ANOVA, F1.8 = 20.5; P = 0.0001, Greenhouse–Geisser corrected for nonsphericity). VAS-experienced fear also correlated with mean Fear of Spider Questionnaire (FSQ) ratings (Spearman r, 0.405; P < 0.038). For the brain imaging data, we computed the difference between the two nearest compartments and two most distant compartments from the participant's foot: (compartment 5 + compartment 4) − (compartment 1 + compartment 2). Supporting our hypothesis, for this contrast indexing increased proximity, we observed augmented activity in the bilateral amygdala, midbrain PAG, ventral striatum, BNST, bilateral anterior insula, and dorsal anterior cingulate cortex [dACC; all P < 0.05, family-wise error (FWE) small volume corrected (svc) using independent coordinates]. For the reverse contrast, we found increased activity in the orbitomedial prefrontal cortex (omPFC; Table S1).
To further quantify the effect of spider proximity, we conducted a parametric regression analysis. As the tarantula was placed closer to the participant's foot (using compartments 1–5 as parametric weights), we again observed increased activity in the bilateral insula, BNST, dACC, ventral striatum, and midbrain (Fig. 2A). With increased tarantula distance, increased activity in omPFC and posterior cingulate cortex was observed (Fig. 2B and Table S2). The degree of increase in midbrain PAG activity with greater proximity was also positively correlated with subject-specific scores on the FSQ (19) and with ratings of the tarantula being scarier than anticipated (Tables S3 and S4).
Fig. 2.
(A) Parametric increases and decreases in BOLD signal. Increased proximity was associated with activity in the midbrain PAG (4, −30, −24; P < 0.028, svc) and dACC (28, 36, 24; P < 0.001, whole-brain corrected). (B) Increased spider distance was associated with a parametric increase in activity in the omPFC (−6, 54, −16; P < 0.016, svc).
Monitoring Approach Versus Retreat of the Tarantula.
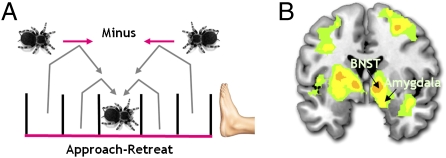
We next investigated the effects of an approaching or looming spider by comparing trials wherein the tarantula was approaching versus retreating from the foot, relative to the previous trial, and irrespective of absolute proximity. For example, for compartment 3, we compared approach to retreat by contrasting those trials in which the previous trial position was 1 or 2 compartments further from the foot (i.e., compartments 1 or 2) versus trials in which the previous position was 1 or 2 compartments closer to the foot (i.e., compartments 4 or 5; Fig. 3A). Independent of absolute proximity, experienced fear ratings were significantly higher when the tarantula approached versus retreated (Wilcoxon Z, −0.3; P = 0.005), as was activity in the right amygdala, bilateral BNST, ventral striatum, and bilateral insula (Fig. 3B). These regions remained significantly activated after covarying out subject-specific FSQ scores (which correlated with the approach minus retreat contrast; r = 0.523; P = 0.009; Table S5) and suggest that the amygdala and BNST keep track of the aversive value of the tarantula via its direction of movement, independent of how close it is.
Fig. 3.
Brain activity for the retreat minus approach contrast. (A) An example of how approach/retreat was quantified. BOLD signal was taken from, for example, compartment three depending on whether the tarantula had moved forward or backward from a previous compartment. Independent of any distance effects, the (B) amygdala (14, −2, −16; P < 0.024, svc) and BNST (right, 12, 0, −4; P < 0.027, svc; left, −12, 4, −4; P < 0.014, svc) were differentially more active for approach versus retreat of the tarantula.
Expectancy Errors: Subject-Specific Differences in Underestimating the Fear Value of the Tarantula.
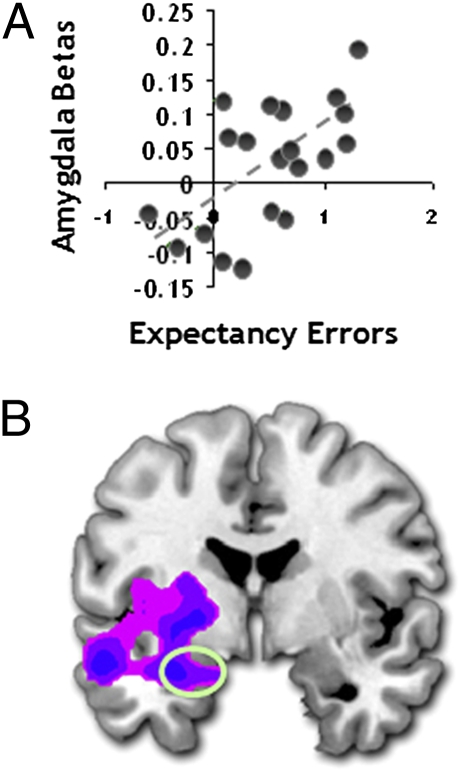
We finally explored the neural substrates of individual tendencies to underestimate how much fear was actually experienced when viewing the tarantula at different proximities (i.e., the degree to which fear on seeing the tarantula exceeded expectations). As with experienced fear scores, expected fear VAS ratings increased with greater proximity (Fig. 1B; F1.3 = 44.5; P = 0.0001, Greenhouse–Geisser corrected) after controlling for tarantula approach/retreat. We also found that postscan ratings of how much scarier the spider was than expected correlated with how large subjects estimated the tarantula to be (r = 0.413; P = 0.035). Expectancy errors—the amount that fear was actually underestimated (calculated by subtracting expected fear ratings from experienced fear reports (16, 19)—were positively correlated with FSQ scores (r = 0.564; P = 0.005), a finding closely allied with theories from clinical research (20–22). The brain imaging data showed that greater subject-specific differences in expectancy errors correlated with greater left amygdala, ventral striatum, and right insula activation. The left amygdala association remained significant after covarying out experienced fear ratings, suggesting that it is independent of the degree of fear experienced when viewing the spider (Fig. 4A). Moreover, this left amygdala relationship was still significant after covarying out FSQ scores (Fig. 4B), supporting the idea that the activity associated with underestimating one's fear responses is not driven by a general fear of spiders (Table S6).
Fig. 4.
Subject-specific differences in expectancy errors and amygdala activity. (A) Increased amygdala activity (−20, 0, −18; P < 0.043, svc) associated with more marked under-prediction of forthcoming fear experience and (B) activity in the amygdala associated with under-prediction of fear after covarying ratings of experienced fear (−26, −6, 18; P < 0.004, svc).
Habituation Effects over the Course of the Experiment.
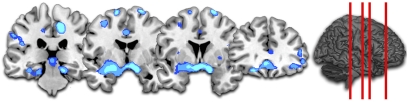
To examine habituation effects over the course of the experiment, we divided the experimental session into four time bins and examined the decreases in fear system response over time. Each time bin, 1 through 4, was used as a parametric weight. Trials across each time bin were matched for spider distance, thus permitting an independent analysis of the effects of time. The brain imaging data showed a general decrease in activity in the fear network including the midbrain PAG, amygdala, insula, and ventral medial PFC (Fig. 5 and Table S8). Conversely, medial PFC activity increased over the course of the experiment (Table S8). Subjective ratings of expected fear decreased over time to a greater extent than experienced fear ratings, thus leading to a mean increase in expectancy errors with time (Fig. S3A). We found that subject-specific differences in this increase in expectancy errors correlated with augmented amygdala activity over time (Fig. S3B), mirroring the previous analysis of expectancy errors (Fig. 3 B and C). We finally examined whether habituating activation would be attenuated in any elements of the fear circuitry by a greater general fear of spiders (e.g., FSQ scores). We found that higher subject-specific FSQ scores were correlated with relatively sustained activation encompassing the midbrain PAG (Fig. S3C).
Fig. 5.
Habituation analysis. Coronal slices moving anterior from the midbrain to the PFC. Blue clusters indicate significantly decreased activity over the course of the experiment in the PAG (12, −32, −14; P < 0.029, svc) and amygdala (−24, −2, −14; P < 0.029, svc).
Discussion
The ubiquitous nature of arachnophobia suggests such fear is shaped by our evolutionary history (19) and that spiders are therefore a potent stimulus with which to interrogate the functioning of the human fear network. As a result of the intense nature of our study, we used subjects with medium to low fear of spiders. Nevertheless, we clearly demonstrate that perceived greater proximity of a live tarantula is associated with both marked subjective fear and pronounced activation in a network of interconnected brain circuitry (11), including the amygdala, BNST, insula, striatum, and midbrain PAG—regions implicated in responses to intense fear-evoking events and fitness-promoting expressions of fear (2, 11, 12). Moreover, our results suggest that the amygdala and BNST play a key role in monitoring whether the threat value of the tarantula is on an escalating versus descending trajectory, as indexed by its direction of movement. Finally, the amygdala may augment attention toward the tarantula when the subject underestimated their expected fear levels, supporting mismatch theories of fear (22).
How the Fear Circuitry Responds to the Close and Distant Tarantula.
A prodigious amount of research suggests that the midbrain PAG is the centerpiece of intense fear and hard-wired threat reactions (2, 8, 13). In the present investigation, midbrain PAG activation was associated with greater tarantula proximity and positively correlated with elevated trait fear of spiders (FSQ scores), suggesting this region comes online when the perceived threat is more extreme. Although no studies have explored the role of the PAG in ecologically valid phylogenetic fears, the current study compares favorably with research showing that stimulation of human PAG results in extreme expressions of fear and panic (23). Despite overall habituation of the fear circuitry (Fig. 5 and Table S7), midbrain PAG activation in the current study remained relatively sustained over the course of the experiment in those subjects with elevated fear of spiders (e.g., higher FSQ scores; Fig S4C and Table S9). Together, our findings suggest that the PAG may be involved in the exaggerated fear observed in phobic subjects and that such fear levels are associated with mitigation of habituation effects.
In contrast to the circuitry activated by tarantula proximity, the omPFC was increasingly active as a function of the tarantula's increased distance. The omPFC projections to the PAG are believed to form a pathway involved in passive coping (e.g., freezing) associated with contextually distant threat, which prompts the inhibition of motor behavior and down-regulation of autonomic arousal (24). Ablation of the nonhuman primate and squirrel omPFC homologues reduces fear reactivity to phylogenetic threats (25, 26). In human arachnophobic subjects, habituated fear to spider pictures has been associated with augmented omPFC activity (27). Conceptually, the omPFC may function to simply suppress primitive fear responses (28), thereby permitting strategic adaptive behaviors when danger is at a safe distance.
Monitoring the Tarantula's Movements.
A number of forebrain components of this fear network, most notably the amygdala, BNST, insula, and striatum, were selectively more active when threat levels were on an escalating trajectory, as indexed by comparing tarantula approach versus retreat, irrespective of absolute distance. In an early study, it was shown that rhesus monkeys respond to such approaching or “looming” objects with fear (1) and recent evidence has shown that looming sounds activate the human amygdala (29), suggesting that the brain is primed to perceive looming as a warning signal. With its intricate connections, the amygdala is perfectly situated to code the oscillating value of external threat and for surveying the sensory landscape for potential danger (30). Indeed, similar mechanisms have been observed in both nonhuman and human primates in which the amygdala keeps track of appetitive (14) and social stimuli (31). Intriguingly, theorists have argued that forward movement (i.e., looming) magnifies the sense of danger and this is be magnified in phobic subjects, leading to distortions in threat magnitude (32).
Several observations of interest have been made concerning the BNST. The BNST is strongly connected to the central nucleus of the amygdala and is believed to act as a relay center for coordinating motor, autonomic, and defense reaction (33). Recently, the BNST has been implicated in monitoring signals representing escalating threat levels in the environment (9, 17, 34). Although the literature is limited, the BNST has also been implicated in phobia. For instance, one study conducted on spider-phobic subjects showed marked activity in the BNST during the anticipation of spiders in pictures. Despite not using real spiders, this study suggests that spider-phobic subjects are hyper-vigilant to the spider's imminent presence (35). These accumulated findings suggest that the amygdala and BNST keep track of escalating threat levels, independent of threat proximity, and signaled in a variety of ways including by direction of threat movement.
Individual Differences in Underestimating the Fear Value of the Tarantula.
In line with consistency (36) and match/mismatch (20) theories, we observed greater amygdala activity when the tarantula elicited fear that exceeded participants’ prior expectations (i.e., expectancy errors). Although correlational, and thus preliminary, these results are consistent with prior evidence that phobic subjects exhibit larger fear responses when fear is underestimated (20). Registering situations in which threat is disproportionate to one's predictions is clearly important in terms of the processing resources allocated to the threat and for longer-term calibration of threat-prediction algorithms. Indeed, theorists posit that such mismatches drive the organism into “control mode” (15), which engenders elevated attention, avoidance, and anxiety toward the threat—operations believed to be dependent on the amygdala (2, 37). Intriguingly, greater expectancy errors were also correlated with increased misperception of the size of the tarantula. This suggests one mechanism by which fear mediates the cognitive biases surrounding threat that are exhibited by phobic subjects (38).
Conclusions.
Our results provide direct evidence of how the human brain monitors and responds to changing proximity of phylogenetic threat. The findings confirm that phylogenetic threats activate similar fear pathways to ontogenetic threats (e.g., shocks) (8), showing a cascade of fear systems that extend from the omPFC to the midbrain PAG. Consistent with theoretical models, we show that the amygdala and BNST are sensitive to escalation of threat levels in terms of the tarantula's direction of movement, independent of proximity. The amygdala was also active to the magnitude of expectancy errors, thus supporting the role of this region in coordinating responses to unexpected threat (2, 20). Identifying how the brain responds to changing intensities of phylogenetic threat may be one fertile source of understanding the exaggerated fear observed in specific phobic subjects.
Experimental Procedures
Participants.
Twenty-five healthy nonphobic volunteers took part in the study. Subjects were rejected if they had any history of neurological damage or psychiatric disorder. After the MRI scan, five participants were excluded as they expressed reservations about whether the study was genuinely in real time during debriefing. This left 20 participants (10 female; mean age, 25.8 ± 3.7 y). Trait anxiety was measured using the Spielberger questionnaire, with a mean score of 40 ± 9.9. These scores are comparable to the published norms for this age group mean (36 ± 10) (39). Subjects were remunerated £30 for time, travel, and inconvenience. All subjects gave informed consent, and the study was approved by the Essex Research Ethics Committee (United Kingdom).
Spider Stimuli Creation and Validation.
We recorded footage of a Brazilian salmon pink tarantula (body size, 22 cm length × 15 cm width; Fig. S1A) being placed in each compartment of the imminence box. Video clips were edited to 4 s and a border was added to the footage to decontextualize the environment. Sixty black-and-white 4-s film clips of the tarantula were then presented in the study (12 in each compartment of the imminence box). Each film was prerated for movement of the tarantula on a four-point scale: 1, no movement; 2, very little movement (<25% of the time); 3, movement 25% to 50% of the time; and 4, movement 50% to 100% of the time. Ratings were made by 10 independent observers. No significant differences were found between compartments (P > 0.05).
There was also no significant relationship between participants’ experienced fear ratings and movement (r = 0.211, P = 0.105). In addition, we tested to see if the direction the spider was facing (toward versus away from the foot) influenced fear ratings. We found no significant correlation between the tarantula's angle of orientation to the foot, from 0° to 180°, and fear ratings (P > 0.05). Finally, mindful of likely habituation effects, we pseudorandomized the presentation of the tarantula in each box so as to not correlate distance from time (Pearson correlation, P < 0.495).
Image Acquisition.
MRI scanning was conducted at the Medical Research Council Cognition and Brain Sciences Unit on a 3-T Tim Trio MRI scanner (Siemens) by using a head coil gradient set. Whole-brain data were acquired with echoplanar T2*-weighted imaging [i.e., echoplanar imaging (EPI)], sensitive to blood oxygen level-dependent (BOLD) signal contrast (48 sagittal slices, 3-mm thickness; repetition time, 2,400 ms; echo time, 30 ms; flip angle, 78°; field of view, 192 mm; voxel size, 3 × 3 × 3 mm). To provide for equilibration effects, the first five volumes were discarded. T1-weighted structural images were acquired at a resolution of 1 × 1 × 1 mm.
Image Preprocessing.
SPM5 software (www.fil.ion.ucl.ac.uk/spm/) was used for data analysis. The echoplaner imaging (EPI) images were sinc interpolated in time for correction of slice timing differences and realignment to the first scan by rigid body transformations to correct for head movements. For each participant, the mean EPI was calculated and examined to guarantee that none exhibited excessive signal dropout in insula and ventral striatum. Using linear and nonlinear transformations, and smoothing with a Gaussian kernel of full-width-half-maximum 8 mm, EPI and structural images were coregistered and normalized to the T1 standard template in Montreal Neurological Institute (MNI) space (International Consortium for Brain Mapping). Moreover, global changes were removed by proportional scaling, and high-pass temporal filtering with a cutoff of 128 s was used to remove low-frequency drifts in signal.
Statistical Analysis.
After preprocessing, statistical analysis was performed by using the general linear model. Our regression matrix included the cue periods (1 s), both expectancy (8 s) and outcome VAS (8 s) time periods, and the 4-s period when the spider was shown in the relevant box. Analysis was carried out to establish each participant's voxel-wise activation during the 4-s presentation of the spider. Activated voxels in each experimental context were identified using an event-related statistical model representing each of the experimental contexts, convolved with a canonical hemodynamic response function and mean-corrected. Six head-motion parameters defined by the realignment were added to the model as regressors of no interest. Multiple linear regression was then run to generate parameter estimates for each regressor at every voxel. For group analysis, a random-effects model was used with a small volume correction for FWE within a priori regions of interest (ROIs) including the amygdala, BNST, medial orbital frontal cortex, and midbrain PAG. Outside of these ROIs, we also present results at P < 0.05, FWE corrected for whole-brain multiple spatial comparisons. When false negative results at these corrected thresholds would be of particular relevance, we also provide results at the exploratory uncorrected threshold P < 0.001 (SI Experimental Procedures).
Questionnaires.
Following the MRI scan, participants were asked to complete the FSQ (40). These mean FSQ scores were in medium to low ranges (mean ± SD, 30.7 ± 18.4; range, 14–86.1). Overall, this mean score is significantly lower than that of diagnosed arachnophobic subjects (89.1 ± 19.6) (41). Subjects were also asked to rate how much scarier than expected the spider was. We also asked subjects to complete a memory for tarantula size test to rate how large they thought the tarantula was by using five different sizes of the spider printed on an A3 sheet of paper (Fig. S4). This was administered between 45 and 60 min after the experiment.
Supplementary Material
Acknowledgments
We thank Simon Strangeways, Mark Townsend, and Gary Chandler for creation of hardware and help with data acquisition. We also thank Ray Dolan and Chris Frith for advice and Jay Wood of the Spider Diaries (www.thespiderdiaries.co.uk). This work was funded by the UK Medical Research Council and Wellcome Trust Grant 077029 (to J.B.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009076107/-/DCSupplemental.
References
- 1.Schiff W, Caviness JA, Gibson JJ. Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science. 1962;136:982–983. doi: 10.1126/science.136.3520.982. [DOI] [PubMed] [Google Scholar]
- 2.McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, NJ: Erlbaum; 1988. pp. 185–211. [Google Scholar]
- 4.Fredrikson M, Annas P, Fischer H, Wik G. Gender and age differences in the prevalence of specific fears and phobias. Behav Res Ther. 1996;1:33–39. doi: 10.1016/0005-7967(95)00048-3. [DOI] [PubMed] [Google Scholar]
- 5.Teghtsoonian R, Frost RO. The effects of viewing distance on fear of snakes. J Behav Ther Exp Psychiatry. 1982;13:181–190. doi: 10.1016/0005-7916(82)90002-7. [DOI] [PubMed] [Google Scholar]
- 6.Craske MGM, Mohlman J, Yi J, Glover D, Valeri S. Treatment of claustrophobias and snake/spider phobias: Fear of arousal and fear of context. Behav Res Ther. 1995;33:197–203. doi: 10.1016/0005-7967(94)p4441-v. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard RJ, Blanchard DC. In: Anxiety. McNaughton N, Andrews G, editors. Dunedin, New Zealand: Otago Univ Press; 1990. pp. 24–33. [Google Scholar]
- 8.Mobbs D, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nili U, Goldberg H, Weizman A, Dudai Y. Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron. 2010;66:949–962. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Price JL. Free will versus survival: Brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 12.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford Univ Press; 1998. [Google Scholar]
- 13.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 14.Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septohippocampal System. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 16.Rachman S, Lopatka C. Match and mismatch of fear in Gray's theory—II. Behav Res Ther. 1986;24:395–401. doi: 10.1016/0005-7967(86)90004-5. [DOI] [PubMed] [Google Scholar]
- 17.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 18.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 19.Coelho CM, Purkis H. The origins of specific phobias: Influential theories and current perspectives. Rev Gen Psychol. 2009;13:335–348. [Google Scholar]
- 20.Rachman S, Lopatka C. Match and mismatch in the prediction of fear—I. Behav Res Ther. 1986;24:387–393. doi: 10.1016/0005-7967(86)90003-3. [DOI] [PubMed] [Google Scholar]
- 21.Gursky D, Reiss S. Identifying danger and anxiety expectancies as components of common fear. J Behav Ther Exp Psychiatry. 1987;18:317–324. doi: 10.1016/0005-7916(87)90045-0. [DOI] [PubMed] [Google Scholar]
- 22.Rachman S, Arnzt A. The overprediction and underprediction of pain. Clin Psychol Rev. 1991;11:339–355. [Google Scholar]
- 23.Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;30:14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- 24.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 25.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 26.Ennis M, Coss RG. Orbital frontal cortex ablations of rock squirrels (Spermophilus variegatus) disinhibit innate antisnake behavior. Behav Neurosci. 2006;120:1299–1307. doi: 10.1037/0735-7044.120.6.1299. [DOI] [PubMed] [Google Scholar]
- 27.Hermann A, et al. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schienle A, Schäfer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and symptom reduction in spider phobia. Eur Arch Psychiatry Clin Neurosci. 2007;257:486–493. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- 29.Bach DR, et al. Rising sound intensity: an intrinsic warning cue activating the amygdala. Cereb Cortex. 2008;18:145–150. doi: 10.1093/cercor/bhm040. [DOI] [PubMed] [Google Scholar]
- 30.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nat Neurosci. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riskind JH, Moore R, Bowley L. The looming of spiders: the fearful perceptual distortion of movement and menace. Behav Res Ther. 1995;33:171–178. doi: 10.1016/0005-7967(94)e0023-c. [DOI] [PubMed] [Google Scholar]
- 33.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 35.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Shepperd JA, Mcnulty JK. The affective consequences of expected and unexpected outcomes. Psychol Sci. 2002;13:85–88. doi: 10.1111/1467-9280.00416. [DOI] [PubMed] [Google Scholar]
- 37.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 38.Riskind JH, Williams NL, Joiner TE., Jr The looming cognitive style: A cognitive vulnerability for anxiety disorders. J Soc Clin Psychol. 2006;25:779–801. [Google Scholar]
- 39.Spielberger CD. Manual for the State Trait Anxiety Inventor. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 40.Szymanski J, O'Donohue W. The fear of spiders questionnaire. J Behav Ther Exp Psychiatry. 1995;26:31–34. doi: 10.1016/0005-7916(94)00072-t. [DOI] [PubMed] [Google Scholar]
- 41.Muris P, Merckelbach H. A comparison of two spider fear questionnaires. J Behav Ther Exp Psychiatry. 1996;27:241–244. doi: 10.1016/s0005-7916(96)00022-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.