Abstract
In recent years, Drosophila melanogaster has emerged as a powerful model for neuronal circuit development, pathology, and function. A major impediment to these studies has been the lack of a genetically encoded, specific, universal, and phenotypically neutral marker of the somatodendritic compartment. We have developed such a marker and show that it is effective and specific in all neuronal populations tested in the peripheral and central nervous system. The marker, which we name DenMark (Dendritic Marker), is a hybrid protein of the mouse protein ICAM5/Telencephalin and the red fluorescent protein mCherry. We show that DenMark is a powerful tool for revealing novel aspects of the neuroanatomy of developing dendrites, identifying previously unknown dendritic arbors, and elucidating neuronal connectivity.
To discover neuronal circuit architecture, genetic tools that specifically mark the pre- and postsynaptic cells and compartments are necessary. Drosophila is a leading genetic model organism in this regard; however, most neuronal circuits remain unmapped. Of particular note is the lack of a universal, phenotypically neutral, and specific marker of the somatodendritic and postsynaptic compartments. Several molecular differences between dendrites and axons, including the presence of different membrane and cytoskeletal proteins in neuronal subregions, have been identified (1, 2). Drosophila neurons exhibit the major kinds of compartmentalization present in mammalian neurons and the fly has emerged as a powerful system to study the establishment and maintenance of neuronal connections (3, 4). Almost all studies of neuronal circuits in the fly have relied on genetic markers such as CD8::GFP that outline the morphology of entire cells rather than particular subcellular compartments (5), as well as presynaptic markers such as Synaptotagmin, Synaptobrevin, and Bruchpilot GFP fusion proteins (6–11). However, more accurate identification and mapping of novel neuronal circuits has been hampered by the lack of a genetically encoded and phenotypically neutral dendritic marker. Over the years, many such markers have been proposed and several were recently examined (12), namely MAP2 (13, 14), Nod::YFP (4, 15–18), Homer::GFP (19), and DSCAM17.1::GFP (20, 21). The analysis of these markers reveals that none of them labels the entire somatodendritic field. Furthermore, it remains unclear whether the markers tested are neutral with respect to dendritic morphology.
Intercellular adhesion molecules (ICAMs) mediate neuronal migration, axon elongation, and fasciculation, synaptogenesis, and synaptic plasticity (22). ICAM5, or Telencephalin, is a 130-kDa type I transmembrane glycoprotein comprising a characteristic extracellular domain, a single transmembrane region, and a short cytoplasmic region (23). The expression of ICAM5 is restricted to the mammalian brain telencephalon (24) but there is no homolog in invertebrates and lower vertebrates. The developmental appearance of ICAM5 parallels the time of dendritic elongation, branching, and synapse formation and ICAM5 is described to selectively target dendrites (25) to endow their protrusions with plastic properties (26).
In this work we show that an ICAM5 fusion protein has no detectable effect on neuronal morphology and function and that it is specifically and highly enriched in the somatodendritic compartment of both peripheral nervous system (PNS) and CNS neurons. Next, we use this marker to gain insights into dendrite development, identify dendritic compartments, and map neuronal connections.
Results
DenMark Fusion Protein Mimics ICAM5 Distribution in Mammalian CNS Neurons.
In mature mammalian hippocampal neurons, ICAM5 specifically marks the somatodendritic compartment as well as the postsynaptic sites where axonal termini form synaptic contacts (Fig. S1 A–A′′). An ICAM5–mCherry fluorescent fusion protein was created by inserting mCherry between the transmembrane domain and the extracellular domain of ICAM5 (Fig. S1A′′′ and S1B′ and Fig. S2). Like endogenous ICAM5, this fusion construct is a specific dendritic marker (henceforth DenMark) in mature cultured hippocampal neurons (Fig. S1B).
To establish the dynamics of dendritic compartmentalization, we traced the localization of DenMark in rat hippocampal neurons at different days in vitro (DIV). Shortly after hippocampal neurons become morphologically polarized, both microtubule-associated protein 2 (MAP2) and Tau are present uniformly throughout the cell. They become gradually restricted to their respective compartments after 1 wk in culture at the time where hippocampal neurons are known to have already established one axon and several dendrites (27–29). In mature neurons, MAP2 is preferentially localized to dendrites, whereas Tau labels axons. We examined DenMark in rat hippocampal neurons in culture at different stages of neurite outgrowth at 1, 3, 5, and 10 DIV, using MAP2 and Tau as dendritic and axonal markers, respectively (Fig. S3). We find that DenMark mimics MAP2 in that it is initially expressed in both axons and dendrites (1 DIV, Fig. S3 A–E) and shows progressive dendritic enrichment at 3 and 5 DIV (Fig. S3 F–O). By 10 DIV, both markers are exclusively dendritic (Fig. S3 P–T). Importantly, however, DenMark appears to completely fill the dendritic compartment, whereas MAP2 is localized to the main dendritic shaft. Interestingly, the most proximal axonal segment behaves more like the dendritic branches than the rest of the axon in terms of the distribution of the markers examined in that it is increasingly enriched for DenMark and MAP2 and depleted from Tau (Fig. S3 F–O).
DenMark as a Neutral and Specific Somatodendritic Marker in Drosophila.
Although ICAM5 has no obvious homologs in the Drosophila genome, we wondered whether it would nevertheless constitute a useful somatodendritic marker in Drosophila. A somatodendritic marker needs to satisfy a number of criteria to be deemed broadly useful. First, it is desirable that the expression of this marker has no significant effect on neuronal morphology and as little effect as possible on neuronal function. Second, it should unambiguously distinguish dendritic from axonal compartments in mature neurons. Third, it should show this specificity independently of neuronal subtype. DenMark expression in the entire nervous system throughout fly development and adult life gives rise to viable and fertile flies. Physiological analyses in the retina (Fig. S4A) and tests for motor behavior show no defects (Fig. S4B). In contrast, pan-neuronal expression of DSCAM17.1::GFP, an existing Drosophila dendritic marker (12), results in complete developmental lethality (n = 250).
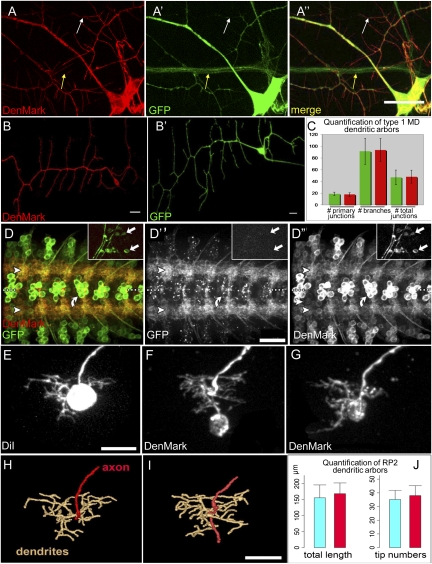
Next, we asked whether DenMark satisfies the criteria of specific localization, general applicability, and phenotypic neutrality at the level of dendritic morphology. To this end, we first focused on two types of larval neurons: multidendritic (MD) sensory neurons and embryonic motor neurons. MD neurons show extensive and well-characterized dendritic arborizations, making them an ideal model to study dendrite morphogenesis (30–32). We first used the GAL4109(2)68 driver in combination with UAS–mCD8::GFP and UAS–DenMark and examined subcellular localization of DenMark expression in third instar larvae. High levels of red fluorescence were detected exclusively in the soma and dendrites of the MD neurons (Fig. 1 A–A′′, white arrow), whereas GFP marked the entire neuron (Fig. 1 A–A′′, yellow arrow). Interestingly, we noted that the full extent of the dendritic arbor as well as small spine-like structures are significantly more visible with DenMark than with GFP. We then tested whether DenMark expression in these cells causes significant alterations to their dendrite morphology, in particular their degree of branching. To facilitate the phenotypic analysis of MD dendritic branches, we restricted either DenMark or mCD8::GFP expression to three MD neurons per segment using the type I MD neuron-specific GAL4221 line and quantified the stereotypical branching of the ventral dendritic arborization (vpda) type I neurons. Two different quantification approaches revealed no significant differences in number of branches and number of junctions between DenMark and mCD8::GFP expressing neurons (Fig. 1 B and C and Table S1).
Fig. 1.
DenMark is a neutral-specific somatodendritic marker in the Drosophila larval PNS and CNS. (A) Multidendritic neurons in the third instar larval body wall. Neurons were labeled by crossing GAL4 109(2)-68, UAS–mCD8::GFP to UAS–DenMark flies. (A) DenMark expression, (A′) GFP expression and (A′′) merge. DenMark and GFP only colocalize in dendrites and cell bodies and not in axons. DenMark appears in spine-like structures, where GFP is weakly present or not at all (white arrow). The axon is marked only with GFP (yellow arrow). Z-projections of confocal image stacks. (Scale bar, 15 μm.) (B) Class I MD neurons (GAL4221 driver) were genetically labeled with DenMark (B) or mCD8::GFP (B′), respectively. Shown are Z-projections of confocal image stacks of vpda neurons. (Scale bars, 50 μm.) (C) Green bars represent the average of the quantifications perfomed in the neurons where GAL4221 drives mCD8::GFP, and red bars represent the same for the neurons where GAL4221 drives DenMark. Quantification of the average number of primary branch points in each genotype (n = 18) reveals no significant differences (Student's T-test, P = 0.209) between the two genotypes. Furthermore the quantification of total number of branches and total number of junctions/branch points (green n = 27; red n = 24) also does not reveal any significant difference (Student's T-test, P = 0.71 and P = 0.74, respectively). (D) Glutamatergic motor and interneurons (marked with OK371–GAL4) in abdominal nerve cord segments were doubly labeled with UAS–mCD8::GFP and UAS–DenMark and imaged at the first larval instar stage. The images are Z-projections of confocal image stacks. In contrast to mCD8::GFP, which labels these neurons homogeneously, the DenMark reporter is highly concentrated in this region (arrowheads), with relatively little present in cell bodies (curved arrow). DenMark does not label distal axons and or presynaptic neuromuscular junctions in the periphery (inset, arrows point to boutons located on muscles VL3-4 and VL2). (Scale bar, 20 μm.) (E–J) DenMark expression in the RP2 motor neurons has no obvious effect on dendrite development. RP2 neurons were labeled either manually with DiI (E) or genetically with UAS–DenMark (F and G) in freshly hatched first instar larvae (20–21 h after egg laying, raised at 29 °C). Dendritic trees were reconstructed digitally (H and I) from confocal image stacks using customized modules from refs. 52, 56 [to obtain quantitative data on total dendritic tree length and number of dendritic tips (end segments)] (Scale bar, 10 μm.) Dendritic trees of RP2 neurons labeled with DenMark show a normal localization in the neuropile and do not differ significantly (Student's T-test) in either total tree length (P = 0.5299) or tip number (P = 0.4565) from manually labeled control RP2 neurons (J; blue, control DiI labeled; red, DenMark labeled; SD is shown; n = 7 for each).
Next, we used the OK371–GAL4 driver line to express DenMark in embryonic and larval glutamatergic motor neurons, a well-defined and frequently used model system. Motor neuron dendrites extend in the CNS where they form a metamerically repeated myotopic map, whereas the axons innervate body wall muscles at the level of the neuromuscular junctions. We find that whereas mCD8::GFP homogeneously labels the membrane of the entire neuron, DenMark is highly concentrated in the dendrites, with fluorescent signal also detected in cell bodies (Fig. 1 D–D′′). Although some DenMark expression was also detected in the proximal portion of the axons, as previously described in young rat hippocampal neurons (Fig. S3 F–O), no DenMark was observed more distally in axons and at the presynaptic neuromuscular junctions (Fig. 1 D–D′′, insets). Finally, we examined the effects of DenMark expression on the dendrites of RP2 motor neurons. In freshly hatched first instar larvae (21 h after egg laying [AEL]), RP2 neurons were labeled either manually with DiI (Fig. 1E) or genetically with UAS–DenMark (Fig. 1 F and G). Dendritic trees of RP2 neurons labeled with DenMark present normal localization in the neuropile and do not differ significantly from manually labeled control RP2 neurons in both total tree length or tip number (Fig. 1 H–J).
DenMark Expression Labels the Entire Dendritic Arbor.
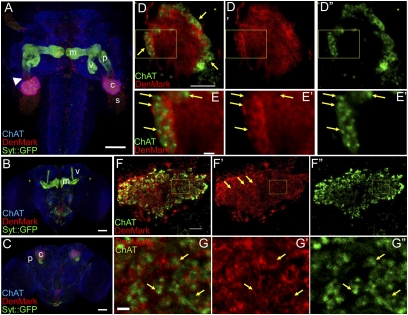
In mammalian neurons DenMark labels the entire dendritic arbor, including the postsynaptic sites. We turned to the Drosophila mushroom bodies (MBs) to test whether this might be the case in flies. MBs are paired neuropile structures involved in olfactory learning and memory in insects. In adult Drosophila, they are composed of about 2,000 intrinsic neurons, termed Kenyon cells (KCs) (33). Each KC extends a single neurite, which bifurcates close to the cell body into dendritic and axonal branches. The axonal branch navigates anteroventrally and branches into medial and vertical lobes. The dendrite ends in the calyx, where it receives inputs, in particular from the cholinergic olfactory projection neurons (PNs). We used the MB 201Y–GAL4 driver line to express both DenMark and synaptotagmin::GFP (syt::GFP), a presynaptic genetically encoded marker (10) and examined their distribution in larval and adult MBs. In both third instar larval and adult MBs DenMark and syt::GFP show a mutually exclusive localization in the MB neuropile. DenMark expression strongly predominates in the calyx and the very proximal region of the axon. In contrast, syt::GFP expression is mostly restricted to axons (Fig. 2 A–C). To quantify the enrichment of DenMark in the calyx, we coexpressed DenMark and the membrane-associated marker mCD8::GFP using 201Y–GAL4. We postulated that CD8::GFP expression level is homogenous in the entire neuron. Therefore, we used mCD8::GFP to normalize the DenMark expression level and quantified the ratio of the DenMark signal to the GFP signal in the calyx and the lobes of larval MBs (see Materials and Methods for details). We detect a 32-fold enrichment of DenMark expression level in the calyx as compared with the lobes (n = 10).
Fig. 2.
DenMark is enriched at putative postsynaptic structures in larval and adult mushroom bodies. (A) 201Y–GAL4 drives expression of DenMark and Syt::GFP in third instar larva mushroom body neurons (horizontal view, anterior is at the Top). DenMark expression is restricted to the calyx, the somas, and the very proximal portion of the peduncle (arrowhead), whereas Syt::GFP is found in the distal portion, the peduncle, and in median and vertical lobes. (B and C) DenMark and Syt::GFP expression in adult mushroom body neurons driven by 201Y–GAL4 (frontal view, dorsal is at the Top). The anteriormost view (B) shows the localization of Syt::GFP in the median and vertical lobes, whereas more posteriorly (C) it is detected in the peduncle. In contrast, DenMark expression is observed only in the calyx. (D–D′′) A single larval calyx showing enriched DenMark expression in the external layer where ChAT-positive PN projections terminate (frontal view, dorsal is at the Top). (E–E′′) High magnification views revealing ChAT positive synaptic terminals surrounded by DenMark-enriched structures (arrows). (F–F′′) A single adult calyx revealing ring-like DenMark-enriched structures surrounding ChAT-positive PN endings (frontal view, dorsal is at the Top). (G–G′′) High magnification of DenMark-enriched ring-like structures surrounding ChAT-positive terminals (arrows).(A–C) Maximal Z-projections of confocal section stacks. (D–G) Single sections. s, soma; c, calyx; p, peduncle; v, vertical lobe; m, median lobe. (Scale bars, 50 μm in A–C; 10 μm in D and F; 2 μm in E–G.)
We next asked whether DenMark labels the entire dendritic compartment. Previous reports showed that synaptic complexes in MB calyces both from Drosophila and in honeybees, present a typical ring-like shape (34–37). To test whether DenMark is indeed present at these ring-like synaptic structures, we labeled the presynaptic terminals by revealing ChAT (Choline Acetyl Transferase) immunoreactivity. These cholinergic terminals belong to the PNs that establish connections into the calyx as well as in the lateral horn. We observe that ChAT-positive boutons are located in the center of the DenMark-enriched ring-like structures resembling the previously described synaptic complexes in both third instar larval (Fig. 2 D and E) and adult (Fig. 2 F and G) MBs, strongly suggesting that DenMark is indeed targeted to the very tip of the dendrite very close to the postsynaptic site.
DenMark Reveals Progressive Developmental Specialization of Drosophila Dendrites.
In mammalian neurons, DenMark shows gradual restriction to dendritic arbors. We asked whether insect CNS neurons show a similar developmental pattern by examining DenMark distribution during MB development at all three larval stages (first, second, and third instar) and in adult flies. In first instar larvae, DenMark is largely homogeneously distributed (Fig. S5 A–A′′). During the second instar, DenMark expression becomes stronger in dendrites and cell bodies than in axons (Fig. S5 B–B′′). By early third instar, DenMark is highly enriched in dendrites and cell bodies and essentially absent from axons (Fig. S5 C–C′′) as it is in adult flies (Fig. S5 D–D′′). Interestingly, DSCAM17.1::GFP also shows gradual restriction to the somatodendritic compartment during development (Fig. S6), suggesting that segregation of dendritic factors is acquired progressively during MB development and is a common feature of fly and mammalian neurons supporting suggestions of a common origin of vertebrate and invertebrate dendrites (4).
Identifying Dendritic Compartments with DenMark.
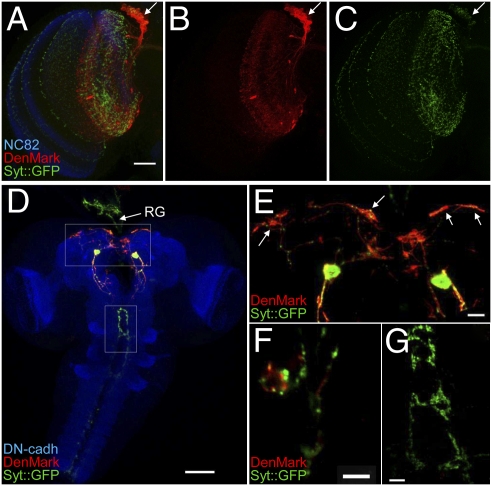
The dendritic patterns of most neurons in the Drosophila CNS remain elusive and the majority of neuronal circuits in the fly brain are unknown. We asked whether DenMark could be used to identify the dendritic compartments of sparse or individual neurons by labeling the dorsal cluster neurons (DCNs) in the adult brain (38) and a pair of eclosion hormone (EH) positive neurons in the third instar larva. DCN axons innervate the lobula and medulla of the optic lobes contralaterally, whereas their dendrites have been proposed to innervate the ipsilateral lobula only (39). Syt::GFP and DenMark were expressed in the DCN and their patterns examined (Fig. 3A). We find that Syt::GFP accumulates in the presynaptic terminals in lobula and medulla, whereas DenMark marks the cell bodies and ipsilateral dendrites that reside in the lobula. Importantly, there is a clear distinction between Syt::GFP and DenMark, demonstrating that DenMark specifically labels the somatodendritic compartment in DCNs, which is clear when the two channels are shown separately (Fig. 3 B and C). The EH–GAL4 line is expressed in a single neuron on each side of ventromedial larval brain. This neuron extends projections toward the ring gland (RG) and the thoracicoabdominal ganglion (40, 41). Using this genetic combination, both DenMark and GFP expression levels were low, leading us to perform double immunostaining with anti-GFP and anti-DsRed antibodies. We detect strong DenMark immunoreactivity in EH neuron soma and extensive neurite arborizations projecting both anteriorly and posteriorly from the cell bodies, forming a stereotyped bilaterally symmetrical dendritic arbor (Fig. 3 D and F). Presynaptic Syt::GFP, on the other hand, was mostly observed in axonal projections in the ring gland (arrow in Fig. 3D) as well as in the thoracicoabdominal ganglion (Fig. 3 D, E, and G). Interestingly, discrete DenMark labeling, juxtaposed to, but largely distinct from Syt::GFP positive boutons, was also observed in the ring gland but not in the thoracicoabdominal presynaptic terminals (Fig. 3 F and G). These data suggest that EH cells project both pre- and postsynaptic terminals into the ring gland and not only axonal terminals as previously assumed.
Fig. 3.
Identifying dendritic compartments with DenMark. (A–C) Dorsal cluster neurons (DCNs) were revealed using ato–GAL4–14a driver in combination with UAS-syt::GFP and UAS–DenMark. Brains were counterstained using an antibody recognizing the neuropilar marker Bruchpilot (mAb nc82).(A) Overview of an adult optic lobe showing the specific distribution of DenMark in DCN cell bodies (arrow) and in the dendrites in the lobula, whereas Syt::GFP is detected in the presynaptic terminals both in the lobula and the medulla. (Scale bar, 30 μm.) (D–G) EH–GAL4-driven expression of DenMark and Syt::GFP in the third instar larval CNS and ring gland (frontal view, dorsal is at the Top). (D) Z-projection of a confocal stack showing an overview of the CNS counterstained using an anti-DNcadherin (DHSB DN-EX#8) antibody and the projections in the ring gland (RG). (Scale bar, 50 μm.) (E) Magnified view of the central brain (white box in D) showing the pair of somas coexpressing both markers as well as medial and lateral DenMark-positive arborizations (arrows). (Scale bar, 10 μm.) (F) Single confocal section showing high magnification of EH neuron terminals in the ring gland. Note the DenMark-positive structures are closely juxtaposed to Syt::GFP positive terminals, suggesting that EH neurons may synapse one onto each other at this level. (Scale bar, 5 μm.) (G) Single confocal section of EH terminals in the thoracicoabdominal ganglion. The exclusive expression of Syt::GFP indicates that these projections could be of “pure” presynaptic type. (Scale bar, 10 μm.)
Using DenMark to Trace Neuronal Connectivity.
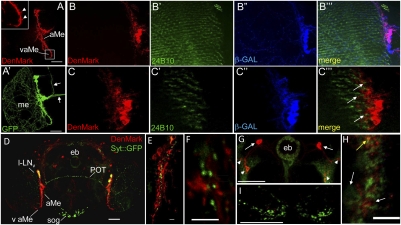
We asked whether DenMark might be a useful tool to attempt a better anatomical description of neuronal connectivity in the fly CNS. We sought to identify features of a less well-described neuronal circuit. The neuronal components involved in the regulation of circadian rhythm in Drosophila have been extensively studied. However, dendritic compartments of many of its components still need to be identified. We selected two GAL4 drivers expressed in the circadian circuitry: pdf–GAL4 (42) and cry–GAL4 (43). We first used pdf–GAL4 to coexpress DenMark and mCD8::GFP in the circadian pacemaker LNv neurons (Fig. 4 A and A′). Previous publications revealed that projections of PDF-expressing LNv neurons elongate ventrally in the ipsilateral accessory medulla (aMe) and arborize on the surface of both ipsilateral and contralateral medulla (42, 44). In addition, the same authors found that the presynaptic marker, nSyb::GFP, was mostly expressed in the projections at the medulla surface, indicating that they correspond to LNvs’ axonal terminations. Here, we show that DenMark is restricted to the projections in the aMe, formally showing that aMe neurites are in fact LNv dendrites. Moreover, we show that, at the level of the LNvs dendrites, DenMark labels fine “spine-like” protrusions (Fig. 4A, inset), suggesting that the dendritic marker penetrates to the very tip of the dendrite arborization. It is well established that flies use light input from the compound eye to entrain the circadian clock (45). However, whether the input from retinal axons onto the clock is direct or indirect remains unknown. We investigated the input onto LNv dendrites from the color and UV sensing retinal axons (R7 and R8) projecting into the medulla (Fig. 4 B–B′′′). We find that R7/8 axons terminate in very close proximity to the LNv dendrites (Fig. 4 C–C′′′), suggesting that the LNv neurons may receive direct input from retinal axons.
Fig. 4.
Using DenMark to map elements of the circadian circuit. (A–A′′) Expression of DenMark and mCD8::GFP were driven in the LNvs (lateral neurons ventral), a subset of clock neurons of the fly CNS, using the pdf–GAL4 driver line. Whereas mCD8::GFP labels all of the neuronal compartments, DenMark is found in somas (s) and in thick bundle of neurites extending in the accessory medulla (aMe) and terminating in the ventral accessory medulla (vaME). In addition, DenMark expression in this level reveals the presence of spine-like protrusions that could correspond to postsynaptic sites (see arrowheads in A′). Please note that, whereas both markers are found at the basis of the axonal tracts projecting toward the central brain (arrowheads in A and A′′), we detect exclusively mCD8::GFP expression in more distal portions of the axons (arrows in A′′). Maximal Z-projection of confocal image stack. Dorsal is at the Top; lateral to the Left. (Scale bars, 50 μm.) (B–C) Here, LNvs are visualized by expressing both DenMark and β-galactosidase using the pdf–GAL4 driver line. In addition, axonal projections of R7 and R8 photoreceptors were labeled using an antibody raised against Chaoptin (mAb 24B10). Single confocal sections of the medulla reveal very close proximity of R7 and R8 axons to the dendritic arbors of the LNv neurons (arrows in C′′′), suggesting potential direct input from the color and UV detecting photoreceptors onto the clock neurons. Dorsal is at the Top; lateral to the Left. (D–H) DenMark and Syt::GFP expression were driven in a larger subset of circadian neurons using the cry–39–GAL4 driver line. (D) Frontal overview of an adult brain shows distinct DenMark and Syt::GFP expression patterns as revealed by anti-DsRed and anti-GFP immunostainings. (E) Higher magnification of the aMe reveals the presence of DenMark in somas and high levels of DenMark expression in elongated ventral projections. In addition, discrete Syt::GFP expression, possibly corresponding to axonal projections of contralateral LNvs, is also detected therein. (F) Higher magnification of the aME region reveals that Syt::GFP and DenMark-positive structures are closely juxtaposed but do not coincide. (G) DenMark and Syt::GFP expression are also detected in R neurons of the ellipsoid body (eb). Anterior somas (arrowheads) send posterior projections that establish DenMark-enriched dendritic terminals (arrows) lateral to the eb, whereas the Syt::GFP expression is confined to somas and to the eb neuropile itself. Note also that a weak expression of DenMark is also detected in the eb neuropile. (H) Higher magnification view of the eb neuropile reveals that DenMark and Syt::GFP expression are largely segregated (white arrows), although some colocalization could also be detected (yellow arrow). The juxtaposition of DenMark and Syt::GFP positive terminals suggests that the two clusters of R neurons may synapse onto one another at the level of the eb neuropile. (I) This closer view of Syt::GFP positive axonal projections in the suboesophageal ganglion shows the complete absence of DenMark expression. Images in D, G, and I correspond to maximal Z-projections of confocal stacks, whereas E, F, and H images correspond to single confocal sections. DenMark expression was revealed by rabbit anti-DsRed antibody (Clontech) and Syt::GFP by mouse anti::GFP antibody except for E, where we used rabbit anti::GFP antibody. Dorsal is at the Top in all panels. (Scale bars, 50 μm in D and G; 5 μm in E, F, and H. aME, accessory medulla; POT, posterior optic tract; eb, ellipsoid body; l-LNvs, large ventrolateral neurons; sog, suboesophageal ganglion.
Next, we used the cry–GAL4 line to express the DenMark and Syt::GFP in LNv neurons as well as other neuronal populations within the circadian circuit. Strong expression of DenMark was observed in the somas of LNv neurons as well as in the aMe, whereas Syt::GFP was mostly found at the surface of the medulla (Fig. 4D). In addition, we detect Syt::GFP positive boutons in the area of the DenMark-labeled LNv dendritic terminations in the aMe. Note that the two markers are closely apposed but do not overlap (Fig. 4 E and F). This result explains the previous observations that LNv neurons establish presynaptic contacts within the aMe (44) and suggests that these contacts are on the postsynaptic sites of the LNv neurons themselves. Thus, similar to DCN neurons (see above), the two clusters of LNv neurons appear to relay information across the two optic lobes. In addition to the LNv, cry–GAL4 labels a group of ellipsoid body (eb) neurons termed the R neurons. The eb is a neuropile structure that regulates locomotor behavior in flies (46–48) and the R neurons were recently demonstrated to control environmentally stimulated arousal in flies (49). Here we show that in these neurons, DenMark expression is present in cell bodies and highly concentrated in dendritic terminals located laterally to the eb, whereas Syt::GFP is only found in the eb itself (Fig. 4 G and H). Strikingly, although at low levels, DenMark was also detected in the ring structure of the eb. Again, DenMark-enriched structures appear juxtaposed to rather than coexpressed with Syt::GFP (Fig. 4H), suggesting that R neurons may establish synapses onto each other within the eb. In contrast, the projections to the suboesophageal ganglion, characterized by strong expression of Syt–GFP (Fig. 4I) are completely devoid of DenMark.
Discussion
Overall, the data presented here strongly indicate that DenMark is a versatile, neutral, and robust marker of the dendritic compartment of probably any neuron in Drosophila. Furthermore, we provide several examples of the use of this tool to examine dendritic morphology, development, maturation, and connectivity.
An interesting and unexpected observation from at least four independent neuronal populations (DCN, EH, LNv, and R) indicates that bilaterally symmetrical neurons with neurites that end at or cross the midline may establish presynaptic terminals onto the dendrites of their reciprocal neurons, perhaps indicating that commisssural neurons with identical functions exchange synaptic information across the two brain hemispheres.
Finally, it is remarkable that a cell adhesion molecule not encoded in any invertebrate genome localizes with such exquisite specificity in Drosophila neurons. This raises the exciting possibility that DenMark may also serve as a dendritic marker in other model organisms such as Xenopus and Zebrafish, in addition to mouse and Drosophila. Interestingly, using DenMark and other markers to compare dendritic development in Drosophila and mouse neurons reveals that dendrites do not acquire their complete molecular specialization until a relatively late stage in neuronal development. This is despite the fact that dendrites are morphologically distinguishable from axons at these stages. We propose that an in-depth comparative characterization of the “dendrome” and the “axome,” perhaps using DenMark localization as a tool, would yield greater insights into the processes that distinguish the axons and dendrites and may lead to a more accurate definition of the pre- and postsynaptic neuronal compartments.
Materials and Methods
A brief description of the materials and methods is presented here. Please see SI Materials and Methods for detailed experimental procedures.
Drosophila Strains and Genetics.
All stocks were raised on standard fly food at 25 °C except for the cross between cry39–GAL4 (43) and UAS–DenMark, UAS–syt::GFP flies, which was maintained at 28 °C. All crosses were made according to standard procedures.
Labeling and Quantitative Analysis of RP2 Neurons.
RP2 neurons in larval nerve cords were labeled genetically using the “FLPout” method outlined in refs. 50 and 51. Dendritic trees of RP2 neurons were reconstructed digitally from confocal image stacks using customized modules to obtain quantitative data on total dendritic tree length and number of dendritic tips (end segments) (52, 53).
Quantification of MD Dendritic Arbors.
Images of MD neurons were skeletonized and subsequently automatically analyzed using the “Skeletonize3D” and “AnalyzeSkeleton” free plugins for ImageJ/FIJI (freely downloadable from the FIJI website: URL: http://pacific.mpi-cbg.de/wiki/index.php/Fiji).
Immunostainings on Drosophila Brains.
Immunostainings on brains were performed as described in ref. 36.
Calculation of the Relative Distribution of DenMark in the Larval Mushroom Bodies.
First the background of confocal sections was corrected on the basis of the offset. For each section, DenMark expression was then normalized to the corresponding CD8::GFP expression on a pixel-by-pixel basis. Finally, the ratio of the normalized DenMark expression in the calyx vs. the lobes was calculated.
Confocal Microscopy and Imaging.
Larval motoneurons were visualized by confocal microscopy (BioRad and Leica DM-RXA) and the final processing was done with Image J.
Primary Hippocampal Neurons.
Primary hippocampal neurons were derived from isolated hippocampi of E17 mouse embryos and maintained in neurobasal medium supplemented with B27 for 14 d postplating. Neurons were fixed for 30 min in 4% PFA in PBS and following a brief permeabilization, blocked and processed for immunocytochemistry as described previously (54).
Electroretinogram Recordings (ERGs).
ERGs were recorded from 7- to 1-d-old flies as described (55), except flies were immobilized with liquid Pritt glue and we used a green LED light digitally controlled to present 1-s light pulses. Data were stored on a personal computer with Clampex 10 and processed with Clampfit and Canvas 7.
Negative Geotaxis Assay.
Motor deficits were assessed by deviation from normal negative geotaxis behavior. Five adult flies per replication were placed in an empty plastic vial. After a 10-min rest period, the flies were tapped to the bottom of the vial, and the number of flies able to climb 10 cm in 15 s was recorded. The assays were repeated three times at 1-min intervals. Four replications were performed for each dataset and 20 flies were tested both for the control (ElavGAL4) and the experimental group (ElavGAL4; UAS–DenMark, UAS–syt::GFP).
Supplementary Material
Acknowledgments
We thank the Drosophila stock centers, P. Taghert (Washington University, Saint Louis) for cry39–GAL4 line, and H. Aberle (The Max Planck Institute, Tübingen, Germany) for the OK371–GAL4 line for providing fly stocks; S. Munck for precious help with confocal microscopy and image quantification; J. Kasprowicz for help with ERGs; M. Fujioka for reagents and help with generating the RN2–FLP transgene for the FLPout system. Work in the Landgraf laboratory was supported by an Isaac Newton Trust Grant (to A.S.M.), Wellcome Trust Programme Grant 075934 (to Michael Bate and M.L.), and a Royal Society University Research Fellowship (to M.L.) Work in the Hassan and Annaert laboratories is funded by the Flanders Institute of Biotechnology (VIB). This work was also supported by Scientific Fund of Flanders (FWO) Grant G.0.543.08.N.10 (to B.A.H.), and a Methusalem grant from Katholieke Universiteit Leuven (KUL), and an Agency for Innovation by Science and Technology (IWT) doctoral fellowship (to L.J.J.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010198107/-/DCSupplemental.
References
- 1.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Establishment and plasticity of neuronal polarity. J Neurosci Res. 1999;57:577–589. [PubMed] [Google Scholar]
- 3.Boyan GS, Ball EE. The grasshopper, Drosophila and neuronal homology (advantages of the insect nervous system for the neuroscientist) Prog Neurobiol. 1993;41:657–682. doi: 10.1016/0301-0082(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Soriano N, et al. Are dendrites in Drosophila homologous to vertebrate dendrites? Dev Biol. 2005;288:126–138. doi: 10.1016/j.ydbio.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: Sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 6.DiAntonio A, et al. Identification and characterization of Drosophila genes for synaptic vesicle proteins. J Neurosci. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly RB. Neural transmission. Synaptotagmin is just a calcium sensor. Curr Biol. 1995;5:257–259. doi: 10.1016/s0960-9822(95)00054-6. [DOI] [PubMed] [Google Scholar]
- 8.Schiavo G, Gmachl MJ, Stenbeck G, Söllner TH, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 9.Südhof TC. The synaptic vesicle cycle: A cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: Synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- 11.Fouquet W, et al. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolls MM, et al. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Develop. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: A difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984;226:203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- 14.Tucker RP, Binder LI, Viereck C, Hemmings BA, Matus AI. The sequential appearance of low- and high-molecular-weight forms of MAP2 in the developing cerebellum. J Neurosci. 1988;8:4503–4512. doi: 10.1523/JNEUROSCI.08-12-04503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen R, Li Y, Resseguie M, Brenman JE. Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. J Neurosci. 2005;25:8878–8888. doi: 10.1523/JNEUROSCI.2005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark IE, Jan LY, Jan YN. Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development. 1997;124:461–470. doi: 10.1242/dev.124.2.461. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Ueda R, Takahashi K, Saigo K, Uemura T. Control of axonal sprouting and dendrite branching by the Nrg-Ank complex at the neuron-glia interface. Curr Biol. 2006;16:1678–1683. doi: 10.1016/j.cub.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 19.Diagana TT, et al. Mutation of Drosophila homer disrupts control of locomotor activity and behavioral plasticity. J Neurosci. 2002;22:428–436. doi: 10.1523/JNEUROSCI.22-02-00428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, et al. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 22.Williams AF, Barclay AN. The immunoglobulin superfamily—domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 23.Tian L, Yoshihara Y, Mizuno T, Mori K, Gahmberg CG. The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J Immunol. 1997;158:928–936. [PubMed] [Google Scholar]
- 24.Oka S, Mori K, Watanabe Y. Mammalian telencephalic neurons express a segment-specific membrane glycoprotein, telencephalin. Neuroscience. 1990;35:93–103. doi: 10.1016/0306-4522(90)90124-m. [DOI] [PubMed] [Google Scholar]
- 25.Mori K, Fujita SC, Wantanabe Y, Obata K, Hayaishi O. Telencephalon-specific antigen identified by monoclonal antibody. Proc Natl Acad Sci USA. 1987;84(11):177–182. doi: 10.1073/pnas.84.11.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian L, et al. Intercellular adhesion molecule-5 induces dendritic outgrowth by homophilic adhesion. J Cell Biol. 2000;150:243–252. doi: 10.1083/jcb.150.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banker G, Waxman A. Hippocampal neurons generate natural forms in culture. In: Lasek RJ, Black MM, editors. Intrinsic Determinants of Neuronal Form and Function. New York: Alan R. Liss; 1988. pp. 61–82. [Google Scholar]
- 28.Cáceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney NT, Li W, Gao FB. Genetic manipulation of single neurons in vivo reveals specific roles of flamingo in neuronal morphogenesis. Dev Biol. 2002;247:76–88. doi: 10.1006/dbio.2002.0702. [DOI] [PubMed] [Google Scholar]
- 32.Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aso Y, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 34.Ganeshina O, Menzel R. GABA-immunoreactive neurons in the mushroom bodies of the honeybee: An electron microscopic study. J Comp Neurol. 2001;437:335–349. doi: 10.1002/cne.1287. [DOI] [PubMed] [Google Scholar]
- 35.Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- 36.Ramaekers A, et al. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Leiss F, Groh C, Butcher NJ, Meinertzhagen IA, Tavosanis G. Synaptic organization in the adult Drosophila mushroom body calyx. J Comp Neurol. 2009;517:808–824. doi: 10.1002/cne.22184. [DOI] [PubMed] [Google Scholar]
- 38.Hassan BA, et al. Atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 39.Srahna M, et al. A signaling network for patterning of neuronal connectivity in the Drosophila brain. PLoS Biol. 2006;4:e348. doi: 10.1371/journal.pbio.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horodyski FM, Ewer J, Riddiford LM, Truman JW. Isolation, characterization and expression of the eclosion hormone gene of Drosophila melanogaster. Eur J Biochem. 1993;215:221–228. doi: 10.1111/j.1432-1033.1993.tb18026.x. [DOI] [PubMed] [Google Scholar]
- 41.McNabb SL, et al. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–823. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 42.Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Klarsfeld A, et al. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helfrich-Förster C, et al. The lateral and dorsal neurons of Drosophila melanogaster: New insights about their morphology and function. Cold Spring Harb Symp Quant Biol. 2007;72:517–525. doi: 10.1101/sqb.2007.72.063. [DOI] [PubMed] [Google Scholar]
- 45.Helfrich-Förster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology (Jena) 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]
- 46.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- 48.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 49.Lebestky T, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy B, et al. Metamorphosis of an identified serotonergic neuron in the Drosophila olfactory system. Neural Dev. 2007;2:20. doi: 10.1186/1749-8104-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ou Y, Chwalla B, Landgraf M, van Meyel DJ. Identification of genes influencing dendrite morphogenesis in developing peripheral sensory and central motor neurons. Neural Develop. 2008;3:16. doi: 10.1186/1749-8104-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evers JF, Muench D, Duch C. Developmental relocation of presynaptic terminals along distinct types of dendritic filopodia. Dev Biol. 2006;297:214–227. doi: 10.1016/j.ydbio.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Evers JF, Schmitt S, Sibila M, Duch C. Progress in functional neuroanatomy: Precise automatic geometric reconstruction of neuronal morphology from confocal image stacks. J Neurophysiol. 2005;93:2331–2342. doi: 10.1152/jn.00761.2004. [DOI] [PubMed] [Google Scholar]
- 54.Annaert WG, et al. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstreken P, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt S, Evers JF, Duch C, Scholz M, Obermayer K. New methods for the computer-assisted 3-D reconstruction of neurons from confocal image stacks. Neuroimage. 2004;23:1283–1298. doi: 10.1016/j.neuroimage.2004.06.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.