Abstract
The Epstein–Barr virus (EBV) latent membrane protein 1 (LMP1) is expressed in multiple human malignancies and has potent effects on cell growth. It has been detected in exosomes and shown to inhibit immune function. Exosomes are small secreted cellular vesicles that contain proteins, mRNAs, and microRNAs (miRNAs). When produced by malignant cells, they can promote angiogenesis, cell proliferation, tumor-cell invasion, and immune evasion. In this study, exosomes released from nasopharyngeal carcinoma (NPC) cells harboring latent EBV were shown to contain LMP1, signal transduction molecules, and virus-encoded miRNAs. Exposure to these NPC exosomes activated the ERK and AKT signaling pathways in the recipient cells. Interestingly, NPC exosomes also contained viral miRNAs, several of which were enriched in comparison with their intracellular levels. LMP1 induces expression of the EGF receptor in an EBV-negative epithelial cell line, and exosomes produced by these cells also contain high levels of EGF receptor in exosomes. These findings suggest that the effects of EBV and LMP1 on cellular expression also modulate exosome content and properties. The exosomes may manipulate the tumor microenvironment to influence the growth of neighboring cells through the intercellular transfer of LMP1, signaling molecules, and viral miRNAs.
Keywords: oncogene, herpesvirus
The Epstein–Barr virus (EBV) is a major human pathogen that potently affects cell growth regulation and is linked to the development of multiple malignancies (1). These cancers contain the viral genome but have different patterns of viral gene expression. Latent membrane protein 1 (LMP1) is considered the major oncogene of EBV because it has transforming properties in cultured cell lines, is essential for B-lymphocyte transformation, and is frequently expressed in EBV-associated cancers (2, 3). LMP1 functions as a constitutively active member of the tumor necrosis factor receptor family and activates multiple signaling pathways, including mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), phosphatidylinositol 3-kinase (PI3K)/Akt, and NF-κB (4). LMP1 induces the expression of specific genes that are involved with apoptosis, cell-cycle progression, cell proliferation, and migration (4). One important target of LMP1 is epidermal growth factor receptor (EGFR), a member of the ErbB receptor tyrosine kinase family (5). Similarly to LMP1, EGFR activates multiple signaling pathways, including Src kinases, JAKs/STATs, Ras/MAPK, and PI3K/Akt (6). As a potent growth-signaling receptor, the EGFR pathway is targeted by several oncogenic viruses, including EBV, to affect cell growth (6). EBV-positive nasopharyngeal carcinoma (NPC) has elevated amounts of EGFR that are directly related to the expression level of LMP1 (5). EGFR can be secreted from cells in exosomes and other microvesicles, and its subsequent uptake by endothelial cells can induce tubule formation, activation of MAPK and Akt pathways, and VEGF expression (7). Interestingly, glioblastoma microvesicles contain a truncated oncogenic form of EGFR that induces proliferation of a human glioma cell line, suggesting potential autocrine and paracrine stimulation (8).
Exosomes are 40- to 100-nm endosomal-derived vesicles that are secreted from many cell types and transfer proteins, mRNAs, and microRNAs (miRNAs) to neighboring or distant cells to modulate immune function, angiogenesis, cell proliferation, tumor-cell invasion, and cell-to-cell communication (9, 10). Exosomes are present in many biological fluids, including the cerebrospinal fluid, blood, and urine, and they likely affect physiologic processes (9, 10). Exosomes are a recently discovered mechanism through which cancer cells and virally infected cells can manipulate their microenvironment. Viruses can use the exosome pathway for virus egress and immune evasion (11–13). Interestingly, EBV-infected cells release exosomes containing LMP1 that induce T-cell anergy (14). LMP1 also increases the concentration and release of FGF-2, a potent angiogenic factor, into exosomes (15).
In this study, the effects of LMP1 on exosome composition and biochemical properties were evaluated. The data indicate that LMP1 increases the release of EGFR into exosomes and that purified exosomes containing LMP1 and EGFR are taken up by epithelial, endothelial, and fibroblast cells, leading to the activation of ERK and PI3K/Akt pathways. It has recently been shown that cellular miRNAs are present in tumor-derived exosomes, and EBV miRNAs have also been detected in exosomes. Similarly, data presented here show that an EBV-positive NPC cell line produces exosomes that contain virus-encoded miRNAs with differing relative abundances in comparison with their intracellular levels in equal amounts of exosomal and intracellular RNA, suggesting that some of the viral miRNAs may be selectively packaged into exosomes. These findings suggest that EBV utilizes the exosomal system to secrete key signaling molecules and viral-encoded proteins and miRNAs. The effects of EBV and LMP1 on exosomes are likely to be important factors in EBV infection through which a small number of cells expressing LMP1 could both modulate the tumor microenvironment and also potentially impact the infected host.
Results
EBV-Infected NPC Produce Exosomes That Activate Growth-Signaling Pathways.
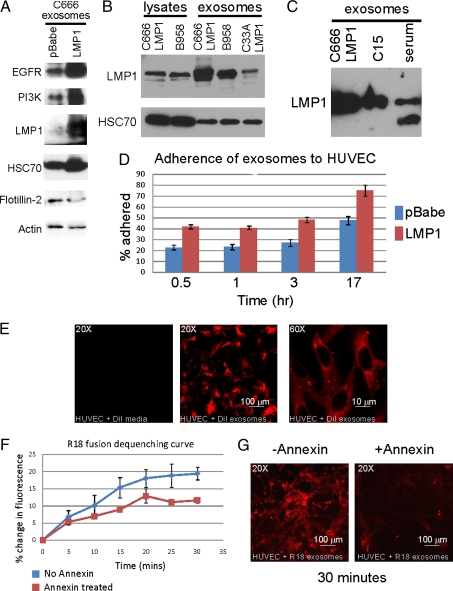
Exosomes containing LMP1 have been detected in the serum of NPC patients, and NPC serum has been shown to have mitogenic activity as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (16). To evaluate the properties of exosomes produced by NPC cells, exosomes were harvested from the media of the NPC C666 cell line, an NPC cell line that has retained EBV but expresses low to undetectable levels of LMP1 (17). Exosomes prepared from the C666 cells and a derivative that stably expresses LMP1 (C666-LMP1) or the pBabe vector control contained standard exosomal markers HSC70 and flotillin-2 (Fig. 1A). LMP1 was abundant in exosomes produced by C666-LMP1 but not detectable in exosomes from the C666 vector control parental line. Both EGFR and PI3K were detected in C666 and C666-LMP1 exosomes (Fig. 1A).
Fig. 1.
Characterization of NPC exosomes and their uptake by HUVECs. (A) Purified exosomes from conditioned media of C666-pBabe and C666-LMP1 were analyzed by immunoblotting with the indicated antibodies and the LMP1 S12 monoclonal antibody. (B) Levels of LMP1 within cellular lysates were compared between C666-LMP1 and B958 cells and in exosomes produced by C666-LMP1, B958, and C33A-LMP1. (C) Exosomes from cultured C666 cells, C15 xenograft, or C15 mouse serum were analyzed by immunoblotting for LMP1 levels by using pooled LMP1 rat monoclonal antibodies. (D and E) Three separate preparations of purified exosomes were labeled with DiI and incubated with HUVECs for the indicated times. Fluorescence was monitored in a plate reader (D) or by confocal microscopy (E). The numbers were normalized to equal DiI totals and represented as a percentage of exosomes adhered. (F and G) Annexin blocking of exosome fusion was measured by fluorescence dequenching of R18-labeled exosomes at the indicated times by using a plate reader (F) or visualized by confocal microscopy (G).
To evaluate the relative levels of LMP1 expression within cells and exosomes, C666-LMP1 was compared with the prototype EBV-transformed lymphoid cell line, B958 (Fig. 1B). LMP1 expression was equivalent in cell lysates of B958 and C666-LMP1 and was abundant within the exosomes produced by these cell lines. LMP1 was also detected in exosomes produced by the C33 epithelial cell line stably expressing LMP1. The C15 NPC tumor is a well-studied model of NPC that must be maintained as a xenograft. The C15 cells can be viably maintained in culture for brief time periods and have been shown to produce exosomes that contain LMP1 and high levels of galectin 9 (13). LMP1 was abundant in exosomes purified from the culture supernatants of both C666-LMP1 and C15 (Fig. 1C). Importantly, LMP1 was also detected in exosomes purified from the serum of mice carrying the C15 tumor (Fig. 1C, serum), consistent with previous work on NPC patient serum (16).
Recent studies have shown that tumor-derived exosomes can be taken up by human umbilical vein endothelial cells (HUVECs). The potential for internalization of NPC exosomes by this important cell type was evaluated by using C666 and C666-LMP1 exosomes produced by equivalent cell numbers that were purified, fluorescently labeled, and incubated with HUVECs for various times. The cells were extensively washed, and binding was evaluated as a percentage of the total fluorescence for each sample added to the cells. Within 30 min, 20% of the parental C666 exosomes and ≈40% of C666-LMP1 exosomes bound to HUVECs, and, after 17-h incubation, binding increased to 60% and 80%, respectively (Fig. 1D). LMP1 modulates the levels of many proteins involved in adhesion and cellular interaction, which could contribute to the potential increased binding efficiency of the LMP1 exosomes. Confocal microscopy of the HUVECs treated with fluorescently labeled exosomes for 16 h revealed punctuate internal staining, suggesting that the exosomes were taken up into endocytic compartments (Fig. 1E). After extensive washing, fluorescence was only detected in confluent monolayers of HUVECs incubated with 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI)- labeled C666 exosomes but not with media containing dye without exosomes, indicating that cell membranes did not nonspecifically take up contaminating dye (Fig. 1E Left).
It has been shown that exosomes contain exposed phosphatidyl serine and their fusion with target cell membranes can be inhibited with annexin V (18). To assess the requirement for exosomal fusion, a fusion assay was performed with the R18 self-quenching dye. Detection of fluorescence reflects dequenching of the dye and is indicative of fusion with the unlabeled cellular membrane. This fluorescence is then compared with the total fluorescence in the sample determined by dequenching with detergent treatment. Fluorescence indicative of fusion was readily detected in HUVECs incubated with R18-labeled C666 exosomes such that 20% of the exosomal membranes had fused in 30 min (Fig. 1F). Preincubation of labeled exosomes with annexin V, a phosphatidyl serine-binding protein, decreased the total amount of fluorescence detected at each time point with an overall decrease of ≈50% (Fig. 1F). This effective blocking is comparable to previous studies and confirms that the NPC exosomes fuse with cellular membranes (7). Confocal microscopy of HUVECs exposed to annexin V-treated and untreated exosomes also confirmed exosomal uptake into punctuate vesicles and indicated that this uptake was specifically blocked by annexin (Fig. 1G).
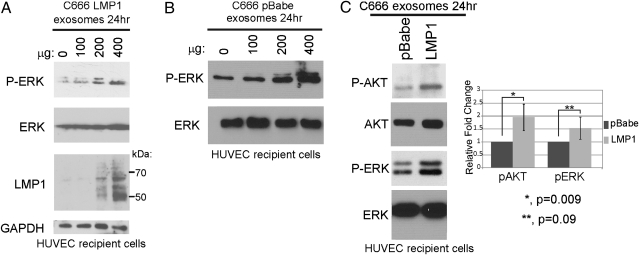
LMP1 activates multiple cellular signaling pathways, and the activation of PI3K, Akt, and ERK are essential for LMP1-mediated transformation of fibroblasts. To assess the potential transfer of LMP1 as well as the activation of ERK and Akt, HUVECs were cultured in serum-free conditions and exposed to increasing amounts of purified C666-LMP1 exosomes for 24 h. Detection of LMP1 in cell lysates, as determined by immunoblotting, was dose-dependent. Additionally, activated phosphorylated ERK was detected and was also proportional to the exosome concentration from both C666-pBabe and C666-LMP1 cells (Fig. 2). To determine whether LMP1 modulates the ability to activate signaling pathways in recipient cells, duplicate cultures of HUVECs were exposed to equivalent amounts of exosomes from C666-pBabe or C666-LMP1 and analyzed for AKT and ERK activation. Although C666-pBabe exosomes were capable of activating these pathways, C666-LMP1 exosomes induced consistently higher levels of activation (Fig. 2C). These findings suggest that NPC exosomes stimulate growth-signaling pathways and that LMP1 can enhance these effects. Importantly, HUVECs are a highly significant cell type whose growth and activation would be important for tumor growth with enhanced vascularization and metastasis.
Fig. 2.
Transfer of LMP1 and activation of ERK and AKT pathways in HUVECs by NPC exosomes. HUVECs were incubated with increasing amounts of purified C666-LMP1 (A) or C666-pBabe (B) exosomes for 24 h in serum-free media. Cell lysates were analyzed by immunoblotting for the indicated proteins and S12 mAb for LMP1. (C) HUVECs exposed to 400 μg of pBabe or LMP1 exosomes for 24 h were analyzed by immunoblotting for activated ERK and AKT. Levels of pAKT and pERK normalized to total AKT and ERK protein levels and represented relative to pBabe exosomes are shown from three independent experiments.
Properties of Exosomes Released from LMP1-Expressing Cells.
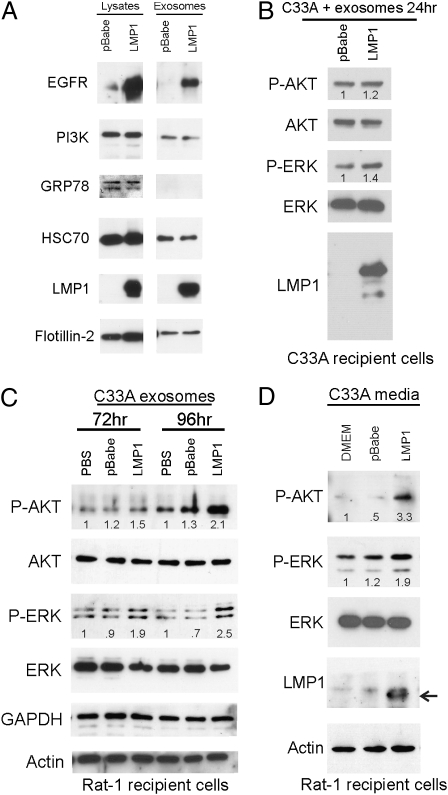
In C33A epithelial cells, LMP1 greatly increases expression of EGFR. To assess the effects of LMP1 on exosomes produced by these cells, exosomes were purified from the media of C33A cells stably expressing LMP1 or vector control by sequential centrifugation. Equivalent total protein levels from the pelleted material were subsequently analyzed for specific exosomal components and signaling molecules. The exosomal components HSC70 and flotillin-2 were detected in exosome preparations from LMP1-expressing and vector control cells (Fig. 3A) (15, 19). The endoplasmic reticulum-localized protein GRP78 was not detected, indicating that the preparations were not contaminated with apoptotic bodies, which contain high levels of endoplasmic reticulum-derived proteins. The purified LMP1-C33A exosomes contained abundant LMP1 and high levels of EGFR (Fig. 3A). PI3K is an important intracellular signal transducer that can bind LMP1 and EGFR and is constitutively activated by LMP1 or EGF treatment. Immunoblotting for p85, the PI3K regulatory subunit, detected the protein in both the pBabe vector control and LMP1-C33A exosome preparations at approximately equivalent levels. Thus, exosomes can contain LMP1, EGFR, and their specific signal transducers.
Fig. 3.
Exosomes and conditioned media from LMP1-expressing C33A cells activate ERK and AKT in recipient cells. (A) Cell lysates and purified exosomes from C33A-LMP1 or C33A-pBabe cells were analyzed by immunoblotting with antibodies against EGFR, p85, GRP78, HSC70, LMP1, flotillin-2, actin, and S12 mAb for LMP1. (B) Untransfected C33A cells were incubated with C33A-pBabe or LMP1 exosomes for 24 h in serum-free media, and the indicated proteins were identified by immunoblotting of cell lysates. (C) C33A exosomes were incubated with Rat-1 cells for 72 or 96 h, and cell lysates were analyzed for the indicated proteins by immunoblotting. Numbers indicate fold change over PBS control. (D) Media alone (DMEM) or conditioned media from C33A-pBabe or C33A-LMP1 was clarified and added to Rat-1 cells every 24 h for 5 d. pAKT and pERK intensities in all experiments were normalized to total protein levels, and the relative values to the control are indicated in each channel. Representative blots from two to four independent experiments are shown.
Purified C33A exosomes were incubated with C33A parental cells for 24 h in serum-free media. After incubation, LMP1 was detected in the lysates of recipient cells with an LMP1-specific antibody (S12) (Fig. 3B). Immunoblots of cells exposed to LMP1 exosomes compared with the control exosomes indicated slightly increased levels of phosphorylated ERK and Akt with LMP1 (Fig. 3B). The modest, yet reproducible, activation of these pathways in the C33A cells exposed to LMP1-containing exosomes likely reflects the elevated endogenous levels of activity in this highly transformed cancer cell line.
Transformation of Rat1 cells by LMP1 requires activation of PI3K and ERK (20). To determine the effects of C33A exosomes in untransformed cells, Rat-1 fibroblasts were exposed to purified LMP1 exosomes, control exosomes, or PBS. Rat-1 cells exposed to LMP1-containing exosomes had elevated levels of phosphorylated ERK and Akt compared with control exosomes or PBS alone. However, the activation of these pathways in Rat-1 cells was not readily detectable until 72 h (Fig. 3C). This delayed activation may reflect differences in the rate of uptake between cell lines.
It is likely that the multiple centrifugation and pelleting steps in exosomal purification damage exosomes and impair their uptake or function. To evaluate this possibility, Rat-1 cells were exposed to C33A-conditioned media, clarified from cell debris, every 24 h for 5 d. Immunoblot analysis detected LMP1 and activated Akt and ERK in the Rat-1 recipient cell lysates exposed to the media from LMP1-C33A cells but not from the pBabe vector control cells or fresh DMEM (Fig. 3D). These data indicate that both purified LMP1-containing exosomes or conditioned media containing LMP1 exosomes can transfer LMP1 and activate ERK and Akt in the recipient cells.
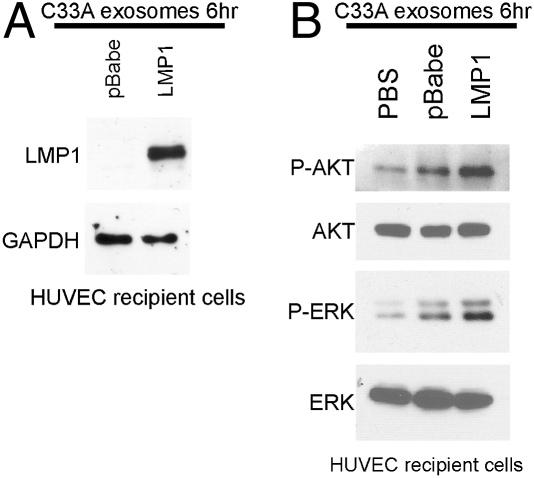
To evaluate the effects of LMP1- and EGFR-containing C33A exosomes on HUVECs and confirm the results obtained with NPC exosomes, cells were exposed to purified C33A exosomes or PBS as described in Materials and Methods. LMP1 was detected by immunoblotting in lysates of HUVEC cells exposed to LMP1-containing exosomes by 6 h posttransfer (Fig. 4A). Activated phosphorylated ERK and Akt were detected in the lysates of HUVEC cells exposed to LMP1-containing exosomes (Fig. 4B) with an average fold increase over pBabe exosomes of 2.0 ± 0.7 for phosphorylated AKT (pAKT) and 2.5 ± 0.9 for phosphorylated ERK (pERK). These data indicate LMP1 can be effectively transferred to endothelial cells through exosomes and can activate the MAPK and Akt pathways.
Fig. 4.
Activation of ERK and AKT pathways in HUVECs exposed to C33A-LMP1 exosomes. HUVECs were incubated for 6 h with PBS, pBabe, and LMP1 exosomes, and recipient cell lysates were immunoblotted for LMP1 with the S12 monoclonal antibody (A) or pAKT and pERK (B) with AKT and ERK as loading controls. Representative results from three independent experiments are shown.
Exosomes Released from EBV-Positive NPC Cells Contain Virus-Encoded miRNAs.
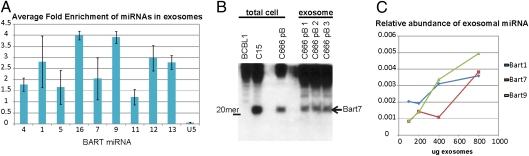
It has been shown that exosomes contain both cellular mRNA and miRNA that can be transferred to other cells to impact cell growth through effects on new protein synthesis or translational inhibition of specific proteins (8, 21). A recent study showed that EBV-infected lymphocytes produced exosomes that contain viral miRNAs and that these miRNAs could be transferred and decrease levels of known cellular targets (22). The majority of the EBV miRNAs are produced from the BART viral transcripts that were initially identified in NPC and are expressed at high levels in NPC and gastric cancer but at low levels in most lymphocyte cell lines or lymphomas (23, 24). To evaluate the incorporation of the viral BART miRNAs in exosomes secreted from EBV-positive NPC, exosomes were purified from the media of C666 cells and the total RNA was extracted from the exosome pellet. An equal amount of total RNA from exosomes and cells was analyzed by using quantitative RT-PCR with primers specific for EBV miRNAs. Comparing the amount of individual EBV exosomal miRNAs to the intracellular levels of the specific viral miRNAs revealed differences in the relative levels, which may indicate that their presence in exosomes is not a random event (Fig. 5A). In addition, the amount of enrichment differed between the individual miRNAs, suggesting that some viral miRNAs may be selectively transported to exosomes. The small nuclear RNA U5 was used as an internal negative control and was not enriched in the exosome preparations. Northern blot analysis for BART 7 using equal amounts of total cellular RNA from C666 cells, the EBV-positive NPC xenograft, C15, and the EBV-negative BCBL1 lymphoma compared with RNA from C666 exosomes indicated that the exosomes contain the processed BART 7 miRNA at levels comparable to NPC tumors that express high levels of the BART miRNAs (Fig. 5B).
Fig. 5.
Viral miRNAs are enriched in NPC exosomes and taken up by HUVECs. (A) Total RNA from C666 cells or exosomes was analyzed by using quantitative RT-PCR for the BART miRNAs with the small nuclear RNA U5A as a negative control. Data are represented as relative levels of the individual miRNAs in comparison with their intracellular levels in equal amounts of C666 total cell RNA and exosomal RNA from three independent experiments run in triplicate. (B) Equal amounts of total cell RNA from C666 cells or three independent exosome preps were separated in tris-borate-EDTA gels and transferred to Hybond N+, hybridized with an end-labeled anti-sense oligo to the miRNA-Bart7, and visualized by autoradiography. EBV-negative BCBL1 cells were used as a negative control, and the C15 xenograft was used as a positive control. (C) HUVEC cells were exposed to C666 exosomes for 6 h, and miRNA uptake was monitored by quantitative RT-PCR of total RNA isolated from HUVECs that had been extensively washed to remove unbound exosomes. miRNA levels are represented as relative to the amount of each miRNA present within an equivalent amount of C666 cellular RNA.
To determine whether viral miRNA could be transferred to cells not harboring EBV, HUVEC cells were exposed to increasing amounts of C666 exosomes for 6 h, extensively washed, and then the RNA was analyzed by RT-PCR for the presence of three different EBV miRNAs. The EBV miRNAs were detected in the total RNA isolated from HUVECs exposed to C666 exosomes in a dose-dependent manner (Fig. 5C). These data indicate that viral miRNAs may be packaged into exosomes for transfer to other cells, which likely provides the virus a unique mechanism for epigenetic effects in neighboring cells. It is possible that the potential selective packaging of individual miRNAs is linked to targeting of specific proteins within the recipient cells.
Discussion
The data presented here reveal that, through exosomal secretion and uptake, a human tumor virus can induce the transfer of a viral oncoprotein, signal transduction molecules, and virus-encoded miRNAs into multiple cell types and activate cell-signaling pathways. These findings suggest that through exosomes EBV could manipulate the tumor microenvironment to enhance tumor progression. LMP1 is not always detected within every tumor cell or sample. However, the exosomal transfer of LMP1 and other signaling molecules could impact growth control of other cells within a tumor. Immune detection would also be impaired by this limited expression within a rare cell. The data described here clearly show that LMP1 can be transferred to cells through exosomes and that NPC exosomes can activate growth-signaling pathways.
It is presently unknown how the levels of exosomes used in these studies compare with the in vivo situation as the concentration of exosomes and transfer efficiency are likely to be much greater at the cell–cell interface, perhaps similar to what has been described for the transfer of components between neurons and immune cells (25). The ability to detect LMP1 in the exosome pellet harvested from serum of mice carrying the C15 tumor supports a physiological role of LMP1-containing exosomes in NPC.
The data presented here indicate that LMP1 is efficiently transported into exosomes and likely affects the components of exosomes, perhaps indirectly, through its effects on cellular expression. The biogenesis of exosomes is linked to both multivesicular bodies and lipid rafts (26, 27). Protein sorting into multivesicular bodies is thought to involve monoubiquitination of cargo proteins for entry into the endosomal sorting complex. LMP1 is ubiquitinated and has been detected in multivesicular bodies (15, 28). Protein targeting into exosomes also involves lipid raft microdomains, and purified exosomal membrane components are enriched with molecules that are characteristic of lipid rafts, including sphingomyelin, cholesterol, and the glycolipid GM3 (27, 29). Thus, the partitioning of some proteins to lipid rafts likely contributes to exosome targeting. LMP1 localizes to lipid raft microdomains, and this localization is critical for LMP1-mediated signaling events (30, 31). The findings presented here indicate that LMP1 is enriched in exosomes and that exosomes also contain signaling proteins such as EGFR and PI3K through which LMP1 affects cell growth.
This study also reveals that the exosomes produced by LMP1-expressing epithelial cells can be taken up by fibroblast, epithelial, and endothelial cells. It is presently unclear exactly how exosomes contact and enter cells and, similarly to viruses, may have diverse entry pathways (32). The confocal images confirm that C666 exosomes both bind and are internalized by endothelial cells, and the punctate internal staining is similar to endocytic uptake of exosomes. An additional property of the LMP1-containing exosomes is their potential enhanced binding to HUVECs. LMP1 affects the expression of multiple cellular proteins that are involved in adhesion and cell communication. The further analysis of the exosomal components modulated by LMP1 may likely identify proteins that are increased within exosomes and facilitate binding and entry.
A recent study showed that EBV-transformed lymphocytes produced exosomes that contained EBV miRNAs and that these miRNAs could modulate their known targets within the recipient cell (22). The findings in this study reveal that the EBV BART miRNAs that are most abundant in NPC and other epithelial tumors are also present within exosomes produced from infected epithelial cells. Of particular interest is the possibility that certain viral miRNAs are detected at higher relative levels within exosomes than within the cell, possibly reflecting preferential sorting into exosomes. Perhaps a subset of the viral miRNAs specifically functions in cells other than those producing them. A recent study demonstrated that components of the miRNA effector complexes associate with multivesicular bodies and are secreted into exosomes (33). One component, GW182, that is required for miRNA function through its association with argonaute 2 (AGO2) was dramatically enriched in exosomes. These findings suggest that exosomes may not only transfer viral miRNAs but also deliver cellular components of the RNA-induced silencing complex to enhance their function.
This study indicates that LMP1, and possibly other viral proteins, may have potent effects on exosome components and functions. It is likely that other viruses that establish long-term, latent, or chronic infections also modulate exosomes to enhance their persistence. The further understanding of how viruses modulate exosome production and function will likely clarify their biogenesis and the mechanisms through which exosomes affect intercellular communication and potentially enable persistent infection.
Materials and Methods
Cell Culture.
C33A cervical carcinoma cells that express pBabe (vector), or pBabe-HA-LMP1 have been described previously (34). C666-1 cells and those stably expressing HA-LMP1 were produced by retrovirus transduction as described (35). HUVECs were cultured in endothelial cell growth media-2 (Lonza) supplemented with the supplied growth factors, 12% FBS, and antibiotic/antimycotic. All cells were maintained at 37 °C with 5% CO2. The C15 xenograft tumor was cultured in vitro for 2 d as previously described (12).
Antibodies.
Antibodies were purchased as indicated: pAkt (Ser473) and anti-Akt (Cell Signaling); anti-GRP78, anti-HSC70, anti-actin, anti-pERK, total ERK, anti-GAPDH, and anti-TSG101 (Santa Cruz Biotechnology); anti-PI3K (Upstate); and anti–flotillin-2 (BD Biosciences). S12 anti-LMP1 was obtained from supernatants of the hybridoma produced by David A. Thorley-Lawson (Tufts University). The pooled anti-LMP1 rat monoclonal antibodies (1G6, 7G8, and 7E10) were obtained from Ascenion.
Exosome Isolation and Transfer.
Confluent monolayers were grown to 1 d postconfluency. Exosomes were collected by differential centrifugation from conditioned media or C15 serum, resuspended into PBS, and stored at −80 °C until use (36). Cell monolayers were washed twice with PBS and incubated with purified exosomes (200–400 μg) or PBS for the indicated times at 37 °C in serum-free media. Cells exposed to exosomes were washed three times with PBS, scraped into cold PBS, pelleted, and lysed in radioimmunoprecipitation assay (RIPA) buffer (20). Subconfluent Rat-1 cells were exposed to conditioned, clarified media from C33A cells every 24 h for 5 d.
DiI-Labeled Exosomes to HUVECs.
C666-1 cells were grown in exosome-free media for 5 d. Purified exosomes from equivalent cell numbers were labeled with 1 μM DiI (Invitrogen) or 1 mM R18 (Invitrogen) as previously described (37). Pelleted exosomes were washed to remove unbound DiI and were resuspended in PBS/5% BSA. Exosomes from equivalent numbers of cells were exposed to HUVECs. Recipient cells were then washed in PBS, fixed in 4% paraformaldehyde, and imaged by confocal microscopy (Olympus FV500), or for adhesion assays fluorescence was determined by using a plate reader with 544/590-nm Ex/Em filters. To correct for potential differences between exosome concentration or labeling, the percentage of exosomes adhered was calculated from the total DiI fluorescence of the individual washed and pelleted samples. SDs were calculated from triplicate wells exposed to three separate preparations of exosomes. For annexin V blocking, R18-labeled exosomes were preincubated with 2 μg/mL annexin V (BD Biosciences) before addition to HUVECs, and SEM was calculated from triplicate wells of two independent experiments.
RNA Preparations and RT-PCR.
Total RNA from cell pellets or exosome pellets of the C666-1 cell line was prepared with TRIzol reagent (Invitrogen) per manufacturer's directions. Quantitative RT-PCR was performed for the BART miRNAs from equal amounts of total C666 exosome and cellular RNA by using the miScript system (Qiagen) with SYBR green dye. Relative abundance of miRNAs was determined by subtracting the cycle threshold of the cellular miRNAs from the exosomal miRNAs to the log base 2. The small nuclear RNA U5 was included as a control.
Northern Blotting.
Northern blots for miRNAs were prepared by using precast 15% urea tris-borate-EDTA gels (Bio-Rad) and transferred to Hybond N+ (GE Biosciences) (38). After UV cross-linking, the membranes were hybridized at 37 °C with an end-labeled anti-sense oligo to the miR-Bart7 by using ExpressHyb solution (Clontech) as directed. Total RNA from the BCBL1 cell line and the C15 xenograft were used as negative and positive controls.
Acknowledgments
This work was supported by Public Service Grants CA32979, CA138811, and CA19014 (to N.R.-T.) and 5059-08 from the Leukemia and Lymphoma Society (to A.R.M.). D.G.M. was supported by training Grant T32CA009156.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Raab-Traub N. Epstein–Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- 2.Kaye KM, Izumi KM, Kieff E. Epstein–Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 4.Morris MA, Dawson CW, Young LS. Role of the Epstein–Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol. 2009;5:811–825. doi: 10.2217/fon.09.53. [DOI] [PubMed] [Google Scholar]
- 5.Miller WE, Earp HS, Raab-Traub N. The Epstein–Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller WE, Raab-Traub N. The EGFR as a target for viral oncoproteins. Trends Microbiol. 1999;7:453–458. doi: 10.1016/s0966-842x(99)01605-4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schorey JS, Bhatnagar S. Exosome function: From tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Mori Y, et al. Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic. 2008;9:1728–1742. doi: 10.1111/j.1600-0854.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keryer-Bibens C, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klibi J, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein–Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 14.Dukers DF, et al. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165:663–670. doi: 10.4049/jimmunol.165.2.663. [DOI] [PubMed] [Google Scholar]
- 15.Ceccarelli S, et al. Epstein–Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494–1506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 16.Houali K, et al. A new diagnostic marker for secreted Epstein Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- 17.Cheung ST, et al. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein–Barr virus. Int J Cancer. 1999;83:121–126. doi: 10.1002/(sici)1097-0215(19990924)83:1<121::aid-ijc21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Keller S, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: Current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 20.Mainou BA, Everly DN, Jr., Raab-Traub N. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol. 2007;81:9680–9692. doi: 10.1128/JVI.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 22.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai X, et al. Epstein–Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilligan K, et al. Novel transcription from the Epstein–Barr virus terminal EcoRI fragment, DIJhet, in a nasopharyngeal carcinoma. J Virol. 1990;64:4948–4956. doi: 10.1128/jvi.64.10.4948-4956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: A focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 28.Rothenberger S, Burns K, Rousseaux M, Tschopp J, Bron C. Ubiquitination of the Epstein–Barr virus-encoded latent membrane protein 1 depends on the integrity of the TRAF binding site. Oncogene. 2003;22:5614–5618. doi: 10.1038/sj.onc.1206497. [DOI] [PubMed] [Google Scholar]
- 29.Wubbolts R, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 30.Ardila-Osorio H, et al. TRAF interactions with raft-like buoyant complexes, better than TRAF rates of degradation, differentiate signaling by CD40 and EBV latent membrane protein 1. Int J Cancer. 2005;113:267–275. doi: 10.1002/ijc.20503. [DOI] [PubMed] [Google Scholar]
- 31.Yasui T, Luftig M, Soni V, Kieff E. Latent infection membrane protein transmembrane FWLY is critical for intermolecular interaction, raft localization, and signaling. Proc Natl Acad Sci USA. 2004;101:278–283. doi: 10.1073/pnas.2237224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh M, Helenius A. Virus entry: Open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 34.Kung CP, Raab-Traub N. Epstein–Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J Virol. 2008;82:5486–5493. doi: 10.1128/JVI.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res. 2008;68:6997–7005. doi: 10.1158/0008-5472.CAN-08-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9:2820–2835. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- 37.Hood JL, Pan H, Lanza GM, Wickline SA, Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN) Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards RH, Marquitz AR, Raab-Traub N. Epstein–Barr virus BART microRNAs are produced from a large intron prior to splicing. J Virol. 2008;82:9094–9106. doi: 10.1128/JVI.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]