Abstract
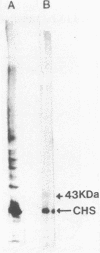
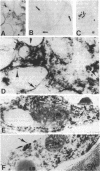
Chalcone synthase [naringenin-chalcone synthase; malonyl-CoA:4-coumaroyl-CoA malonyltransferase (cyclizing), E.C. 2.3.1.74], the key enzyme of flavonoid pathways that was believed to be soluble, has been localized on ribosome-bearing endoplasmic reticulum membranes in the epidermis of buckwheat (Fagopyrum esculentum M.) hypocotyls. Enzyme activity measurement and immunoblots of buckwheat hypocotyl homogenates that were fractionated on linear sucrose density gradients and developed with a specific chalcone synthase antibody and a 20-nm ImmunoGold conjugate showed the presence of chalcone synthase in fractions enriched in endoplasmic reticulum membranes. The presence of chalcone synthase on these membranes was not caused by nonspecific adsorption or entrapment of proteins. Immunocytochemical investigations with both a 5-nm and a 20-nm ImmunoGold conjugate showed that chalcone synthase was associated with the cytoplasmic face of rough (ribosome bearing) endoplasmic reticulum membranes. Plasma membrane, nucleus, plastids, mitochondria, golgi, and the tonoplast were not labeled. These data are consistent with our earlier described model suggesting that the synthesis of phenylpropanoids and flavonoids takes place partially or fully on membrane-associated enzyme complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Demuth G., Hinz H., Seligmann O., Wagner H. Investigations on anthrachinonglycosides of Rhamnus species, V Emodin-8-O-beta-gentiobiosid,e a new O-glycoside from Rhamnus frangula. Planta Med. 1978 Feb;33(1):53–56. doi: 10.1055/s-0028-1097358. [DOI] [PubMed] [Google Scholar]
- Fossel E. T., Solomon A. K. Ouabain-sensitive interaction between human red cell membrane and glycolytic enzyme complex in cytosol. Biochim Biophys Acta. 1978 Jun 16;510(1):99–111. doi: 10.1016/0005-2736(78)90133-5. [DOI] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Lifson E., Weeden N. F. Isolation and characterization of buckwheat (Fagopyrum esculentum M.) chalcone synthase and its polyclonal antibodies. Arch Biochem Biophys. 1986 Jun;247(2):414–419. doi: 10.1016/0003-9861(86)90600-4. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Wagner G. J. Metabolic pathways as enzyme complexes: evidence for the synthesis of phenylpropanoids and flavonoids on membrane associated enzyme complexes. Arch Biochem Biophys. 1985 Feb 15;237(1):88–100. doi: 10.1016/0003-9861(85)90257-7. [DOI] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Specificity in the association of glyceraldehyde 3-phosphate dehydrogenase with isolated human erythrocyte membranes. J Biol Chem. 1973 Dec 25;248(24):8457–8464. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Moses V. The tentative identification in Escherichia coli of a multienzyme complex with glycolytic activity. Eur J Biochem. 1976 Jun 15;66(1):25–36. doi: 10.1111/j.1432-1033.1976.tb10421.x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Solti M., Bartha F., Halász N., Tóth G., Sirokmán F., Friedrich P. Localization of glyceraldehyde-3-phosphate dehydrogenase in intact human erythrocytes. Evaluation of membrane adherence is autoradiographs at low grain density. J Biol Chem. 1981 Sep 10;256(17):9260–9265. [PubMed] [Google Scholar]
- Titus D. E., Becker W. M. Investigation of the glyoxysome-peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J Cell Biol. 1985 Oct;101(4):1288–1299. doi: 10.1083/jcb.101.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J., Hrazdina G. Endoplasmic reticulum as a site of phenylpropanoid and flavonoid metabolism in hippeastrum. Plant Physiol. 1984 Apr;74(4):901–906. doi: 10.1104/pp.74.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wombacher H. Molecular compartmentation by enzyme cluster formation. A view over current investigations. Mol Cell Biochem. 1983;56(2):155–164. doi: 10.1007/BF00227216. [DOI] [PubMed] [Google Scholar]