Abstract
Development of the mammalian heart is mediated by complex interactions between myocardial, endocardial, and neural crest-derived cells. Studies in Drosophila have shown that the Slit-Robo signaling pathway controls cardiac cell shape changes and lumen formation of the heart tube. Here, we demonstrate by in situ hybridization that multiple Slit ligands and Robo receptors are expressed in the developing mouse heart. Slit3 is the predominant ligand transcribed in the early mouse heart and is expressed in the ventral wall of the linear heart tube and subsequently in chamber but not in atrioventricular canal myocardium. Furthermore, we identify that the homeobox gene Nkx2-5 is required for early ventral restriction of Slit3 and that the T-box transcription factor Tbx2 mediates repression of Slit3 in nonchamber myocardium. Our results suggest that patterned Slit-Robo signaling may contribute to the control of oriented cell growth during chamber morphogenesis of the mammalian heart.
Keywords: Slit/Robo pathway, cardiac development, mouse, Tbx, atrioventricular canal
INTRODUCTION
Cardiogenesis is one of the earliest and most critical steps during vertebrate organogenesis. Heart development begins when cardiac progenitor cells in the anterior lateral mesoderm cluster in the primary heart field (Harvey, 2002). These cells give rise to the cardiac crescent and linear heart tube containing the future left ventricle and atrioventricular canal (AVC; see Buckingham et al., 2005). Subsequently the heart tube undergoes rightward looping (Harvey, 2002). As looping progresses, cells of the second heart field in splanchnic mesoderm are added to the heart tube to form the outflow tract (OFT), right ventricle, atria and inflow tract regions (Buckingham et al., 2005). Subsequently, atrial and ventricular chambers form through a localized process that involves differential growth or “ballooning” of the outer curvature of the heart tube (Christoffels et al., 2000). Importantly, part of the heart tube, including the OFT, inner curvature, AVC, and inflow tract, escapes this developmental chamber program through the repressive action of the T-box factors, Tbx2 and Tbx3 (Habets et al., 2002; Christoffels et al., 2004b; Harrelson et al., 2004; Bakker et al., 2008). Regionalized gene expression provides evidence for the presence of dorsoventral patterning in the early tube that precedes chamber development (Christoffels et al., 2004a). A retrospective clonal analysis of cardiac cells has shown that myocardium, from the time of its formation, is a polarized and regionalized tissue in which oriented cell growth may be important in shaping the chambers (Meilhac et al., 2004). Although key factors that play a role in forming the heart tube have been identified, including GATA4, NKX2-5, dHAND, TBX5, or RALDH2 (Harvey, 2002), the molecular effectors of cell polarity and cell shape changes remain unknown.
The extracellular-matrix molecule Slit and its Robo (roundabout) family receptors have been implicated in the regulation of cell polarity and morphogenesis during formation of the cardiac tube in Drosophila (Qian et al., 2005; MacMullin and Jacobs, 2006; Medioni et al., 2008; Santiago-Martinez et al., 2008). In particular, the Slit-Robo pathway is required for progressive polarization of cardiac cells during migration to the midline (Medioni et al., 2008). In contrast to the single Slit and three Robo genes in Drosophila, three distinct Slit genes (Slit1, Slit2, and Slit3) and four distinct Robo genes (Robo1, Robo2, Rig1/Robo3, and Robo4) are found in mammals (Chedotal, 2007). Slit functions as a repulsive ligand for the Robo-family receptors in the central nervous system (CNS), and acts both attractively and repulsively in somatic muscles (Kidd et al., 1998, 1999; Brose et al., 1999; Simpson et al., 2000; Wu et al., 2001). In addition, both gene families display distinct expression patterns outside the CNS (Holmes et al., 1998; Yuan et al., 1999; Strickland et al., 2006). Remarkably, Slit3 is widely expressed in different organs, including the tongue, kidney, pharynx, umbilical cord vein, heart, lung, and diaphragm (Yuan et al., 1999; Liu et al., 2003; Yuan et al., 2003). Consistent with its expression in non-neural tissues, studies have established that Slit3 is required for angiogenesis and formation of the diaphragm and kidney (Liu et al., 2003; Yuan et al., 2003; Zhang et al., 2009).
The role of the Drosophila Slit-Robo pathway in regulating changes in cell shape during cardiac tube formation prompted us to conduct a detailed analysis of murine Slit and Robo gene expression during mouse heart development. We describe that Slit3 in particular, shows a specific localization in the ventral region of the forming heart tube and subsequently restriction to chamber myocardium. We show that the homeobox gene Nkx2-5 is required for the ventral restriction of Slit3 in the linear heart tube. In addition, we demonstrate that the T-box factors Tbx2 and Tbx20 mediate the restriction of Slit3 expression to the chamber myocardium. Given the spatial and temporal expression profile of Slit3 and its role in polarized growth and migration in other tissues, we propose that Slit-Robo signaling may be required for cardiac chamber expansion.
RESULTS AND DISCUSSION
Expression of Mouse Slit and Robo Genes During Heart Development
Previous studies in Drosophila have shown that the Slit-Robo signaling pathway controls cardiac cell polarity and formation of the cardiac lumen (Medioni et al., 2009). Therefore, we decided to analyze the expression pattern of murine Slit and Robo genes in the developing heart. In contrast to other tissues, detailed analysis of the cardiac expression pattern of Slit and Robo genes has not been reported (Holmes et al., 1998; Yuan et al., 1999; Holmes and Niswander, 2001; Liu et al., 2003; Yuan et al., 2003). Mouse embryos between 7.5 and 12.5 days of development (E7.5–E12.5) were hybridized with antisense riboprobes for Slit1, Slit2, Slit3, Robo1, Robo2, Rig1/Robo3, and Robo4 (Figs. 1, 2; also see Supp. Fig. S1, which is available online).
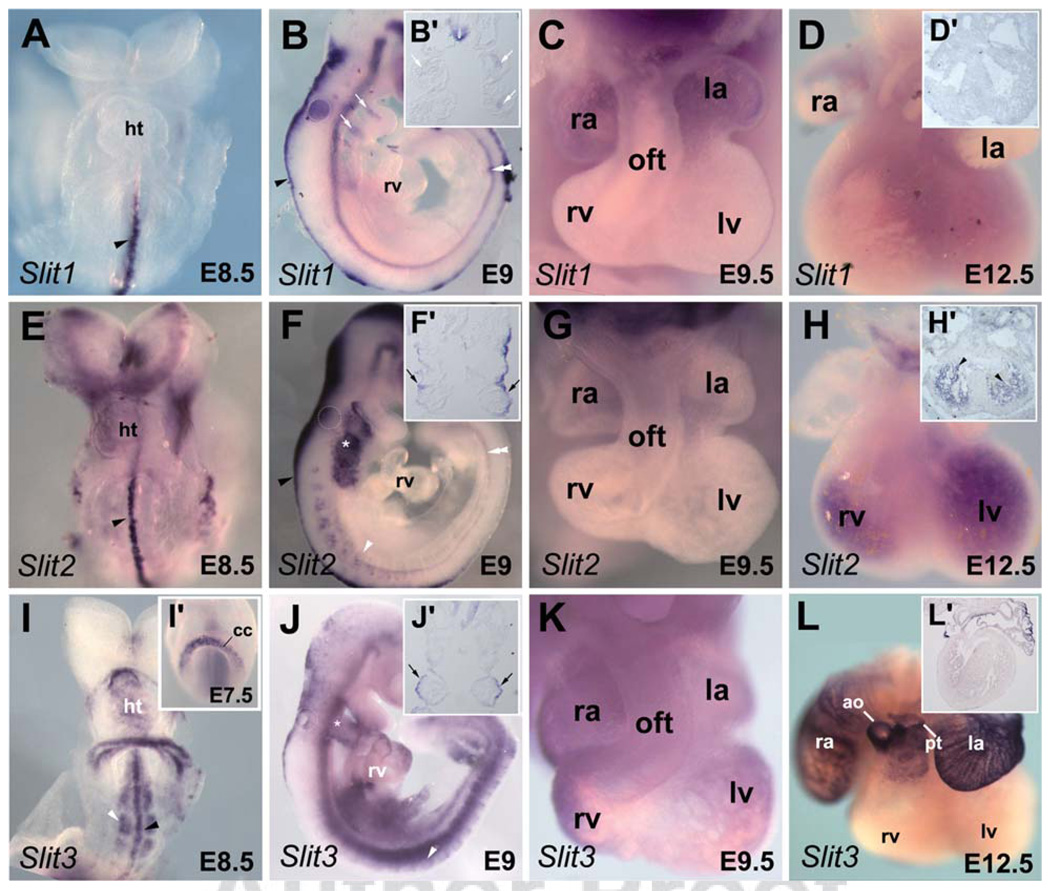
Fig. 1.
Expression pattern of Slit genes during embryonic heart development. Whole-mount in situ hybridization analysis of embryos with Slit1 (A–D), Slit2 (E–H), and Slit3 (I–L) probes. A: Ventral view of embryonic day (E) 8.5 embryo showing Slit1 expression in the ventral midline (arrowhead). Slit1 is not detected in the forming heart tube. B: Lateral view of E9 embryo showing Slit1 expression in the roof plate (arrowhead), in the floor plate (double arrowhead), and the pharyngeal region (white arrows). B′: Frontal section (same embryo depicted in B) showing Slit1 expression in the mesodermal core of the pharyngeal arch (white arrows) and in the floor plate of the spinal cord. C: High magnification picture of heart at late E9.5 showing Slit1 in atrial wall. D: Dissected E12.5 heart showing no expression of Slit1. D′: In situ hybridization with Slit1 on section of E12.5 heart. E: Slit2 was not detected in the heart of embryo at E8.5 stage. Note its expression at the ventral midline (arrowhead). F: Lateral view of early E9.5 embryo showing Slit2 expression in the pharyngeal region (asterisk). Slit2 is also detected in the roof plate (arrowhead), the notochord (double arrowheads) and the somites (white arrowhead). F′: Frontal section (same embryo depicted in F) showing Slit2 expression in the pharyngeal ectoderm surface (arrows). G: Dissected E9.5 heart showing no expression of Slit2. H: A robust expression of Slit2 is detected in trabeculae of both ventricles at E12.5. H′: In situ hybridization on section of E12.5 heart shows expression in the trabeculae (arrowhead). I,I′: Ventral views of E7.5 and E8.5 embryos showing Slit3 expression in the cardiac crescent and the forming heart tube. Expression of Slit3 is also detected in the ventral midline (black arrowhead) and the somites (white arrowhead) of E8.5 embryo. J: Lateral view of early E9.5 embryo showing Slit3 expression in the heart and the pharyngeal ectoderm surface (asterisk) and in the somites (white arrowhead). J′: Frontal section (same embryo depicted in J) showing expression of Slit3 the pharyngeal ectoderm surface (arrows). K: High magnification picture of the heart at late E9.5. Expression of Slit3 is seen in the outflow tract, the atria and ventricles of the embryonic heart. L: At E12.5, expression of Slit3 is maintained in the right and left atria and in great arteries, the aorta and pulmonary trunk. L′: Section of the heart shown in L. ao, aorta; cc, cardiac crescent; ht, heart tube; la, left atrium; lv, left ventricle; oft, outflow tract; pt, pulmonary trunk; ra; right atrium; rv, right ventricle.
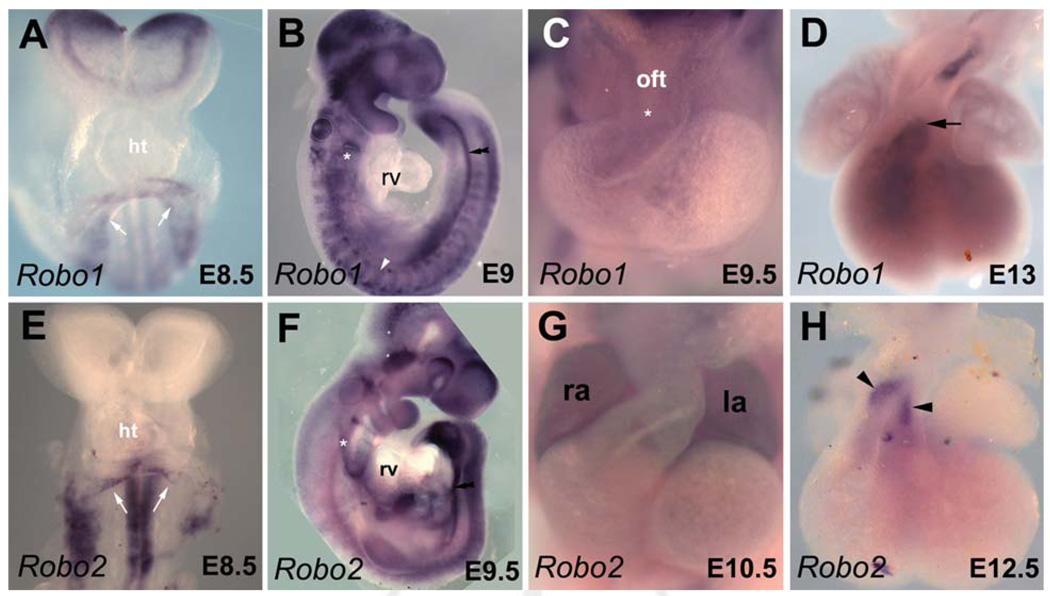
Fig. 2.
Expression pattern of Robo genes during embryonic heart development. Whole-mount in situ hybridization analysis of embryos with Robo1 (A–D) and Robo2 (E–H) probes. A: Ventral view of early embryonic day (E) 8.5 embryo showing Robo1 expression in the venous pole (white arrows) of the forming heart tube. B: Lateral view of early E9 embryo. Robo1 expression is detected in the notochord (double arrowhead), the somites (white arrowhead), and the ectodermal pouches of the pharyngeal arches (asterisk) but not in the heart. C: High magnification picture of the heart at late E9.5 showing Robo1 in the cushions (asterisk) of the outflow tract. D: Dissected E12.5 heart showing expression of Robo1 at the base of the great arteries (arrow). E: Ventral view of early E8.5 embryo. High Robo2 expression levels are visible in the neural tube and the venous pole (white arrows) of the forming heart tube. F: Lateral view of E9.5 embryo. Weak expression of Robo2 is detected in the looped heart, the ectoderm of the pharyngeal arches (asterisk) and the notochord (double arrowheads). G: High magnification of the heart at E10.5 showing Robo2 in both atria. H: Dissected E12.5 heart showing expression of Robo2 in the great arteries (arrowheads). ht, heart tube; la, left atrium; lv, left ventricle; oft, outflow tract; ra; right atrium; rv, right ventricle.
During this period of development, Slit1 expression was primarily observed in the roof plate and the floor plate (Fig. 1A,B). Slit1 transcripts were also observed in the mesodermal core of the pharyngeal arches (Fig. 1B,B′). Interestingly, pharyngeal mesoderm has been shown to contribute to the formation of the OFT as well as the pharyngeal arch artery (PAA) development (see Kelly and Buckingham, 2002). At E9.5, Slit1 expression was detected in the developing heart in the left and right atria (Fig. 1C). However, at E12.5 we did not observe any expression in the heart (Fig. 1D,D′).
Similarly to Slit1, Slit2 expression was observed prominently in neural tissue (Fig. 1E,F). From E8.5 to E9.5, Slit2 expression was not detected in the myocardium; however, it was highly expressed in the pharyngeal region at these stages (Fig. 1F) as reported by others (Yuan et al., 1999; Calmont et al., 2009). Strong expression of Slit2 was seen in the pharyngeal surface ectoderm (Fig. 1F′). This tissue has been shown to be a crucial source of signals for fourth PAA formation and remodeling (Kirby, 2007). Slit2 has also been identified as a downstream target of Tbx1, and is implicated in cardiac neural crest cells (NCC) migration at the time of PAA formation (Calmont et al., 2009). While no clear expression in the embryonic heart was detected at E9.5 (Fig. 1G), a strong expression of Slit2 was observed in the trabecular region of the ventricular chambers at E12.5 (Fig. 1H,H′). Of interest, the trabecular formation occurs when cardiomyocytes migrate toward the endocardium, which is coincident with upregulation of cell adhesion molecules (Ong et al., 1998). Thus, our observation suggests that other cell signaling molecules such as Slit may be involved in this process.
Slit3 is the earliest Slit gene to be expressed in the developing heart. Transcripts were observed in the cardiac crescent at E7.5 and in the linear heart tube at E8.5 (Fig. 1I,I′). At E8.5, Slit3 expression is observed on the ventral wall of the linear heart tube (see Fig. 3A,B). Slit3 is also expressed in the ventral midline and developing somites (Fig. 1I,J). Unlike the other Slit genes, Slit3 expression was observed in all compartments of embryonic heart at E9.5, restricted to the outer curvature of the looped heart (Fig. 1J,K). The myocardium of the outer curvature is known to give rise to the ventricular chamber or “working” myocardium (de la Cruz and Markwald, 1999; Christoffels et al., 2000). Analysis of the distribution of clonally related myocytes has demonstrated that different patterns of oriented cell growth underlie regional differences in morphogenesis within the embryonic heart (Meilhac et al., 2004). The expression pattern of Slit3 and its established role in polarized growth and migration in other tissues suggest implication of Slit-Robo signaling in the oriented cell growth that accompanies ballooning of the ventricular chambers (Christoffels et al., 2004a). By E12.5, expression of Slit3 was observed only in myocardium of the atria and at the base of the great arteries (Fig. 1L,L′), in agreement with published expression data (Liu et al., 2003). Remarkably, during early heart development, Slit2 and Slit3 are both distributed within the ventricular chambers, suggesting requirement of a specific Slit ligand during ventricular and trabecular formation of the embryonic heart. Despite that difference, Slit2 and Slit3 were detected in the surface pharyngeal ectoderm of embryo at E9.5 (Fig. 1F,F′,J,J′), suggesting redundancy of these molecules during cardiac NCC migration and PAA formation. The Slit-Robo signaling pathway is known to be involved in trunk neural crest migration (Jia et al., 2005). It would be interesting to identify whether Slit-Robo signaling mediates repulsive or attractive signals during cardiac NCC migration.
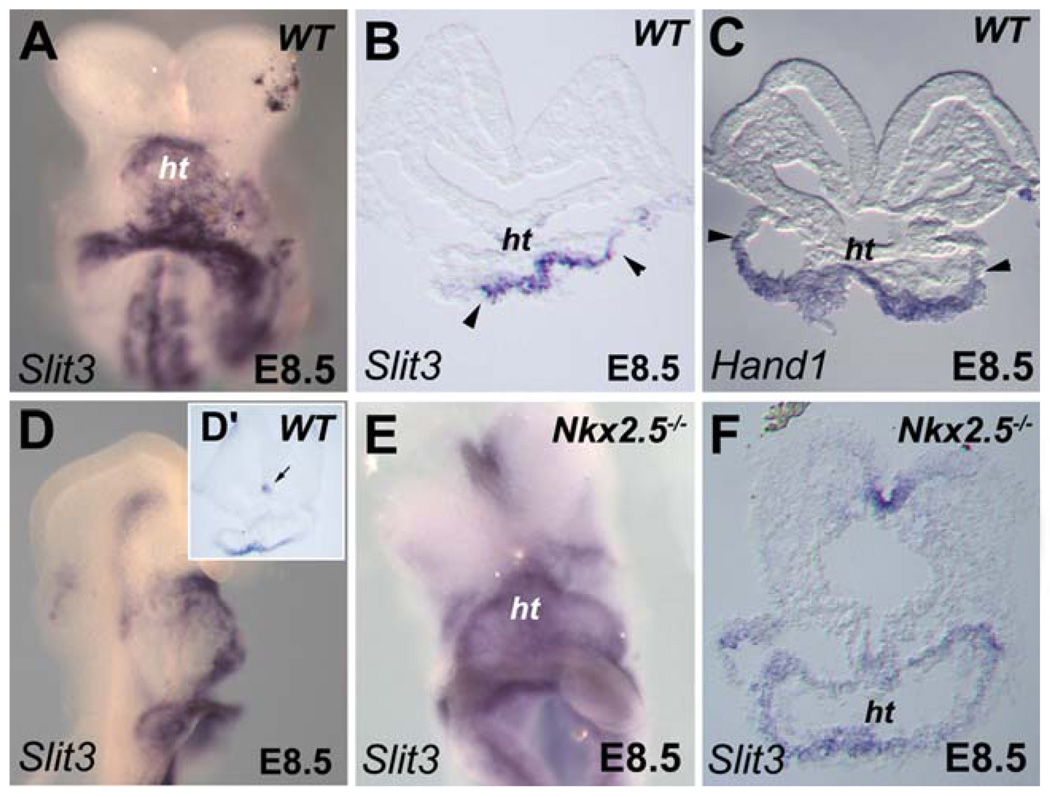
Fig. 3.
Dorsoventral patterning of Slit3 expression in the linear heart tube requires Nkx2-5. A: Ventral view of embryo at early embryonic day (E) 8.5. Slit3 is highly expressed in the ventral side of the forming heart tube. B: Section (same embryo as depicted in A) showing that Slit3 expression in the heart tube is strictly ventral as delimited by the arrowheads. C: Expression of Hand1 is shown as a reference to indicate the ventral side (arrowheads) of the heart tube at E8.5. D,E: Comparison of Slit3 expression in wild-type (WT) and Nkx2-5−/− embryos at E8.5. D: Lateral view of WT embryo showing high expression of Slit3 in the ventral side of the forming heart tube. D′: Section of the embryo shown in D. Note the expression of Slit3 in the floor plate (arrow). E: Slit3 expression is maintained in Nkx2-5−/− embryo. F: Section (same embryo as shown in E) reveals that Slit3 is uniformly expressed in heart of Nkx2-5−/− embryo. ht, heart tube; nt, neural tube.
Robo family members Robo1-4 are the putative receptors for Slit ligands. Whereas Robo4 is specifically expressed in the vascular endothelium (Supp. Fig. S1; Park et al., 2003), the expression profile of the three other members during heart development has not been analyzed in detail (Yuan et al., 1999; Camurri et al., 2004; Jia et al., 2005). In situ hybridization with Robo1 and Robo2 riboprobes showed that these receptors were both expressed in the venous pole of the linear heart tube at E8.5 (Fig. 2A,E). In agreement with a recent study (Calmont et al., 2009), we found Robo1 expression in migrating NCCs (Fig. 2B). At E9.5, Robo1 expression was faintly detected in the endocardial cushions of the OFT (Fig. 2C). Later, Robo1 expression was maintained in the great arteries (Fig. 2D). Unlike other Robo genes, a consistent expression of Robo2 was observed in both atria of the heart at E10.5 (Fig. 2G). At E12.5, Robo2 expression was observed in the endocardium of the great arteries (Fig. 2H). The specific expression of Slit ligand and Robo receptor genes in the venous pole at E8.5 and in the atria from E9.5 to E12.5 suggests a role for these molecules in oriented cell growth and migration during atrial morphogenesis (Meilhac et al., 2004). Finally, from our in situ hybridization analysis we did not detect any expression of Rig1/Robo3 and Robo4 in the developing mouse heart at any time-point analyzed (Supp. Fig. S1).
Dorsoventral Patterning of Slit3 Is Controlled by Nkx2-5
As noted above, whole-mount in situ hybridization analysis revealed expression of Slit3 transcripts in the linear heart tube at E8.5 (Fig. 1I). At this stage, Slit3 expression is comparable to the expression of the basic helix–loop–helix (bHLH) transcription factor gene, Hand1, which is restricted to the ventral wall of the forming heart tube (Fig. 3A–D; Biben and Harvey, 1997; Christoffels et al., 2000; Togi et al., 2004). Although Slit3 is expressed at high levels in the developing heart, early cardiac defects have not been reported in Slit3 mutant embryos (Liu et al., 2003; Yuan et al., 2003). However, the expression of Slit3 detected in the outer curvature of the looped heart and the enlarged right ventricle observed in the hearts of Slit3 mutant mice (Liu et al., 2003), suggest a role for Slit signaling in ventricular chamber formation. Further studies are required to determine whether subtle changes may exist in the heart of these mutants especially during the formation of the myocardium chambers and the great arteries.
We subsequently examined expression of Slit3 in mutant mice affecting cardiac morphogenesis. Mutations in the NK-like homeobox gene, Nkx2-5/Csx, causes early embryonic lethality with cardiac development arrested at the linear heart tube stage, before looping (Komuro and Izumo, 1993; Lyons et al., 1995; Biben and Harvey, 1997). In Nkx2-5−/− embryos, the early expression of Slit3 was indistinguishable from that in control embryos (Fig. 3E). However, we found that the dorsoventral pattern of Slit3 expression in the linear heart tube was perturbed and expression was observed throughout the mutant heart tube (Fig. 3E,F). This result suggests that the myocardium of Nkx2-5−/− embryos is competent to express Slit3 but not to interpret signals that restrict expression on the ventral side of the heart. Of note, two putative Nkx2-5 binding motifs (TGAAGTGATG and TAAAGTGGGT) are found in a 3,000 bp Slit3 5′ proximal fragment as predicted using the TFSEARCH program (http://mbs.cbcr.jp/research/db/TFSEARCH.html).
Expression of Robo receptor genes in the venous pole (Fig. 2A,E) incited us to examine their expression in Nkx2-5−/− embryos. Of interest, we did not detect expression of Robo2 in the linear heart tube of Nkx2-5 mutant embryos (Supp. Fig. S2). This observation suggests that Nkx2-5 regulates in a different way Slit ligand and Robo receptor genes during the formation of the embryonic heart tube.
Regulation of the Chamber-Specific Expression Profile of Slit3
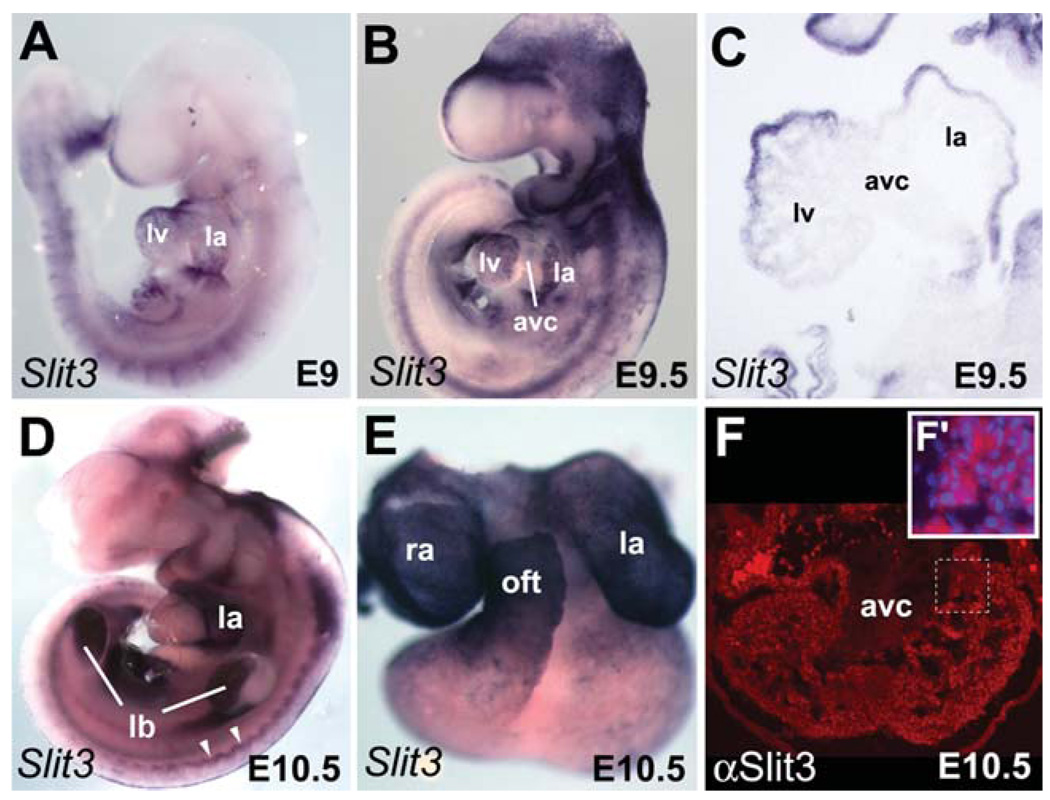
In looped hearts (E9–E9.5), Slit3 expression was confined to the atrial and ventricular myocardium but was clearly absent from the AVC (Fig. 4A–C), a pattern resembling that of atrial natriuretic factor (ANF; see Supp. Fig. S3A), Chisel, and Connexin40 at this stage (Christoffels et al., 2000). At E10.5 and E12.5 Slit3 expression was restricted to the atria and the OFT (Figs. 1L, 4C–E). Immunohistochemistry revealed Slit3 protein expression in both atrial and ventricular myocardium at E10.5 (Fig. 4F). The AVC region was negative, confirming our results by in situ hybridization. Detection of Slit3 protein but not mRNA in the ventricles of the heart at E10.5 (Fig. 4E,F) indicates a persistence of the protein in the chamber myocardium. Although Slit3 expression overlaps with Robo1 and Robo2 in the venous pole and later only with Robo2 in the atria, no Robo receptor genes were detected in the ventricular myocardium of the heart (Fig. 2). Despite its favorite link with Robo receptors, Slit contains domains that suggest association with the extracellular-matrix receptors (Chedotal, 2007). Furthermore, recent study in Drosophila have proposed that Slit is localized on cardiac cells by association with Dystroglycan (Dg), a proteoglycan (Medioni et al., 2008), and possibly also with αPS3/βPS1 Integrin (MacMullin and Jacobs, 2006).
Fig. 4.
Dynamic expression pattern of Slit3 during heart development. In situ hybridization (A–E) and immunofluorescence (F) were used to detect spatial expression of Slit3 in hearts from embryonic day (E) 9 to E10.5. A,B: Expression of Slit3 is detected in the whole heart of E9 embryo, whereas it is down-regulated in the AVC of late E9.5 embryo. C: Section (same embryo as depicted in B) showing expression of Slit3 in the chambers but not in the AVC. Note weak expression of Slit3 in the trabeculae of the left ventricle. D: Lateral view of E10.5 embryo. High expression of Slit3 is detected in the left atrium. Note Slit3 expression in the limb buds and the dermomyotome (white arrowheads). E: Dissected heart from the embryo shown in D. High expression is observed in the right and left atria and the outflow tract. F: Expression of Slit3 protein (red) is detected in the ventricular myocardium and trabeculae but not in the AVC of heart at E10.5. Higher magnification of the trabeculae region delimited by the dotted line is shown in the inset. F′: Immunodetection of Slit3 (red) and DAPI (blue) staining. Note Slit3 expression in the cytoplasm. ao, aorta; avc, atrioventricular canal; la, left atrium; lb, limb bud; lv, left ventricle; oft, outflow tract; pt, pulmonary trunk; ra, right atrium; rv, right ventricle.
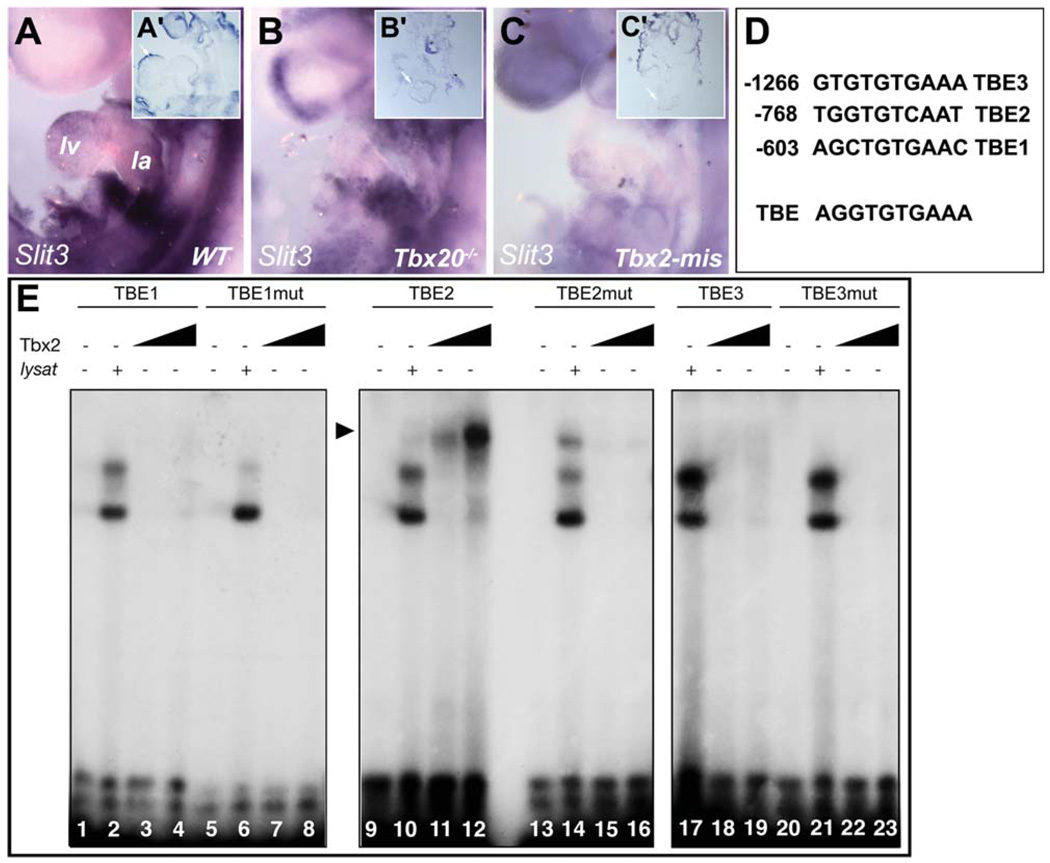
The absence of Slit3 gene expression and protein in AVC myocardium suggested potential regulation by the T-box transcriptional repressors Tbx2 and Tbx3 that are restricted to nonchamber myocardium (Supp. Fig. S3B,C), where they repress the chamber transcriptional program (Habets et al., 2002; Christoffels et al., 2004b; Harrelson et al., 2004; Bakker et al., 2008; Mesbah et al., 2008). Therefore, we examined Slit3 expression in mutant embryos deficient for Tbx2 and Tbx3. In situ hybridization on stage-matched embryos revealed activation of Slit3 in the AVC of E10.5 Tbx2−/− but not Tbx3−/− hearts (Supp. Fig. S4). This observation indicates that Tbx2 alone is sufficient to repress Slit3 in the nonchamber myocardium of Tbx3−/− hearts, consistent with previous findings on other chamber-specific genes (Bakker et al., 2008; Mesbah et al., 2008). The T-box factor Tbx20 is essential for embryonic chamber formation through its negative regulation of Tbx2 in the myocardium and endocardium (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005). To test the hypothesis that ectopic Tbx2 expression may be able to repress Slit3 in the forming heart, we analyzed Slit3 expression in Tbx20−/− embryos. Mutant embryos showed severe cardiac abnormalities including rudimentary ventricular chambers that did not further differentiate (Stennard et al., 2005). We could not detect any Slit3 expression in Tbx20−/− hearts at E9 (compare Fig. 5B,B′ with 5A,A′). Of interest, Drosophila Slit is also perturbed in the embryonic heart tube of Tbx20-ortho-log (H15nmr) mutants (Qian et al., 2005), suggesting a high evolutionary conservation of this regulation pathway. We also examined Slit3 expression in embryos misexpressing Tbx2 throughout the embryonic heart under the control of the XMLC2 promoter (XMLC2-rtTA/tetO-Tbx2; (Dupays et al., 2009). Consistent with our observation in Tbx20−/− embryos, Slit3 expression was significantly reduced (Fig. 5C,C′). The residual weak expression of Slit3 observed in these embryos may be explained by the mosaic expression of the transgene at this stage (Dupays et al., 2009). Moreover, we found three conserved T-box binding elements (TBEs; Kispert and Herrmann, 1993; Sinha et al., 2000) in the Slit3 5′ proximal promoters (Fig. 5D). The requirement of these TBEs for repression in the AVC and the complementary expression pattern between Tbx2 (Supp. Fig. S3) and Slit3 (Fig. 4B) prompted us to study the interaction of Tbx2 with the TBE motifs identified in the Slit3 promoter using electrophoretic mobility shift assay (EMSA) experiments. Oligonucleotide probes corresponding to the three TBE motifs were used. Tbx2 bound to the wild-type TBE2 motif but not TBE1 and TBE3, because a shifted band was detected only in lanes 11 and 12 (Fig. 5E). However, when the EMSA was performed with a mutated TBE2 motif this binding was abolished (Fig. 5E). Together these results suggest that Slit3 expression may be directly repressed by Tbx2 during AVC formation.
Fig. 5.
Disrupted cardiac expression of Slit3 in Tbx20 mutant and in Tbx2-misexpression embryos. A–C: Lateral views of wild-type (WT), Tbx20−/− mutant and Tbx2-misexpressing embryos. A′–C′: Sections of embryos shown in A–C. While Slit3 is present in the forming heart of WT embryo (A,A′), its expression is not detected in the heart of E9 Tbx20−/− embryo (B,B′). C,C′: Slit3 expression is highly reduced in heart misexpressing Tbx2. D: Sequence of three-conserved T-box binding elements (TBE) found in a Slit3 5′ proximal promoter. Numbers indicate position of the sequences from the ATG. TBE sequence shows the T-box binding site as determined previously (Kispert and Herrmann, 1993). E: Electrophoretic mobility shift assay shows that Tbx2 binds to the wild-type TBE2 motif (lanes 9–12) but not to the TBE1 (lanes 1–8) and TBE3 (lanes 17–23) motifs. Mutation of the TBE2 motif impairs Tbx2 binding (lanes 13–16). la, left atria; lv, left ventricle.
Conclusion
In this study, we have characterized Slit ligand and Robo receptor gene expression in the developing mouse heart. Our results suggest that Slit-Robo signaling, essential for morphogenesis of the Drosophila heart tube, may play roles in oriented cell growth during atrial and ventricular morphogenesis in vertebrates. Furthermore, we identify two upstream regulators of Slit3 expression, the predominant Slit ligand expressed in the early mouse heart: analysis of Slit3 expression in different mutant mouse embryos reveals that Slit3 is restricted to the ventral wall of the linear heart tube by Nkx2-5 regulated mechanisms and excluded from AVC myocardium by the transcriptional repressor Tbx2.
EXPERIMENTAL PROCEDURES
Animals and Tissue Preparation
All experiments involving animals were performed in accordance with French guidelines on the care and use of laboratory animals. After death by CO2 asphyxiation, embryo mice were removed from timed-pregnant CD1 or mutant mice. The day of vaginal plugging was defined as 0.5. Embryos were genotyped by polymerase chain reaction (PCR) using genomic DNA isolated from yolk sacs. The null alleles Tbx2tm1Pa, Tbx3tm1Pa, Tbx20lacZ, and Nkx2-5gfp (hereafter referred to as Tbx2−, Tbx3−, Tbx20−, and Nkx2-5−) were maintained on a mixed genetic background (Biben et al., 2000; Davenport et al., 2003; Harrelson et al., 2004; Stennard et al., 2005). Somites were counted for developmental staging and a sample of the yolk sac was taken for PCR genotyping using the following primers. Tbx3: the primers 5′-GGC CTC AAG TAG CTT GGA A-3′, 5′-AGG CCA ACA GAA GAG CAG A-3′, and 5′-CTA AGC CTG ATG GTG TGA G-3′ result in a 350 bp wild-type band and a 500 bp mutant band. Tbx2: the primers 5′-CCA GCC AGG GAA CAT AAT GAG G-3′, 5′-CTG TCC CCT GGC ATT TCT GG-3′, and 5′-CCT GCA GGA ATT CCT CGA CC-3′ result in a 180 bp wild-type band and a 88 bp mutant band. Nkx2-5: the primers 5′-GAA CCT GGA GCA GCA GCA GCG TAG C-3′ and 5′-CAG AAG GGA AGA GCT TGA GGT TCT C-3′ result in a 308 bp wild-type band and a 1,376 bp mutant band.
The tetO-Tbx2 and xMlc2-rtTA transgenes have been previously described (Dupays et al., 2009). Doxycycline was administered to pregnant females either by intraperitoneal injection (2 mg of Dox in 0.5 ml of 0.9% aqueous NaCl) at the indicated stage or by means of food (2 mg of Dox in 0.5 ml of 0.9% aqueous NaCl) from stages specified.
For early developmental stages whole embryos were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) overnight at 4°C, dehydrated and kept in methanol. Hearts or trunks were dissected and fixed in 4% PFA in PBS overnight at 4°C, transferred to 15% sucrose in PBS, followed by 15% sucrose 7% gelatin, and frozen in liquid nitrogen before cryo-sectioning at 10 µm.
Whole-Mount In Situ Hybridization
Whole-mount in situ hybridization was carried out as published (Zaffran et al., 2004). Probes were labeled according to the manufacturer’s instruction using the digoxigenin (DIG) -RNA labeling mix (Roche). The probes used for in situ hybridization were mSlit2 and mSlit3 (Yuan et al., 1999), rat Slit1, Robo1, Robo2 (Kidd et al., 1998), Rig1/Robo3, and mRobo4 3′-untranslated region (obtained by PCR). Hybridization signals were then detected by alkaline phosphatase (AP) -conjugated anti-DIG antibodies (1/2,000; Roche), which were followed by color development with NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate) substrate (Promega). After staining, the samples were washed in PBS and post-fixed. Embryos were imaged using a Zeiss Lumar stereomicroscope coupled to an Axiocam digital camera (AxioVision 4.4, Zeiss). The number of embryos examined was at least 3 for each stage.
Immunohistochemistry
For immunohistochemistry, sections were incubated as described previously (Zaffran et al., 2004). The Slit3 antibody, C-20, was a polyclonal antibody raised against a peptide mapping within an internal region of Slit3 of human origin from Santa Cruz, used at 1/50 dilution. The Robo1 and Robo2 antibodies were polyclonal antibody raised against ectodomains of rat Robo1 (amino acids 1–892) and rat Robo2 (amino acids 1–855) as described previously (Tamada et al., 2008). Secondary antibodies were donkey anti-goat or anti-rabbit, Alexa 488 used at 1/500 dilution (Molecular Probes). Sections were photographed using a Zeiss Axiovert 200M microscope with an Axiocam camera (AxioVision 4.4, Zeiss).
DNA-Binding Assay
For EMSA, the Tbx2 protein was produced with the TNT (T7) -coupled in vitro transcription/translation system (Promega). Production yields of Tbx2 protein was estimated by [35S]methionine labeling. EMSAs were performed in a 20-µl volume on ice with 104 cpm (0.5 ng) of either probes. Probes used were double-stranded: TBE1 (5′-TTTTTGTGTGTGAAAGTGCA), TBE 1mut (5′-TTTTTGTcaaaGAAAGTGCA), TBE2 (5′-TAGTGATGGTGTCAATACCGG), TBE2mut (5′-TAGTGATGcaaaCAATACCGG), TBE3 (5′-TGCCTTAGCTGTGAACACTAAT) and TBE3mut (5′-TGCCTTAGCTcaatACACTAAT) from the Slit3 promoter. Briefly, 3 or 9 µl of Tbx2 protein was gently added and incubated for 30 min with labeled probes and 0.1 mg of nonspecific competitor poly[(dC)] in a binding buffer 5× composition of 20% glycerol, 50 mM Tris-HCl pH7.5, 250 mM NaCl, 2.5 mM ethylenediaminetetraacetic acid, 2.5 mM dithiothreitol, and 0.25 mg BSA then loaded on a 4% polyacrylamide gel in 0.25× TBE buffer. The gel was dried and analyzed with a PhosphoImager.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. David Ornitz for his kind and generous gift of the Slit2 and Slit3 vectors, and Dr. Alain Chedotal for providing the Slit1 and Robo1, Robo2, and Robo3 vectors. We thank Dr. Fugio Murakami for generously providing Robo1 and Robo2 antibodies. We are grateful to Dr. Samir Merabet for allowing Bruno Hudry to carry out part of this work. We thank Dr. Robert Kelly for discussion and comments on the manuscript. S.Z. was funded by grants from the “Agence Nationale pour la Recherche” and V.E.P. was funded by the NIH. K.M. was supported by the European Commission under the FP7 CardioGeNet project.
Grant sponsor: Agence Nationale pour la Recherche; Grant number: ANR-007-MRAR-003; Grant sponsor: NIH; Grant number: 5R37HD033082; Grant sponsor: The European Commission; Grant number: HEALTH-2007-B-223463.
REFERENCES
- Bakker ML, Boukens BJ, Mommersteeg MT, Brons JF, Wakker V, Moorman AF, Christoffels VM. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ Res. 2008;102:1340–1349. doi: 10.1161/CIRCRESAHA.107.169565. [DOI] [PubMed] [Google Scholar]
- Biben C, Harvey RP. Homeodomain factor Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand during murine heart development. Genes Dev. 1997;11:1357–1369. doi: 10.1101/gad.11.11.1357. [DOI] [PubMed] [Google Scholar]
- Biben C, Weber R, Kesteven S, Stanley E, McDonald L, Elliott DA, Barnett L, Koentgen F, Robb L, Feneley M, Harvey RP. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circ Res. 2000;87:888–895. doi: 10.1161/01.res.87.10.888. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Zhou W, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S. T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development. 2005;132:2475–2487. doi: 10.1242/dev.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, Scambler PJ. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development. 2009;136:3173–3183. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camurri L, Mambetisaeva E, Sundaresan V. Rig-1 a new member of Robo family genes exhibits distinct pattern of expression during mouse development. Gene Expr Patterns. 2004;4:99–103. doi: 10.1016/s1567-133x(03)00142-x. [DOI] [PubMed] [Google Scholar]
- Chedotal A. Slits and their receptors. Adv Exp Med Biol. 2007;621:65–80. doi: 10.1007/978-0-387-76715-4_5. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP, Moorman AF. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol. 2000;223:266–278. doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Burch JB, Moorman AF. Architectural plan for the heart: early patterning and delineation of the chambers and the nodes. Trends Cardiovasc Med. 2004a;14:301–307. doi: 10.1016/j.tcm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn. 2004b;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- de la Cruz M, Markwald R, editors. Living morphogenesis of the heart. Boston: Birkhauser; 1999. [Google Scholar]
- Dupays L, Kotecha S, Angst B, Mohun TJ. Tbx2 misexpression impairs deployment of second heart field derived progenitor cells to the arterial pole of the embryonic heart. Dev Biol. 2009;333:121–131. doi: 10.1016/j.ydbio.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 1inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- Harvey RP. Organogenesis: patterning the vertebrate heart. Nat Rev Genet. 2002;3:544–556. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- Holmes G, Niswander L. Expression of slit-2 and slit-3 during chick development. Dev Dyn. 2001;222:301–307. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Holmes GP, Negus K, Burridge L, Raman S, Algar E, Yamada T, Little MH. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech Dev. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev Biol. 2005;282:411–421. doi: 10.1016/j.ydbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–216. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kirby M, editor. Cardiac development. Oxford: Oxford University Press; 2007. [Google Scholar]
- Kispert A, Herrmann BG. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 1993;12:3211–3220. doi: 10.1002/j.1460-2075.1993.tb05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- MacMullin A, Jacobs JR. Slit coordinates cardiac morphogenesis in Drosophila. Dev Biol. 2006;293:154–164. doi: 10.1016/j.ydbio.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Medioni C, Astier M, Zmojdzian M, Jagla K, Semeriva M. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J Cell Biol. 2008;182:249–261. doi: 10.1083/jcb.200801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Senatore S, Salmand PA, Lalevee N, Perrin L, Semeriva M. The fabulous destiny of the Drosophila heart. Curr Opin Genet Dev. 2009;19:518–525. doi: 10.1016/j.gde.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kerszberg M, Moss JE, Buckingham ME. Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol. 2004;164:97–109. doi: 10.1083/jcb.200309160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Theveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong LL, Kim N, Mima T, Cohen-Gould L, Mikawa T. Trabecular myocytes of the embryonic heart require N-cadherin for migratory unit identity. Dev Biol. 1998;193:1–9. doi: 10.1006/dbio.1997.8775. [DOI] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Qian L, Liu J, Bodmer R. Slit and Robo control cardiac cell polarity and morphogenesis. Curr Biol. 2005;15:2271–2278. doi: 10.1016/j.cub.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Santiago-Martinez E, Soplop NH, Patel R, Kramer SG. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J Cell Biol. 2008;182:241–248. doi: 10.1083/jcb.200804120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Singh MK, Christoffels VM, Dias JM, Trowe MO, Petry M, Schuster-Gossler K, Burger A, Ericson J, Kispert A. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development. 2005;132:2697–2707. doi: 10.1242/dev.01854. [DOI] [PubMed] [Google Scholar]
- Sinha S, Abraham S, Gronostajski RM, Campbell CE. Differential DNA binding and transcription modulation by three T-box proteins, T, TBX1 and TBX2. Gene. 2000;258:15–29. doi: 10.1016/s0378-1119(00)00417-0. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OW, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- Strickland P, Shin GC, Plump A, Tessier-Lavigne M, Hinck L. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development. 2006;133:823–832. doi: 10.1242/dev.02261. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Mileikovskaia M, Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M, Georges R, Davidson L, Mo R, Hui CC, Henkelman RM, Nemer M, Black BL, Nagy A, Bruneau BG. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development. 2005;132:2463–2474. doi: 10.1242/dev.01827. [DOI] [PubMed] [Google Scholar]
- Tamada A, Kumada T, Zhu Y, Matsumoto T, Hatanaka Y, Muguruma K, Chen Z, Tanabe Y, Torigoe M, Yamauchi K, Oyama H, Nishida K, Murakami F. Crucial roles of Robo proteins in midline crossing of cerebellofugal axons and lack of their up-regulation after midline crossing. Neural Dev. 2008;3:29. doi: 10.1186/1749-8104-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togi K, Kawamoto T, Yamauchi R, Yoshida Y, Kita T, Tanaka M. Role of Hand1/eHAND in the dorso-ventral patterning and interventricular septum formation in the embryonic heart. Mol Cell Biol. 2004;24:4627–4635. doi: 10.1128/MCB.24.11.4627-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z, Zhang X, Rao Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci U S A. 2003;100:5217–5222. doi: 10.1073/pnas.0730709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right Ventricular Myocardium Derives From the Anterior Heart Field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009;114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.