Abstract
It is well established that healthy aging is accompanied by structural changes in many brain regions and functional decline in a number of cognitive domains. The goal of this study was to determine 1) whether the regional distribution of age-related brain changes is similar in gray matter (GM) and white matter (WM) regions, or whether these two tissue types are affected differently by aging, and 2) whether measures of cognitive performance are more closely linked to alterations in the cerebral cortex or in the underlying WM in older adults (OA). To address these questions, we collected high-resolution MRI data from a large sample of healthy young adults (YA; aged 18–28) and OA (aged 61–86 years). In addition, the OA completed a series of tasks selected to assess cognition in three domains: cognitive control, episodic memory, and semantic memory. Using advanced techniques for measuring cortical thickness and WM integrity, we found that healthy aging was accompanied by deterioration of both GM and WM, but with distinct patterns of change: Cortical thinning occurred primarily in primary sensory and motor cortices, whereas WM changes were localized to regions underlying association cortices. Further, in OA, we found a striking pattern of region-specific correlations between measures of cognitive performance and WM integrity, but not cortical thickness. Specifically, cognitive control correlated with integrity of frontal lobe WM, whereas episodic memory was related to integrity of temporal and parietal lobe WM. Thus, age-related impairments in specific cognitive capacities may arise from degenerative processes that affect the underlying connections of their respective neural networks.
Keywords: aging, white matter, cortical thickness, DTI, MRI, cognitive control processes, episodic memory
1. Introduction
Healthy aging is characterized by myriad cognitive changes. Some of the most pronounced and consistently reported deficits are on tasks that require long-term memory or that challenge cognitive control processes and working memory (Hedden and Gabrieli, 2004; Piguet and Corkin, 2007). While the pattern of age-related cognitive changes is relatively well characterized, less is known about the neural bases of age-related changes in cognition.
1.1. Age-related structural changes in gray matter
Age-related cognitive decline is frequently attributed to deterioration of cortical gray matter (GM) structures. Magnetic resonance imaging (MRI)-based studies point to reduced global GM volumes in OA (Allen et al., 2005; Bartzokis et al., 2001; Pfefferbaum et al., 1994; Raz et al., 1997; Raz et al., 2005; Salat et al., 1999; Walhovd et al., 2005). Several studies that examined regional effects of age found that frontal areas showed greater volumetric reduction than posterior regions (Allen et al., 2005; Bartzokis et al., 2001; Raz et al., 1997; Salat et al., 1999). Particularly notable are volumetric losses in prefrontal cortex (PFC) (Grieve et al., 2005; Raz et al., 2004a; Raz et al., 1997; Salat et al., 1999). These findings have led to the hypothesis that age-related GM loss occurs along an anterior-to-posterior gradient (Jernigan et al., 1991; Raz et al., 1997; Raz and Rodrigue, 2006; Sowell et al., 2003). While this hypothesis tends to dominate the aging literature, degeneration has also been documented in all of the major lobes of the brain (Allen et al., 2005; Bartzokis et al., 2001; Cowell et al., 1994; Raz et al., 2004a; Tisserand et al., 2002; Van Petten, 2004; Van Petten et al., 2004).
Advanced methods now allow assessment of changes in cortical thickness across the lifespan (Fischl and Dale, 2000). Similar to results from volumetric studies, cortical thickness of lateral PFC is reduced in OA. At the same time, cortical thinning is also found in the occipital lobe and precentral gyrus—areas that have not generally been associated with volumetric decline (Salat et al., 2004). The histopathological underpinnings of these macroscopic changes in cortical GM remain elusive: While early studies reported a loss of cortical neurons and decreased cell packing density (Pakkenberg and Gundersen, 1997), more advanced methods indicate that cell loss is relatively minimal in old age, overshadowed by a drastic loss of neuropil (Peters et al., 1998a).
1.2. Age-related structural changes in white matter
In addition to GM degeneration, WM changes likely play an important role in explaining age-related cognitive deficits (Hinman and Abraham, 2007; Peters, 2002). Many volumetric studies have documented reduced global and regional WM volumes in OA (Allen et al., 2005; Bartzokis et al., 2001; Courchesne et al., 2000; Guttmann et al., 1998; Jernigan et al., 2001; Piguet et al., 2007; Salat et al., 1999), but see (Good et al., 2001; Pfefferbaum et al., 1994; Sullivan et al., 2004). Evidence that WM volume loss is greatest in the frontal lobes is equivocal (Allen et al., 2005; Piguet et al., 2007; Raz et al., 1997; Raz et al., 2004b; Salat et al., 1999).
Other MRI-based markers of WM degeneration include an increase in hyperintensities on T2- and proton density-weighted images, with the greatest volume of hyperintensities typically found in the WM underlying the frontal lobes (de Groot et al., 2000; DeCarli et al., 1995; Gunning-Dixon and Raz, 2000; Nordahl et al., 2006; Pfefferbaum et al., 2000; Tullberg et al., 2004; Yoshita et al., 2006). In addition, microstructural deterioration of WM has been assessed using diffusion tensor imaging (DTI), with numerous studies documenting widespread age-related decreases in fractional anisotropy (FA) (Benedetti et al., 2006; Charlton et al., 2006; Madden et al., 2004; O’Sullivan et al., 2001; Salat et al., 2005a; Sullivan and Pfefferbaum, 2003). FA measures the degree to which the diffusion of water molecules is restricted by microstructural elements, such as cell bodies, axons, or myelin and other glial cells (Beaulieu, 2002). Like WM hyperintensities, FA reductions tend to be most prominent anteriorly, such as in the genu and anterior portions of the corpus callosum and in the WM underlying PFC (Ardekani et al., 2007; Head et al., 2004; Madden et al., 2007; O’Sullivan et al., 2001; Pfefferbaum et al., 2005; Salat et al., 2005b; Sullivan and Pfefferbaum, 2006; Yoon et al., 2007). Other notable loci of decreased integrity include the internal capsule (Good et al., 2001; Salat et al., 2004), auditory pathways of the temporal lobes (Lutz et al., 2007), and cingulum bundle (Yoon et al., 2007). Postmortem studies reveal a number of pathologic factors that may cause changes in FA, including loss of small myelinated fibers (Marner et al., 2003; Tang et al., 1997) and myelin degradation (Peters, 2002), which likely contribute to volumetric change (Double et al., 1996; Guttmann et al., 1998; Ikram et al., 2007; Piguet et al., 2007).
In summary, evidence of morphological and microstructural changes in frontal areas appears consistently in the aging literature. In addition, regional alterations have been noted across wide regions of GM and WM, although the exact nature and magnitude of these changes remains a topic of debate. To explicitly test whether WM and GM exhibit similar or distinct patterns of age-related change, measures of both structures must be examined in a single group of participants. The present study achieved that goal.
1.3. Linking GM changes to cognitive performance in OA
A prevalent view contends that age-related decline in episodic memory is related to deterioration of the hippocampus and other medial temporal lobe structures, and that cortical losses are more highly correlated with decrements in cognitive control processes (i.e., the frontal aging hypothesis) (Tisserand and Jolles, 2003; West, 1996). While a number of studies have reported correlations between hippocampal volume and episodic memory (Golomb et al., 1996; Kramer et al., 2007), some concerns have been raised about the robustness of the effect (Van Petten, 2004). Direct evidence in favor of the frontal aging hypothesis has also been difficult to demonstrate in humans (Greenwood, 2000; Raz and Rodrigue, 2006; Van Petten et al., 2004). Diminished attention and executive function in OA have been associated with decreased global cortical volumes and reduced volumes of lateral PFC and OFC (Kramer et al., 2007; Zimmerman et al., 2006), although an inverse correlation between working memory function and OFC volume has also been reported (Salat et al., 2002). In addition, PFC volume has been inversely correlated with perseverative errors in OA (Gunning-Dixon and Raz, 2003; Raz et al., 1998). In contrast, spatial and object working memory correlated with visual cortex volume (Raz et al., 1998), but neither spatial and object or verbal working memory (Gunning-Dixon and Raz, 2003) showed significant correlations with PFC volume.
Less is known about the cognitive correlates of cortical thinning. An experiment in monkeys found that age-related cortical thinning was associated with deficits in recognition memory and overall cognitive function (Peters et al., 1998b). In humans, OA with high fluid intelligence scores had large regions of thicker cortex in the right hemisphere, most notably in posterior cingulate cortex, compared to OA with average scores (Fjell et al., 2006). In contrast, the same study found virtually no thickness differences between high and low performers on tests of executive function.
1.4. Linking WM changes to cognitive performance in OA
Given the distributed nature of the neural networks that support the cognitive functions that decline most with age, degradation of the connections in these networks could have a dramatic effect on the processing abilities of OA. One study of older rhesus monkeys found a correlation between measures of executive function and DTI-based measures of WM integrity in long-distance corticocortical association pathways (Makris et al., 2006). Similarly, several investigations of humans have linked deficits in processing speed, executive function, immediate and delayed recall, and overall cognition to an increased burden of periventricular WM hypertintensities in OA (Gunning-Dixon and Raz, 2000; Gunning-Dixon and Raz, 2003; Soderlund et al., 2003). WM hyperintensities have also been associated with decreased frontal lobe metabolism (DeCarli et al., 1995; Tullberg et al., 2004), and with diminished BOLD responses in PFC during performance of episodic and working memory tasks (Nordahl et al., 2006). When measured using DTI, functional correlates of decreased WM integrity included working memory impairments (Charlton et al., 2006), slowed processing speed (Bucur et al., 2007; Sullivan et al., 2006), and executive dysfunction (Deary et al., 2006; Grieve et al., 2007; O’Sullivan et al., 2001). In one study of OA, WM integrity was negatively correlated with the magnitude of the BOLD response in PFC in individuals performing an episodic memory task (Persson et al., 2006). While these studies suggest that degeneration of WM pathways may contribute to the etiology of age-related cognitive decline to an equal or greater extent than GM atrophy (Hinman and Abraham, 2007; O’Sullivan et al., 2001), a strong test of this hypothesis requires measures of GM and WM integrity in the same group of participants, and then relating those measures to cognitive test scores. To date, no study has provided a direct test of the hypothesis that cognitive performance in OA correlates more strongly with WM than with GM changes.
1.5. The present study
Our study was asked two specific questions: 1) Do the patterns of age-related change differ between WM and GM structures, and 2) Are changes in discrete regions of GM and WM related to specific cognitive measures in OA? To address the first question, we used high-resolution structural MRI to obtain measures of cortical thickness and DTI-based indices of WM integrity in a single sample of young adults (YA) and OA. We hypothesized that the patterns of change in WM and GM would largely overlap, with frontal regions showing the most widespread losses. At the same time, we expected the patterns to diverge slightly, with cortical thinning also extending to primary sensory and motor cortices, while loss of WM integrity was expected to be more restricted to frontal areas. We chose not to limit our DTI analyses to WM regions and intentionally performed exploratory analyses of GM regions as well. This decision was based on emerging evidence that DTI data contain rich information about microstructural characteristics of all brain tissues (Rose et al., 2008), and may be capable of detecting age-related changes in GM structures (Abe et al., 2008). To answer the second question, our sample of OA completed a series of tasks designed to measure three cognitive domains: cognitive control, episodic memory, and semantic memory. We predicted that cognitive control and episodic memory in OA would correlate with cortical thickness in PFC and association areas of parietal and temporal lobes, respectively, as well as with the integrity of WM underlying these cortical areas. Because semantic memory tends to remain relatively stable throughout the lifespan, we did not expect to find robust structure-function correlations for this domain.
2. Materials and Methods
2.1. Participants
The participants in this study were 36 YA (16F/20M), aged 18–28 years (mean age = 21.9 ± 2.6 years), and 38 OA (20F/18M), aged 61–86 years (mean age = 70.3 ± 7.2) (Table 1). Most of the YA were recruited from the MIT and Harvard communities; OA came primarily from the MIT and Harvard alumni associations. OA had more years of education (17 ± 3.0) than YA (15 ± 2.0), due to the fact that the majority of YA had not completed their education. Our exclusion criteria were: history of neurological or psychiatric disease, use of psychoactive medications, substance misuse, and presence of serious medical conditions, including history of heart disease, diabetes, and untreated hypertension. Those participants whose hypertension was controlled by prescription medication were admitted into the study. All participants were screened for dementia using the Mini Mental State Examination (MMSE) (Folstein et al., 1975), and any individual scoring below 26 was excluded from the study. The two groups were well matched on MMSE scores (mean scores: YA = 29.2 ± 1.0; OA = 29.2 ± 2.0). All participants gave informed consent using methods approved by the MIT Committee on the Use of Humans as Experimental Subjects and by the Partners Human Research Committee (Massachusetts General Hospital).
Table 1.
Participant characteristics: mean ± SD and (range)
| Group | Age (yrs) | Education (yrs) | MMSEa |
|---|---|---|---|
| Young Adults (YA) (n = 36, 16F) | 21.9 ± 2.6 (18–28) | 15 ± 2.0 (12–18) | 29.2 ± 1.0 (27–30) |
| Older Adults (OA) (n = 38, 20F) | 70.3 ± 7.2 (61–86) | 17 ± 3.0 (14–23) | 29.2 ± 1.2 (27–30) |
Mini Mental State Examination
2.2. MRI acquisitions
We collected whole-head MRI scans using a 1.5 Tesla Siemens Sonata imaging system (Siemens Medical Systems, Iselin, NJ). Tightly padded clamps attached to the head coil minimized head motion during the scan. For analyses of WM integrity, we obtained high-resolution DTI scans from each participant (TR = 9100 ms, TE = 68 ms, slice thickness = 2 mm, 60 slices, acquisition matrix 128 mm × 128 mm, FOV 256 mm × 256 mm yielding 2 mm3 isotropic voxels, seven averages of eight directions with b-value = 700 s/mm2, and 5 T2-weighted images with no diffusion weighting, b-value = 0 s/mm2). The effective diffusion gradient spacing was Δ = 64 ms with a bandwidth of 1445 Hz/pixel. Images were collected in an oblique axial plane. The DTI acquisition used a twice-refocused balanced echo, developed to reduce eddy current distortions (Reese et al., 2003). For morphometric analyses of cortical thickness, we collected two high-resolution T1-weighted anatomical (MPRAGE) scans from each participant (voxel size = 1.0 × 1.0 × 1.33 mm, TR = 2530 ms, TE = 2.6 ms, TI = 7100 degrees, flip angle = 7 degrees).
2.3. DTI analyses
We chose FA as an indirect measure of the integrity of WM fiber bundles and GM structures because FA values are dependant upon the microstructural composition of different tissues (Beaulieu, 2002). We tested for differences in FA between YA and OA using a whole-brain atlas-based analysis, and by comparing mean FA values derived from manually delineated regions of interest (ROIs).
2.3.1 Processing of DTI data
All DTI data were processed using tools from the FSL (http://www.fmrib.ox.ac.uk/fsl) and FreeSurfer (http://surfer.nmr.mgh.harvard.edu) image analysis packages. We first applied motion and eddy current correction to all DTI scans. To this end, we registered each participant’s diffusion-weighted images to the T2-weighted image using FMRIB’s Linear Image Registration Tool (FLIRT), available as part of the FSL analysis package (Jenkinson et al., 2002; Jenkinson and Smith, 2001). FLIRT employs a 12-parameter affine transformation and a mutual information cost function to achieve a globally optimized registration. Diffusion tensor and FA metrics were derived as described previously (Basser et al., 1994; Pierpaoli and Basser, 1996). For atlas-based statistical analyses, we used trilinear interpolation to resample all maps and nearest-neighbor resampling for ROI analyses. To avoid partial volume effects associated with the inclusion of cerebrospinal fluid in WM voxels, all voxels with trace diffusion values greater than 6 μm2/ms were excluded from the analyses.
2.3.2 Whole-brain Statistical Analyses of DTI data
To perform voxelwise statistical analyses of DTI data, FA volumes were spatially normalized to MNI space with FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001) by registering each participant’s T2-weighted volume to the MNI’s 152-subject T2-weighted template (Mazziotta et al., 1995) and then applying this transformation to individual FA maps. To increase statistical power, we performed minimal spatial smoothing of FA maps using a 3D Gaussian kernel with 4-mm full width at half maximum. Independent t tests were calculated at each voxel to test for differences in FA between groups. The tools that we used to perform statistical analyses of our DTI data were developed in-house and are not equipped with a False Discovery Rate correction procedure that is appropriate for voxel-wise analyses of WM regions. We did, however, enforce a strict cutoff of p < .001 for all statistical comparisons of FA measures to minimize the potential confound of multiple comparisons.
2.3.3 ROI analysis of DTI data
We manually defined 14 ROIs (Figure 1b) that were either selected a priori, based on age-related changes previously documented in the literature, or to confirm the results from our whole-brain analyses of FA, thereby ensuring that any group differences were not confounded by registration or smoothing procedures. Based on previous reports (Abe et al., 2008; Lutz et al., 2007; Madden et al., 2004; O’Sullivan et al., 2001; Salat et al., 2005a; Sullivan et al., 2006), we expected FA to be decreased in OA in the following regions: the genu of the corpus callosum, posterior sagittal striatum, and radiate WM regions underlying PFC and OFC. In contrast, we expected to find little or no age-related change in FA values in the splenium of the corpus callosum and in the radiate WM underlying occipital, temporal, and parietal areas (Head et al., 2004; Salat et al., 2005a; Sullivan et al., 2006), and increased FA in OA in the putamen (Abe et al., 2008). All ROIs were placed individually in each participant’s native, unsmoothed FA volume, thus avoiding any errors due to misregistration and any confounding effects of spatial smoothing. With the exception of callosal labels, all ROIs were drawn separately in each hemisphere. Each label contained the same number of voxels in each participant. We provide detailed anatomical descriptions of ROI placement and label size online as Supplementary Material. To help ensure that no GM voxels were included in any of the WM ROIs, we created individual WM masks by thresholding each participant’s FA volume at 0.25, thus masking out the majority of GM voxels. To further minimize partial volume effects, we positioned all labels near the center of each WM region or tract, avoiding border voxels. The GM ROIs were drawn in participants’ native, unthresholded maps; to ensure that these labels contained only GM voxels, we did not include any voxels with FA values above 0.5, essentially masking out most WM voxels. A multivariate repeated measures general linear model (GLM) tested for significant differences between YA and OA in mean FA for each ROI. In order to compare the magnitude of effects across ROIs, we calculated effect sizes ([OA mean – YA mean]/pooled standard deviation) for the differences in means between OA and YA.
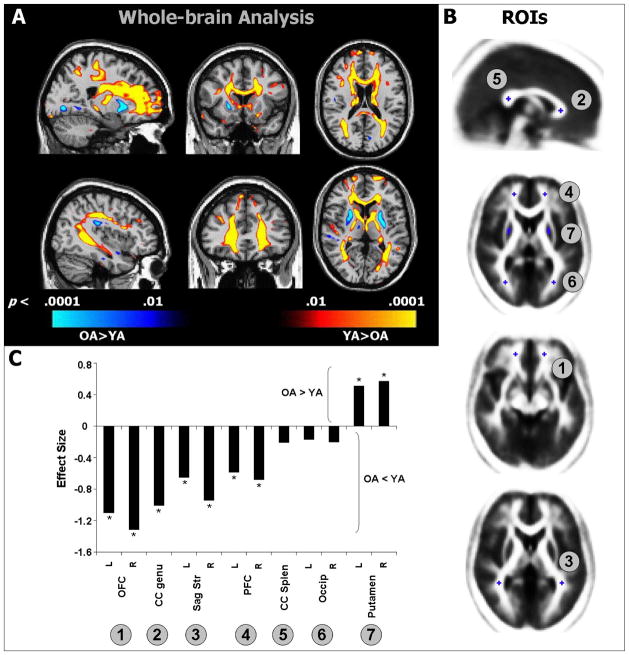
Figure 1.
A) Voxel-wise t maps showing differences between YA and OA in FA overlaid on representative sagittal (left), coronal (middle), and axial (right) slices. Regions depicted in red-yellow indicate areas where FA was lower in OA compared to YA; regions depicted in blue indicate areas where FA was higher in OA compared to YA. B) Placement of manually defined ROIs. C) Effect sizes ([OA mean – YA mean]/pooled SD) for all ROIs; negative values are regions where FA was lower in OA compared to YA and positive values are area where FA was greater in OA; asterisks indicate significant differences between OA and YA (p < .05). Abbreviations: CC, corpus callosum; PFC, prefrontal cortex; OFC, orbitofrontal cortex; Sag Str, sagittal stratum.
2.4. Cortical thickness analyses
We used advanced tools to derive measures of cortical thickness across the entire cortical mantle. We tested for regional differences in cortical thickness between YA and OA using an automated, surface-based approach, as well as by analyzing cortical thickness measures derived from manually delineated ROIs.
2.4.1 Analyses of regional cortical thinning
T1-weighted MRI data were processed using the FreeSurfer (http://surfer.nmr.mgh.harvard.edu) morphometric analysis tools. We performed motion correction and averaged the two scans from each participant, yielding a single volume with high contrast- and signal-to-noise ratios. Cortical surfaces were reconstructed using a semi-automated procedure that has been described at length in previous work (Dale et al., 1999; Fischl et al., 2001; Fischl et al., 1999a). We derived thickness measures at each vertex along the reconstructed surface by calculating the shortest distance from the gray/white border to the outer cortical (pial) surface (Fischl and Dale, 2000). Thickness measures were then mapped back onto each participant’s inflated cortical surface and were averaged across all participants using a spherical averaging procedure (Fischl et al., 1999b).
2.4.2 Statistical Analyses of cortical thickness data
For analyses of differences between OA and YA, an average surface was derived using our entire sample. The average surface ensures that the data are displayed on a model that is representative of the overall population, but lacks individual anatomical idiosyncrasies, thus maximizing the chance of accurate regional localization of effects. For correlations between cortical thickness and measures of cognition, a separate average surface was derived using only the sample of OA who completed the cognitive tasks. All statistical comparisons were performed in a vertex-wise fashion across the entire cortical surface. We tested for group differences and for correlations between thickness and measures of cognition using GLMs. For correlations between thickness and cognitive performance, age and years of education were included as continuous covariates. Between-group comparisons and correlations were subject to False Discovery Rate correction (q = .05) for multiple comparisons (Benjamini and Hochberg, 1995; Genovese et al., 2002).
2.4.3 ROI analysis of cortical thinning
In addition to the cortex-wide comparisons of cortical thickness between YA and OA, we manually defined ROIs (Figure 2b) in regions that we predicted would show the greatest degree of thinning (precentral gyrus, calcarine, PFC, OFC) (Fjell et al., 2006; Raz et al., 1997; Salat et al., 2004), in two regions that were not expected to show reduced cortical thickness in OA (middle temporal gyrus and anterior cingulate cortex)(Fjell et al., 2006; Salat et al., 2004), and in two regions (inferior temporal gyrus and posterior cingulate cortex) that have been associated with inconsistent results based on the volumetric and thickness literatures (Raz et al., 1997; Salat et al., 2004). We tested each ROI for differences in mean cortical thickness between YA and OA using a multivariate repeated measures GLM. We then calculated an ES for each ROI.
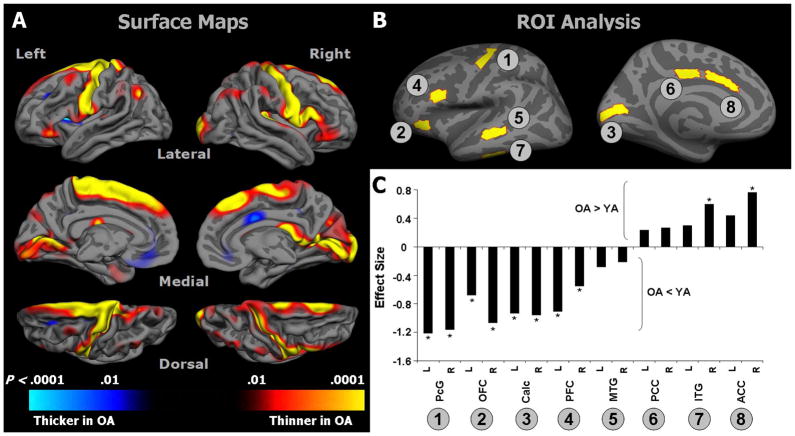
Figure 2.
A) Surface-based vertex-wise GLM maps showing differences between OA and YA in cortical thickness. Regions depicted in red-yellow indicate areas where cortex was thinner in OA compared to YA; regions depicted in blue indicate areas where cortex was thicker in OA compared to YA. B) Placement of manually defined cortical ROIs. C) Effect sizes ([OA mean – YA mean]/pooled SD) for all ROIs; negative values indicate regions with thinner cortex in OA, compared to YA, and positive values represent regions with thicker cortex in OA; asterisks indicate significant differences between OA and YA (p < .05). Abbreviations: MTG, middle temporal gyrus; ITG, inferior temporal gyrus; PFC, prefrontal cortex; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; PcG, precentral gyrus; Calc, calcarine cortex.
2.5. Cognitive testing
Participants who underwent MRI scanning were asked to return for a second visit to complete a series of cognitive tasks. Over 90% of the OA returned to complete the cognitive tasks, compared to 60% of the YA. This difference resulted in a less representative YA sample and reduced statistical power for brain-behavior correlations. Thus, correlations between cognitive scores and neuroanatomical measures were restricted to the OA group.
We selected a series of tasks to assess frontal lobe and long-term memory functions in OA (Table 2). For frontal lobe function, we chose tasks that require working memory or cognitive control processes; within the realm of long-term memory, we included tasks to assess episodic and semantic memory capacities. The cognitive control composite was designed to provide and index of a range of frontal lobe functions and included several tests that are know to be sensitive to aging (Glisky et al., 1995). The cognitive control composite included a measure robustly associated with inhibitory control, the Stroop interference score (i.e., difference between the color-word card score and the color card score) (Stroop, 1935), and two tests of working memory, the total raw score from the Wechsler Memory Scale-III Backward Digit Span (Wechsler, 1997b) and the Trail Making Test, B-A score (Reitan, 1958). In addition, to provide an index of abstract mental operations, we included the total number of words produced in the Controlled Oral Word Association Test (COWAT) (Benton and Hamsher, 1989), a test of verbal fluency that is thought to rely on search strategies rather than on semantic knowledge (Lezak, 1995). The episodic memory composite was comprised of two measures known to be sensitive to impairment in long-term memory: the delayed recall scores for the Wechsler Memory Scale-III Logical Memory and Word List subtests (Wechsler, 1997b). To achieve a broad assessment of semantic memory function in OA, this composite included scores for the Wechsler Adult Intelligence Scale-III Vocabulary subtest (Wechsler, 1997a), which tend to remain stable or even improve with age, and the total number of words for the Boston Naming Test (Kaplan et al., 1983), which tends to show some variability across the lifespan (Zec et al., 2007; Zec et al., 2005). All test scores were converted to z scores and then averaged to create a composite score for each of the three cognitive domains: cognitive control processes, long-term episodic memory, and semantic memory
Table 2.
Scores for OA on the individual cognitive comprising each composite score.
| Composite score | Test | Mean | SD | Range |
|---|---|---|---|---|
| Episodic memory | Word lists | 8.0 | 3.0 | (3 – 12) |
| Logical memory | 29.9 | 8.2 | (13 – 45) | |
| Semantic memory | Boston naming | 40.6 | 1.7 | (36 – 42) |
| Vocabulary | 59.1 | 6.1 | (44–66) | |
| Cognitive control | COWAT | 49.6 | 11.8 | (30 – 76) |
| Trails B-A | 47.4 | 30.5 | (8 – 129) | |
| Digit span backward | 8.8 | 2.8 | (3 – 14) | |
| Stroop interference | 101.8 | 8.2 | ( 86 – 115) |
2.6. Brain-behavior correlations in OA
For the OA group, we performed whole-brain and ROI-based multiple regression analyses to examine correlations between each cognitive composite score and measures of FA and cortical thickness. The whole brain analyses consisted of voxelwise correlations between FA or cortical thickness and cognitive composite scores. The ROI-based regression models included age as a continuous covariate. To ensure that any observed correlations were not simply the result of individual differences in FA values, we took the following approach: For any ROI that showed a correlation between FA and a cognitive measure, we calculated the correlation between age and FA in that ROI and then calculated partial correlation coefficients for FA and age from the multiple regression model. We then computed squared partial correlation coefficients to determine the percentage of variance in the cognitive measure that was due to FA, age, or both.
3. Results
3.1. Differences between YA and OA in WM integrity
We found widespread reductions in FA values in OA, compared to YA (Figure 1a, yellow and red areas). As anticipated, FA was reduced in anterior regions, including the genu and anterior body of the corpus callosum, and in the WM underlying the superior and middle frontal gyri and OFC. We also noted reduced FA in the WM underlying the middle and superior temporal gyri and posterior parietal cortex. In contrast, FA values in the putamen were significantly greater in OA than in YA (blue areas).
In our ROI analysis (Figure 1b) of FA values, a multivariate repeated measures GLM revealed a significant main effect of age (F1,61 = 17.2, p < .001) and a significant age by region interaction (F11,61 = 9.1, p = .004). Effect sizes for each ROI are presented in Figure 1c. Post-hoc comparisons confirmed our predictions: FA was lower in OA relative to YA in the following ROIs: radiate OFC on the left (p < .001) and right (p, 0.001); genu of the corpus callosum (p < .001); sagittal striatum on the left (p = .008) and right (p < .001); and radiate PFC on the left (p = .02) and right (p = .01). In contrast, FA was significantly greater in OA, compared to YA, in the putamen on the left (p = .04) and right (p = .02). We found no significant differences between YA and OA for mean FA values in the splenium of the corpus callosum or in the radiate occipital WM bilaterally.
3.2. Differences between YA and OA in cortical thickness
A surface-based GLM revealed regional changes in cortical thickness between YA and OA (Figure 2a); all areas reported showed significant differences that survived False Discovery Rate correction for multiple comparisons (q < .05). Cortical thinning was found bilaterally in the following regions: the lateral aspect of the superior frontal gyrus, the precentral gyrus and banks of the central sulcus, and in the calcarine sulcus and cuneus in the occipital lobeand in lateral PFC and inferior parietal cortex. We also found age-related thinning in the transverse temporal gyri, but with a rightward predominance. A small, circumscribed region of cortex in the right posterior cingulate gyrus was thicker in OA.
We used a multivariate repeated measures GLM to test for differences in mean thickness in selected ROIs (Figure 2b). These analyses revealed a significant main effect of age (F1,69 = 12.9, p < .001) and a significant age by region interaction (F15,69 = 10.9, p = .001). Effect sizes for each ROI are presented in Figure 2c. Post-hoc comparisons indicated the greatest degree of thinning occurred, bilaterally, in the precentral gyrus, followed by OFC, calcarine sulcus, and PFC (all p < .01). In contrast, OA showed significantly thicker cortex in the anterior cingulate on the right (p < .001), but not on the left (p = .06), and in the inferior temporal gyrus on the right (p = .01), but not on the left (p = .22). No significant differences were found in thickness of the middle temporal gyrus on the left (p = .25) or right (p = .33) or in the posterior cingulate on the left (p = .33) or right (p = .27). The pattern of these results agreed largely with the predicted pattern of change, as well as with the results from our map-based cortical thickness analysis.
3.3. Brain-behavior correlations in OA
To determine whether changes in discrete regions of GM and WM were associated with specific cognitive impairments in OA, we tested for correlations between each cognitive composite score and measures of FA and cortical thickness. For each measure, we used an automated, whole-brain approach, and a complimentary ROI-based approach.
3.3.1 Correlations with DTI data
Voxel-based regression analyses revealed a significant positive correlation between performance on cognitive control tasks and FA in frontal lobe WM (Figure 3a). By contrast, performance on episodic memory tasks correlated positively with FA in more posterior regions, in particular the WM underlying the temporal and parietal lobes (Figure 3b). No significant correlations were found between semantic memory composite scores and FA. Multiple regression analyses of FA values derived from manually delineated ROIs revealed significant, regionally-specific, correlations between cognitive composite scores and FA in two ROIs (Figure 3c): episodic memory composite scores correlated significantly with mean FA in the temporoparietal WM on the left (R2 = .46, p = .003; r2[FA] = .40; r2[age] = .10) and right (R2 = .29, p = .04; r2[FA] = .27; r2[age] = .05). FA in the temporoparietal ROI was negatively correlated with age on both the left (r2= .14, p = .002) and right (r2= .23, p = .001). In contrast, cognitive control scores were positively correlated with mean FA in the PFC on the left (R2 = .36, p = .01; r2[FA] = .29; r2[age] = .09) and right (R2 = .30, p = .04; r2[FA] = .19; r2[age] = .10). FA in the PFC ROI was negatively correlated with age on both the left (r2= .13, p = .003) and right (r2= .18, p < .001).
Figure 3.
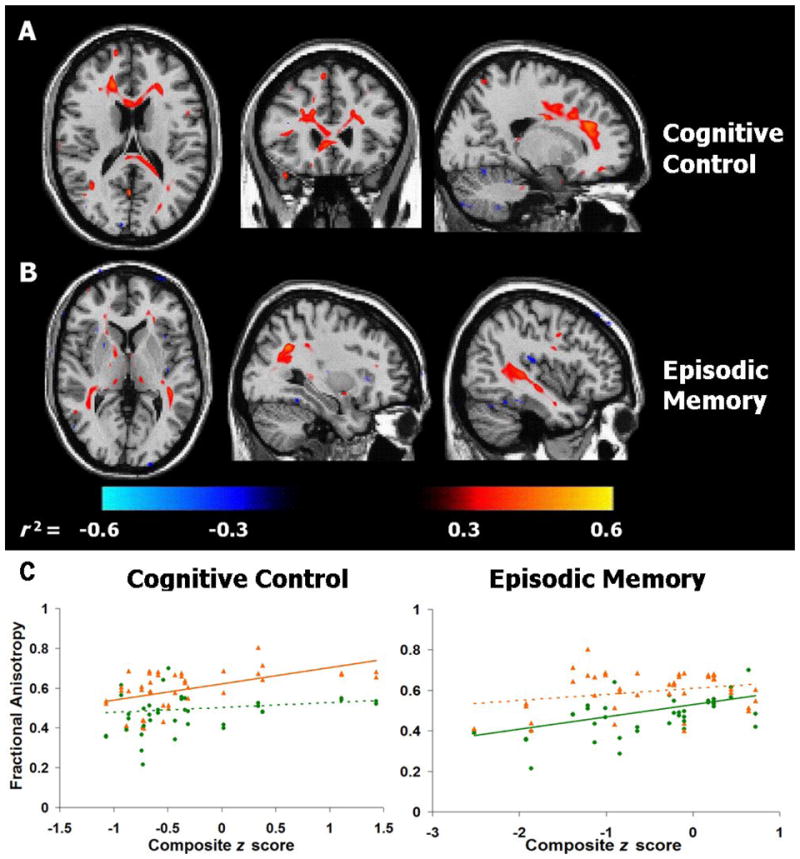
Voxel-wise signed-r2 maps for correlations between FA and composite scores on A) cognitive control tasks, and B) episodic memory tasks in OA. Positive correlations are shown in red, negative correlations in blue. C) Composite z scores on cognitive control and episodic memory tasks plotted against mean FA for frontal (▲, orange lines) and temporo-parietal (●, green lines) ROIs, bilaterally (see text for anatomical definitions). Significant correlations (p < 0.01) are indicated by solid lines, non-significant correlations by dashed lines. Due to the similar patterns of correlation, right and left ROIs are combined for graphical purposes.
To test the regional specificity of these brain-behavior correlations, we performed additional multiple regression analyses for each of these two cognitive measures, including FA values from both the anterior and posterior ROIs as regressors. These models demonstrated that FA in left (p = .018) and right (p = .028) PFC correlated with performance on cognitive control tasks, independently of any contribution from temporoparietal WM on the left (p = .59) or right (p = .51). In contrast, episodic memory scores were correlated with FA values in left (p = .004) and right (p = .021) temporoparietal WM, but not PFC WM on the left (p = .32) or right (p = .6).
3.3.2 Correlations with cortical thickness
Surface-based regressions revealed a few modest correlations between semantic and episodic memory performance and measures of cortical thickness in OA. None of these correlations, however, exceeded our significance cutoff of p < .001. Further, we found no significant correlations between thickness in any manually defined cortical ROI and any cognitive composite score (all p > .06).
4. Discussion
This study addressed two open questions about cognitive aging: 1) Do the distributions of age-related change in cortical thickness and WM integrity overlap, or are these brain regions affected differently; and 2) What are the cognitive effects of these brain changes? Here we consider the specific age-related alterations in brain structure, discuss the possible implications of the brain-behavior correlations, and relate our findings to the literature on microstructural factors that may contribute to the etiology of cognitive and neural decline in aging.
4.1. Age-related changes in WM integrity
Our predictions concerning age-related differences in WM were largely confirmed: We found widespread regions of reduced FA in the WM underlying the frontal lobes, including OFC, the genu of the corpus callosum, forceps major, and the anterior corona radiata of PFC. The finding of lower FA in the frontal lobes of OA is compatible with the results of several other studies that used a combination of ROI and voxel-based analyses similar to those employed here (Salat et al., 2005a), or that used ROI- or tractography-based methods (Head et al., 2004; Nusbaum et al., 2001; O’Sullivan et al., 2001; Ota et al., 2006; Pfefferbaum et al., 2005). The observed pattern of change in FA also parallels studies that report reduced frontal WM volumes (Allen et al., 2005; Bartzokis et al., 2001; Courchesne et al., 2000; Guttmann et al., 1998; Jernigan et al., 2001; Salat et al., 1999) and increased WM hyperintensities on FLAIR and T2-weighted images (de Groot et al., 2000; DeCarli et al., 1995; Gunning-Dixon and Raz, 2000; Nordahl et al., 2006; Tullberg et al., 2004; Yoshita et al., 2006). An increased burden of WM hyperintensities is associated with decreased FA values, both within the hyperintense regions, as well as in normal appearing WM (O’Sullivan et al., 2004; Taylor et al., 2007). The present study did not exclude areas of hyperintense signal from FA analyses. While it is possible that a similar relation exists in the present sample, we have examined the quantitative effects of hyperintense signal on DTI measures using statistical models, and found that this variable did not have a significant effect on patterns of FA change in patients with Alzheimer’s disease (Salat et al., 2008).
We also found age-related decreases in WM adjacent to temporal and parietal cortices. The few DTI-based studies that have examined temporoparietal WM regions have reported mixed results. In agreement with our findings, one study showed a modest decrease in temporal and parietal lobe FA, but this decline was not proportionate to that in frontal lobe areas (Head et al., 2004). Our results indicate that age-related changes in FA are particularly widespread in WM regions underlying multimodal association cortices. In support of this view, histopathological evidence points toward a degenerative process whereby small diameter myelinated fibers are more vulnerable to aging than larger diameter axons (Tang et al., 1997). The interhemispheric callosal connections between frontal and temporoparietal areas appear to consist predominantly of small diameter fibers (Aboitiz and Montiel, 2003). Thus, the age-related vulnerability of the WM connecting association areas may reflect the high sensitivity of these small diameter fibers to aging processes.
In contrast to the significant decreases noted above, occipital FA values did not differ reliably between OA and YA, but FA values in the putamen were significantly greater in OA compared to YA. This finding is similar to one other report of reduced striatal FA (Abe et al., 2008). Studies using T2-weighted MRI have documented signal changes that are believed to arise from an accumulation of heavy metals in the striatum with increasing age (Ketonen, 1998). Iron deposition in neural tissue has been associated with neurodegenerative disorders, such as Parkinson’s disease (Ke and Ming Qian, 2003; Zecca et al., 2004), and there is some evidence that a notable amount of buildup also occurs in subcortical GM structures during the course of healthy aging (Bartzokis et al., 1994; Bartzokis et al., 2007; Hallgren and Sourander, 1958; Ketonen, 1998). While the mechanism by which iron deposition would cause a change in the FA metric is not entirely understood, a well-documented decrease or shortening of the T2 signal has been linked to heavy metal accumulation in the putamen in the sixth decade of life (Ketonen, 1998). T2 shortening is typically found using gradient echo sequences, which are related to the DTI acquisitions used here (i.e., pulsed-gradient, spin-echo sequences). Thus, iron deposition in the putamen provides a putative explanation for the increased striatal FA that we see in our sample of OA.
4.2. Parallels between reduced FA and microstructural changes in aging
Our results indicate that DTI-based measures of WM integrity are sensitive to pathological changes that occur with advanced age. While we do not yet fully understand which specific tissue-level properties give rise to the MR signals used to derive FA values (Beaulieu, 2002), evidence from histopathological studies suggests that alterations in myelin structure and integrity likely contribute to the age-related differences in FA values reported here. Studies of aged monkey brains show numerous abnormalities in myelin (Peters, 2002), including the formation of cytoplasmic inclusions following splitting of the myelin lamellae, the accumulation of “balloons” or holes inside the myelin sheath, formation of redundant myelin sheaths (Rosenbluth, 1966; Sturrock, 1976), and loss of small myelinated fibers (Kemper, 1994; Marner et al., 2003; Sandell and Peters, 2001; Tang et al., 1997). Further, myelin abnormalities have been linked to cognitive dysfunction in aged monkeys (Moss and Killiany, 1999). Thus, although the evidence is indirect, decreased FA values in the present study are likely due to cellular changes in myelin. Conclusive evidence will require further investigation combining MRI and histopathological techniques in the same brains.
4.3. Age-related changes in cortical thickness
Consistent with another study (Salat et al., 2004), we found large regions of cortical thinning in sensory and motor areas, including the precentral gyrus, the pericalcarine region, and the medial aspect of the superior frontal gyrus. We also noted smaller regions of thinning in the lateral PFC, inferior parietal cortex, and transverse temporal gyri. In contrast, other frontal and temporal areas were largely devoid of significant age-related cortical thinning, and small areas of the right anterior cingulate and right inferior temporal gyrus showed modestly increased thickness in OA. These findings are in partial agreement with previous studies that documented significant age-related GM decrements in primary sensory and motor cortices using voxel- or ROI-based approaches (Good et al., 2001; Lemaitre et al., 2005; Raz et al., 2004a; Resnick et al., 2003; Salat et al., 2004; Tisserand et al., 2004), as well as with one study that used the same cortical thickness tools employed here (Salat et al., 2004). Other studies, however, reported the greatest degree of volumetric loss in frontal areas, with primary sensory and motor cortices showing less age-related degeneration (Jernigan et al., 1991; Raz et al., 1997; Raz and Rodrigue, 2006; Sowell et al., 2003), possibly reflecting a pattern of atrophy that occurs in reverse of the developmental trajectory of growth (Raz et al., 1997).
The discrepancy in findings could be the result of differences in analytic techniques, whereby measures of cortical thickness and volume are detecting separate degenerative processes. Because cortical volume is a product of thickness and surface area, degenerative processes that selectively affect surface area would not necessarily be detected using measures of cortical thickness. For example, age-related sulcal expansion (Kemper, 1994) could, in theory, be related to changes in cortical volume but not thickness. Alternatively, the discrepancy across laboratories may be related to differences in participant characteristics, such as exclusionary criteria or the ages of the OA groups. Several studies indicate that some frontal lobe damage is more closely related to vascular disease than to healthy aging processes (Artero et al., 2004; Raz et al., 2007). Thus, results from studies such as the present one, which excluded any OA with untreated hypertension, should include fewer changes that are specifically related to vascular disease processes.
We also found several small areas where cortical thickness was greater in OA compared to YA. While not directly comparable to the present study, which examined differences between YA and OA, one study did report a regionally specific increase in cortical thickness in high functioning OA, compared to OA with average fluid intelligence scores (Fjell et al., 2006). The high functioning group had thicker cortex in the right posterior cingulate gyrus. Although slightly more posterior to the area of the cingulate gyrus where we found increased thickness in OA, these two regions are adjacent. Taken together, these findings support the idea that some areas of cortex do not show age-related loss, and that thickening in specific cortical regions may impart some protective advantage to OA in terms of performance in selected cognitive domains.
4.4. Cognitive correlates of decreased WM integrity
We found a reliable pattern of correlations between WM integrity and cognitive performance in this sample of healthy OA. These results are striking because the ROI-based analysis showed a putative double dissociation between cognitive control and episodic memory function vis-à-vis WM integrity in anterior and posterior regions, respectively. Namely, we found a positive correlation between FA in anterior WM regions, including PFC, and scores on tasks that assessed cognitive control processes. In contrast, episodic memory performance correlated positively with the integrity of WM underlying temporal and posterior parietal areas, but not frontal areas, in our sample of OA. It is important to note that, in a cross-sectional study, one cannot completely disentangle the effects of age and the effects of inherent variability in a brain measure when the brain measure itself is negatively correlated with age. From careful analysis of our data, however, we find that age has a negative effect on FA (both through our group comparisons and correlations with age in selected ROIs) and that cognition is predicted by FA. Strengthening this conclusion is our finding that the correlations between cognition and FA were found in brain areas in which OA also showed significantly lower FA, when compared to YA.
The findings of a DTI study of WM in old rhesus monkeys support our conclusion: Age-related decline in executive function was significantly correlated with FA in long-distance corticocortical association pathways, including the anterior corpus callosum and the superior longitudinal fasciculus (Makris et al., 2006). In older humans, increased numbers of WM hyperintensities in PFC are associated with greater executive dysfunction (Gunning-Dixon and Raz, 2003), and FA in anterior WM correlates with selected measures of executive function and mnemonic control (O’Sullivan et al., 2001; Sullivan et al., 2006). The present results confirm and extend these previous reports suggesting a link between the integrity of frontal lobe WM and performance on tests requiring the top-down control of cognition. By virtue of our combined whole-brain and ROI-based approach, we have also demonstrated that performance on these tasks is not correlated with WM integrity in more posterior regions. The brain-behavior correlations described here underscore the necessity of frontal lobe WM in supporting cognitive control processes in OA.
Other evidence supporting the association between temporoparietal areas and episodic memory performance comes from functional neuroimaging studies that have demonstrated a critical role for parietal lobe GM in mediating episodic memory retrieval (Buckner and Wheeler, 2001; Rugg et al., 2002; Shannon and Buckner, 2004; Wagner et al., 2005). The cortical loci that are consistently implicated include lateral posterior parietal cortex (including the intraparietal sulcus and inferior parietal lobule), precuneus, posterior cingulate, and retrosplenial cortices (Wagner et al., 2005). In the present study, the locus of the correlation between temporoparietal WM and episodic memory fell at the junction of several WM tracts that connect these previously identified brain regions. This WM region includes the sagittal stratum, which originates in the caudal portions of the superior and inferior parietal lobules and superior temporal gyrus, and the inferior longitudinal fasciculus, which contains projections that connect posterior parietal cortex with the inferior temporal gyrus and occipital lobe (Schmahmann and Pandya, 2006). One would expect this area to include fibers that link many of the cortical areas that are active during episodic retrieval, and it is, therefore, not surprising that decreased integrity of this WM region is associated with lower episodic memory performance in OA.
4.5. Cognitive correlates of age-related cortical thinning
Contrary to our expectations, we found no significant correlations between measures of cortical thickness and composite scores from any of the three cognitive domains examined. Prior evidence suggesting a link between age-related cortical changes and cognitive decline comes from a variety of sources. Functional neuroimaging studies in OA show dramatic changes in cortical activity during performance of tasks that require cognitive control (Gazzaley et al., 2005; Grady et al., 2006; Velanova et al., 2007), episodic memory (Cabeza et al., 1997; Grady et al., 1999; Stebbins et al., 2002), and semantic memory processes (Cabeza, 2001; Dennis et al., 2006; Madden et al., 1996). Such studies, however, rely on indirect measures of neural activity, and not structural integrity per se. Thus, these fMRI studies cannot rule out contributions from other factors, such as decreased integrity of the connections between nodes in these functional networks, similar to the pattern we report here using DTI-based indices of WM integrity.
More direct evidence for a link between cortical thinning and cognitive decline comes from a study in monkeys, showing that cortical thinning in PFC was a good predictor of performance on a delayed non-match to sample memory test (Peters et al., 1998b). In humans, however, the evidence for a relation between cortical atrophy and cognitive decline is equivocal. A review of the literature on structure-function correlations in aging concluded that “the magnitude of the observed associations is modest” and “not easily replicated” (Raz and Rodrigue, 2006). One experiment that was limited to YA suggested a link between cortical thickness and verbal recall (Walhovd et al., 2006), but at delay intervals (months) much longer than those employed here (minutes). Consistent with our results, another study found no association between cortical thickness and measures of executive function (Fjell et al., 2006). Although some investigators have advocated searching for a link between memory decline and changes in the size of the hippocampus or other medial temporal lobe structures (Golomb et al., 1996), a meta-analysis of volumetric studies found little evidence for an association between hippocampal atrophy and memory decline in healthy OA (Van Petten, 2004). Unfortunately, our thickness analyses are limited to cortical regions, and thus is is not possible to determine whether hippocampus proper is related to episodic memory function in our sample of OA. Our analyses do, however, include other medial temporal lobe structures, such as entorhinal and parahippocampal cortices, and we did not find evidence for age-related changes or correlations with episodic memory scores in these areas.
These negative findings with respect to correlations between cortical thickness and cognition raise the possibility that MRI-based measures of thickness or volume are simply not sensitive enough to detect the age-related alterations that are functionally significant. It is important to note, however, that unlike many previous studies, the methods employed here are not constrained by the size of ROIs selected a priori (i.e., we did not rely on pre-defined cortical parcellation units), but rather combined an ROI analysis with a point-by-point examination of cognitive correlations across the entire cortical surface. Thus, we would have been capable of detecting correlations between cognitive scores and changes in small, discrete areas of cortex, had such correlations existed. It is possible that the cognitive processes that are measured by our three composite scores do not rely on the function of easily localizable cortical foci, but instead tend to recruit numerous nodes, distributed across large regions of cortex (McIntosh, 2000; Mesulam, 1990). Because efficient communication is essential for the proper function of such large scale networks, disruption in the integrity of the links could introduce catastrophic interference. This hypothesis is in accordance with our finding of stronger structure-function correlations when considering WM integrity.
5. Conclusions
Our data suggest that WM degeneration, rather than cortical (i.e., GM) thinning, may contribute more to explaining age-related deterioration of cognitive control processes and episodic memory. While healthy aging was associated with cortical thinning and loss of WM integrity, the loci of these changes were distinct. WM changes occurred in tissue underlying association cortices, whereas cortical thinning was greatest in primary sensory and motor cortices. Thus, the contribution of cortical thinning to decline in the domains of episodic memory and cognitive control may be secondary to deterioration of WM integrity, or may instead correlate with lower-level processes that rely more on the primary sensory areas where cortical thinning is most pronounced. Further, this spatial dissociation suggests that separate degenerative processes may be at play in GM and WM. These processes may follow different time courses, and GM and WM abnormalities may even respond to different therapeutic interventions. The identification of new therapeutic opportunities applicable to healthy aging will require interdisciplinary research efforts that combine theoretical perspectives from cognitive science with histology, neuroimaging, genetics, and psychopharmacology.
Supplementary Material
Acknowledgments
This work was supported by NIH grants: AG021525 (SC), K01 AG24898 (DS), and T32 GM007484 (DZ). Imaging facilities at the Athinoula A. Martinos Center for Biomedical Imaging are supported by grants from the NCRR (P41RR14075) and the MIND Institute. Oliver Piguet was supported by a National Health and Medical Research Council of Australia Neil Hamilton Fairley Postdoctoral Fellowship (ID# 222909). He is now at now at the Prince of Wales Medical Research Institute, Sydney, Australia. Emily Connally is now at the University of Arizona.
We thank Meredith Brown for helpful editorial comments, Julie Proulx for help with data collection, Nicholas Harrington for help with data analysis, and Joseph Locascio and Paymon Hosseini-Varnamkhasti for statistical consultation.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the cns: Comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Braz J Med Biol Res. 2003;36:409–20. doi: 10.1590/s0100-879x2003000400002. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–82. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25:154–67. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry. 2004;75:1304–8. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–5. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Sultzer D, Marx P, Herzberg JS, Phelan CK, Marder SR. In vivo mr evaluation of age-related increases in brain iron. AJNR Am J Neuroradiol. 1994;15:1129–38. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–23. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the nmr spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, mt, and dt mri. Neurology. 2006;66:535–9. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Benton AL, Hamsher Kd. Multilingual aphasia examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–34. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: Contributions of functional neuroimaging. Scand J Psychol. 2001;42:277–86. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–22. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: Quantitative analysis at in vivo mr imaging in healthy volunteers. Radiology. 2000;216:672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–55. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: The rotterdam scan study. Ann Neurol. 2000;47:145–51. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–12. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–84. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Halliday GM, Kril JJ, Harasty JA, Cullen K, Brooks WS, Creasey H, Broe GA. Topography of brain atrophy during normal aging and alzheimer’s disease. Neurobiol Aging. 1996;17:513–21. doi: 10.1016/0197-4580(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. Ii: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM. Selective increase of cortical thickness in high-performing elderly--structural indices of optimal cognitive aging. Neuroimage. 2006;29:984–94. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittelman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–3. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999;9:805–14. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–41. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–26. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor mr imaging study. AJNR Am J Neuroradiol. 2007;28:226–35. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective mri study. Neuropsychologia. 2003;41:1929–41. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–8. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–23. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hinman JD, Abraham CR. What’s behind the decline? The role of white matter in brain aging. Neurochem Res. 2007 doi: 10.1007/s11064-007-9341-x. [DOI] [PubMed] [Google Scholar]
- Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, Niessen WJ, Breteler MM. Brain tissue volumes in the general elderly population the rotterdam scan study. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on mri, part i: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The boston naming test. Philadelphia: 1983. [Google Scholar]
- Ke Y, Ming Qian Z. Iron misregulation in the brain: A primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–53. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert ML, Knoefel JE, editors. Clinical neurology of aging. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Ketonen LM. Neuroimaging of the aging brain. Neurol Clin. 1998;16:581–98. doi: 10.1016/s0733-8619(05)70082-7. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal mri and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–8. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age-and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–11. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lutz J, Hemminger F, Stahl R, Dietrich O, Hempel M, Reiser M, Jager L. Evidence of subcortical and cortical aging of the acoustic pathway: A diffusion tensor imaging (dti) study. Acad Radiol. 2007;14:692–700. doi: 10.1016/j.acra.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fmri and dti study. Neurobiol Aging. 2007;28:459–76. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Coleman RE, Provenzale JM, DeGrado TR, Hoffman JM. Adult age differences in regional cerebral blood flow during visual world identification: Evidence from h215o pet. Neuroimage. 1996;3:127–42. doi: 10.1006/nimg.1996.0015. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–81. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, Benner T, Wald LL, Wu O, Tuch DS, Caviness VS, Moore TL, Killiany RJ, Moss MB, Rosene DL. Frontal connections and cognitive changes in normal aging rhesus monkeys: A dti study. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: Theory and rationale for its development. The international consortium for brain mapping (icbm) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–70. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ. Age-related cognitive decline in rhesus monkey. In: Peters A, Morrison JH, editors. Cerebral cortex vol. 14: Neurodegenerative and age-related changes in structure and function of the cerebral cortex. Vol. 14. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 21–48. [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–29. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol. 2001;22:136–42. [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “Disconnection” As a mechanism of age-related cognitive decline. Neurology. 2001;57:632–8. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–52. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–15. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol. 2002;31:581–93. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998a;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998b;8:671–84. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–9. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Piguet O, Corkin S. The aging brain. In: Feinstein S, editor. Learning and the brain: A comprehensive guide for educators, parents, and teachers. Lanham, MD: Rowman & Littlefield Education; 2007. [Google Scholar]
- Piguet O, Double KL, Kril JJ, Harasty J, Macdonald V, McRitchie DA, Halliday GM. White matter loss in healthy ageing: A postmortem analysis. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging. 2004a;25:377–96. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 2004b;62:433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–57. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion mri using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of brain damage. Perceptual & Motor Skills. 1958;8:271–276. [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Chalk JB. Gray and white matter changes in alzheimer’s disease: A diffusion tensor imaging study. J Magn Reson Imaging. 2008;27:20–6. doi: 10.1002/jmri.21231. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Redundant myelin sheaths and other ultrastructural features of the toad cerebellum. J Cell Biol. 1966;28:73–93. doi: 10.1083/jcb.28.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and alzheimer disease. Arch Neurol. 1999;56:338–44. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005a;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005b;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol. 2001;429:541–53. doi: 10.1002/1096-9861(20010122)429:4<541::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–92. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund H, Nyberg L, Adolfsson R, Nilsson LG, Launer LJ. High prevalence of white matter hyperintensities in normal aging: Relation to blood pressure and cognition. Cortex. 2003;39:1093–105. doi: 10.1016/s0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Bennett DA, Wilson RS, Glover G, Gabrieli JD. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol Gen. 1935;18:643–662. [Google Scholar]
- Sturrock RR. Changes in neuroglia and myelination in the white matter of aging mice. J Gerontol. 1976;31:513–522. doi: 10.1093/geronj/31.5.513. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–9. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–55. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–92. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: A stereological investigation. Neurobiol Aging. 1997;18:609–15. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: An mri study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–73. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–53. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–35. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–46. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I. Regional cortical thickness matters in recall after months more than minutes. Neuroimage. 2006;31:1343–51. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale--third edition. San Antonio, TX: Harcourt Assessment; 1997a. [Google Scholar]
- Wechsler D. Wechsler memory scale--third edition. San Antonio, TX: Harcourt Assessment; 1997b. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: A diffusion-tensor analysis. Arch Gerontol Geriatr. 2007 doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, mci, and ad. Neurology. 2006;67:2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec RF, Burkett NR, Markwell SJ, Larsen DL. A cross-sectional study of the effects of age, education, and gender on the boston naming test. Clin Neuropsychol. 2007;21:587–616. doi: 10.1080/13854040701220028. [DOI] [PubMed] [Google Scholar]
- Zec RF, Markwell SJ, Burkett NR, Larsen DL. A longitudinal study of confrontation naming in the “Normal” Elderly. J Int Neuropsychol Soc. 2005;11:716–26. doi: 10.1017/S1355617705050897. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14:823–33. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.