Abstract
Understanding the development of nociceptive circuits is important for the proper treatment of pain and administration of anesthesia to prenatal, newborn and infant organisms. The spinothalamic tract (STT) is an integral pathway in the transmission of nociceptive information to the brain, yet the stage of development when axons from cells in the spinal cord reach the thalamus is unknown. Therefore, the retrograde tracer Fluoro-Gold was used to characterize the STT at several stages of development in the mouse, a species in which the STT was previously unexamined. One week old, two day old and embryonic day 18 mice did not differ from adults in the number or distribution of retrogradely labeled STT neurons. Approximately 3,500 neurons were retrogradely labeled from one side of the thalamus in each age group. Eighty percent of the labeled cells were located on the side of the spinal cord contralateral to the injection site. Sixty-three percent of all labeled cells were located within the cervical cord, 18% in thoracic cord and 19% in the lumbosacral spinal cord. Retrogradely labeled cells significantly increased in diameter over the first postnatal week. Arborizations and boutons within the ventrobasal complex of the thalamus were observed after the anterograde tracer biotinylated dextran amine was injected into the neonatal spinal cord. These data indicate that while neurons of the STT continue to increase in size during the postnatal period, their axons reach the thalamus before birth and possess some of the morphological features required for functionality.
Keywords: spinal cord, dorsal horn, thalamus, neonate, embryo, pain
INTRODUCTION
Organisms exposed to noxious stimuli early in life can develop abnormal pain experience during adulthood (Anand et al., 1999; Ruda et al., 2000; Sternberg et al., 2005). This may reflect a sensitive period during which noxious stimuli can shape the development of neural circuits mediating nociception (Fitzgerald 2005). Knowledge of the developing neuroanatomy responsible for the processing of nociceptive stimuli is important because potentially stressful and injurious procedures are increasingly being performed on fetuses and neonates (Grunau et al., 2006). An additional concern is the level of the neuraxis that nociceptive information is able to reach at advancing developmental time points, and therefore the increasing potential for the experience of pain (Lee et al., 2005). Recent hemodynamic imaging and electrophysiological studies suggest that information specific to nociceptive processing can reach cortical areas in preterm humans (Fernandez et al., 2003; Bartocci et al., 2006; Slater et al., 2006). However, conclusions about pain experience from these studies must be tempered because of an incomplete understanding of the anatomic development of nociceptive circuits in the central nervous system. In particular, the nociceptive pathways that ascend from the spinal cord to supraspinal targets and that are required for the perception of pain have not been examined during the perinatal period.
One ascending pathway that is critical for direct nociceptive transmission to the forebrain is the spinothalamic tract (STT). Neurons of the STT presumably derive from a population of precursor cells that migrate from the ventricular zone of the spinal cord and then begin to differentiate on embryonic day 12 in rats (E12; Altman and Bayer 1984). The majority of STT neurons in the lumbar cord of rats were found to be generated by E14 (Beal and Bice, 1994; Bice and Beal 1997). In adult rats, STT neurons are predominantly located within several areas of the spinal cord including the marginal zone, lateral cervical and spinal nuclei, nucleus proprius, intermediate zone and ventral horns (Giesler et al., 1979; Kevetter and Willis, 1983; Granum 1986; Burstein et al., 1990; Kobayashi 1998; Al-Khater et al., 2008). Axons of STT neurons typically decussate in the spinal cord and terminate in the contralateral thalamus where they transmit nociceptive information about thermal, mechanical and chemical stimuli (Willis and Coggeshall 2004). However, the developmental time point at which STT axons reach their destination in the thalamus and could possibly transmit this nociceptive information is unknown.
The STT has not been examined during the perinatal period in any species, nor has the STT of the adult mouse been fully characterized. One study has shown that the lateral cervical nucleus projects to the thalamus in mice (Giesler et al., 1987), but nothing is known of the projections from spinal cord gray matter to the thalamus. Yet the mouse has become an important organism in studies on the molecular mechanisms of pain because of the growing availability of genetically altered models (Harvey et al., 2004; Zhang and Bao 2006; Graham et al., 2007; Lacroix-Fralish et al., 2007). Moreover, the identification of transcription factors involved in the genesis and differentiation of sensory, including nociceptive, neurons has lead to novel characterizations of neural populations in the developing mouse (Chen et al., 2001; Helms and Johnson 2003; Ding et al., 2004). Presently, a lack of neuroanatomical studies in mice has limited the ability to extrapolate the contributions that molecularly identified cell populations might make to functional pathways. In this study we used retrograde and anterograde tracers to quantify the cells of origin and observe the axon terminations of STT neurons in mice during early development and adulthood.
METHODS
CD-1 mice (Charles River Laboratories, Wilmington, MA) were used for all procedures. Adult mice were male; neonates (born on P0) and embryos (plug visible on E0) were of either sex. All procedures were conducted in accordance with the guidelines of the Institutional Care Facility at the University of Minnesota.
Adult Mice
Adult mice weighing 28 – 40g were anesthetized with pentobarbital (60 mg/kg, IP) and fixed in a stereotaxic frame. An incision was made along the midline of the skull and a small bur hole was drilled through the bone over the approximate location of the thalamus. A pulled borosilicate glass pipette with a tip diameter of ~20 µm was attached to a 5 µL Hamilton syringe filled with mineral oil. A solution of 4% Fluoro-Gold (Fluorochrome LLC, Denver, CO) was drawn into the pipette. The syringe was attached to a manipulator and moved to the coordinates (in mm from Bregma): [AP −2.0, ML 1.8, and depth from brain surface: −3.0] and then to [AP −1.8, ML 1.8 and depth: −2.8]. Two separate pressure injections of 80 nL of Fluoro-Gold were administered from each of the above locations into the thalamus. The incision was sutured and the mouse recovered on a warm pad and upon waking was returned to the home cage. Mice were sacrificed five to seven days later with an overdose of pentobarbital and perfused with physiological saline and then 4% paraformaldehyde. The brain and spinal cord were removed and post-fixed in 4% paraformaldehyde overnight. Brains and spinal cords were sectioned transversely on a freezing microtome at 75 µm and 50 µm respectively.
Neonatal and Embryonic Mice
Injections into the thalamus of perinatal mice were performed as described previously (Davidson et al., 2010). Briefly, neonates were anesthetized by hypothermia and positioned on a stage designed for stereotaxic injections in neonatal mice. The transverse and superior sagittal sinuses are visible through the skin and skull and were used as landmarks. For P0-P5 neonates, the pipette insertion point was 0.7 mm lateral of the superior sagittal sinus and 1.2 mm rostral of the transverse sinus. The pipette was inserted to a depth 2.2 mm measured from the surface of the skin. For neonates P5 and older, a small puncture at the pipette insertion point was first made with a 30 gauge needle to allow the micropipette to penetrate the skull more easily. 50 nL of 4% Fluoro-Gold was delivered over 15 s and the pipette was removed after 60 s. After the injection the neonate was immediately placed under a warming lamp and on a warming pad. Approximately one minute later, color, movement, and reaction to toe pinch returned and the neonate was returned to the home cage with its mother.
In neonatal mice (P0 to P5) the anterograde tracer biotinylated dextran amine (BDA, 5%, 3,000 MW, lysine fixable, Molecular Probes, Eugene, OR) was injected by pressure into the spinal cord gray matter. Because of the longer duration of the surgical procedure relative to thalamic injections, mice were anesthetized with intramuscular pentobarbital (50mg/kg). A laminectomy was performed over the cervical spinal cord and the dorsal portion of one or two vertebrae removed with fine forceps. A pipette attached to a Hamilton syringe was lowered into one side of the spinal cord to a depth just below the surface and 30 nL of BDA was delivered. After the injection a single suture and a small amount of cyanoacrylate glue were used to close the wound. Neonates spent one hour on a warm heating pad, then were returned to their home cage with their mother. After 48 hours the neonates were deeply anesthetized with pentobarbital and perfused as described below.
Timed pregnant females carrying embryonic day 17 (E17) embryos were anesthetized with pentobarbital (60 mg/kg IP) and placed on a heating pad. A laparotomy was performed and a portion of the uterus containing 2 or 3 embryos was gently extracted (Davidson et al., 2010). Ritodrine hydrochloride (100 µL at 14 mg/ml in saline) was applied directly to the surface of the exposed uterus to relax the muscle. An individual embryo was manipulated in utero under a dissecting microscope using hand-held ring forceps. The head of the embryo was held in direct contact with the uterine wall so that the transverse and superior sagittal sinuses could be clearly seen. A pipette was lowered through the uterine wall, through the skull of an embryo and into the thalamus where 30 nL of Fluoro-Gold was ejected by pressure. The pipette remained in place for 10 seconds after completion of the injection and then was slowly withdrawn. A few easily accessible embryos on each side of the uterus were injected and then placed back into the body cavity. The dam was sutured and recovered on a warming pad for 1 hour and then moved back to her home cage. The dam was re-anesthetized 24 hours later with pentobarbital and the abdominal incision reopened to access the embryos for hypothermia induced anesthesia and fixation by perfusion.
Fixation and Histology
For embryonic injections the transport time was one day and for neonatal injections two days. After this time the embryo or neonate was placed on ice to induce anesthesia by hypothermia. Mice were perfused with 2 mL 0.9% saline through the heart and then for fixation, 3 mL of 4% paraformaldehyde in 0.2 M, 7.2 pH phosphate buffer. Solutions were delivered by pump at 0.33 mL/minute. Partially dissected neonates/embryos were placed in 4% paraformaldehyde overnight. The next day the nervous system was removed under a dissecting microscope, and the brain and spinal cord was separated at the level of the tectum and embedded in cutting medium. Both were sectioned transversely at 50 µm with a cryostat. For retrograde experiments the tissue was mounted onto a gel coated slide, dried and coverslipped. After injection sites were confirmed to be contained within the thalamus, labeled neurons were mapped in the spinal cord using a camera lucida drawing tube attached to a microscope equipped for ultraviolet reflected illumination. Labeled cells in every 50 µm section of spinal cord were counted. Counting began on the first cervical segment on the section immediately caudal to the pyramidal decussation and ended in the rostral sacrum. Cells were categorized by location in the spinal cord using a classification system similar to that of Burstein et al. (1990b). For total cell counts, the Abercrombie correction factor was multiplied to the total cell counts to reduce error from double counting cells split between sections (Abercrombie, 1946; Burstein et al., 1990; Klop et al., 2004). Additional adult, P7, P2, and E18 mice were injected into the thalamus with Fluoro-Gold following the protocols detailed above and the spinal cords were sectioned in the sagittal plane to determine the diameter of labeled cells from superficial and deep dorsal horn. These diameters were used in the equation: Thickness of section / (thickness of section + mean cell diameter) = correction factor.
In anterograde tracing experiments, BDA was visualized in the spinal cord and brain using the ABC-DAB reaction applied to floating sections. The tissue was washed with 0.1 M phosphate buffered saline and then incubated for 1 hr at room temperature with reagents as directed (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Tissue was rinsed with 0.1 PBS containing 0.2% Triton-X and then incubated with 0.05% DAB, 0.005% H2O2, 0.05 M TBS (pH 7.6). After 3 – 5 min, sections were rinsed in 0.1 PBS and then mounted on gel coated slides. The location and extent of the injection site in the spinal cord were determined and the thalamus was examined for evidence of axonal labeling.
Micrographs were made digitally using Scion Capture v2.0 software with a Scion 1.4 megapixel grayscale camera mounted to an Olympus BX50 microscope. Brightness and contrast of whole images were uniformly adjusted using Adobe Photoshop CS3. Anatomical borders in the perinatal mouse brain were determined with the assistance of Paxinos et al. (2007).
RESULTS
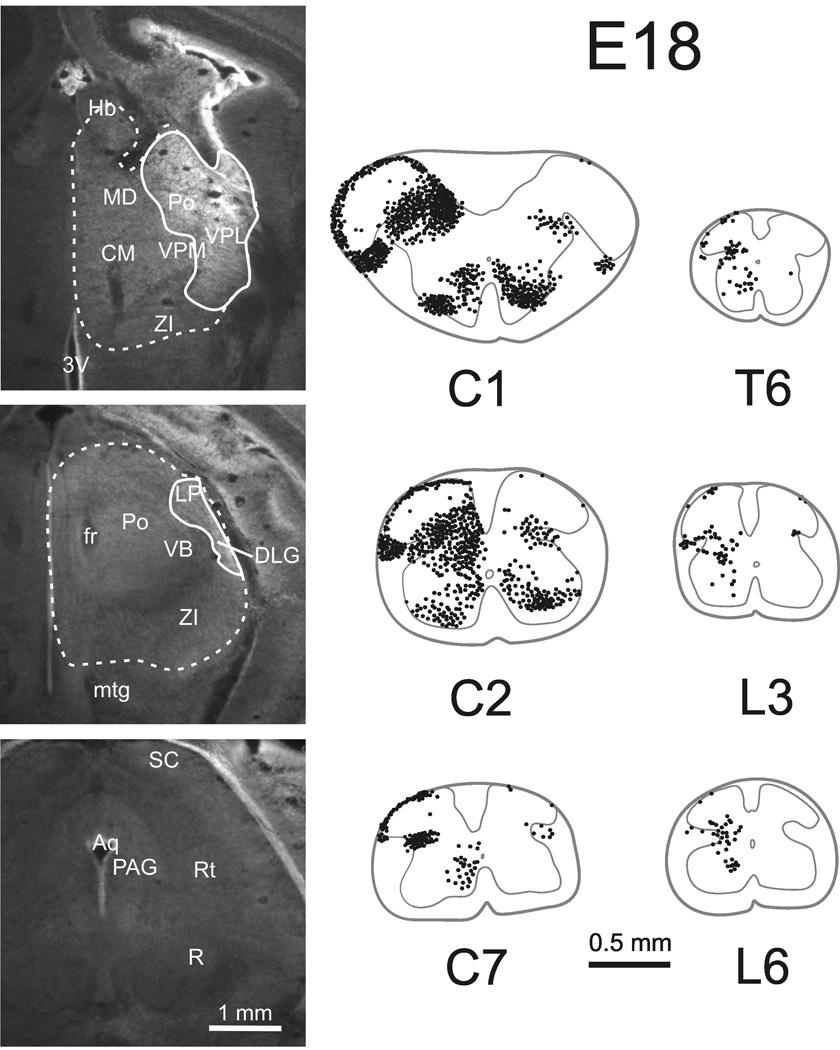
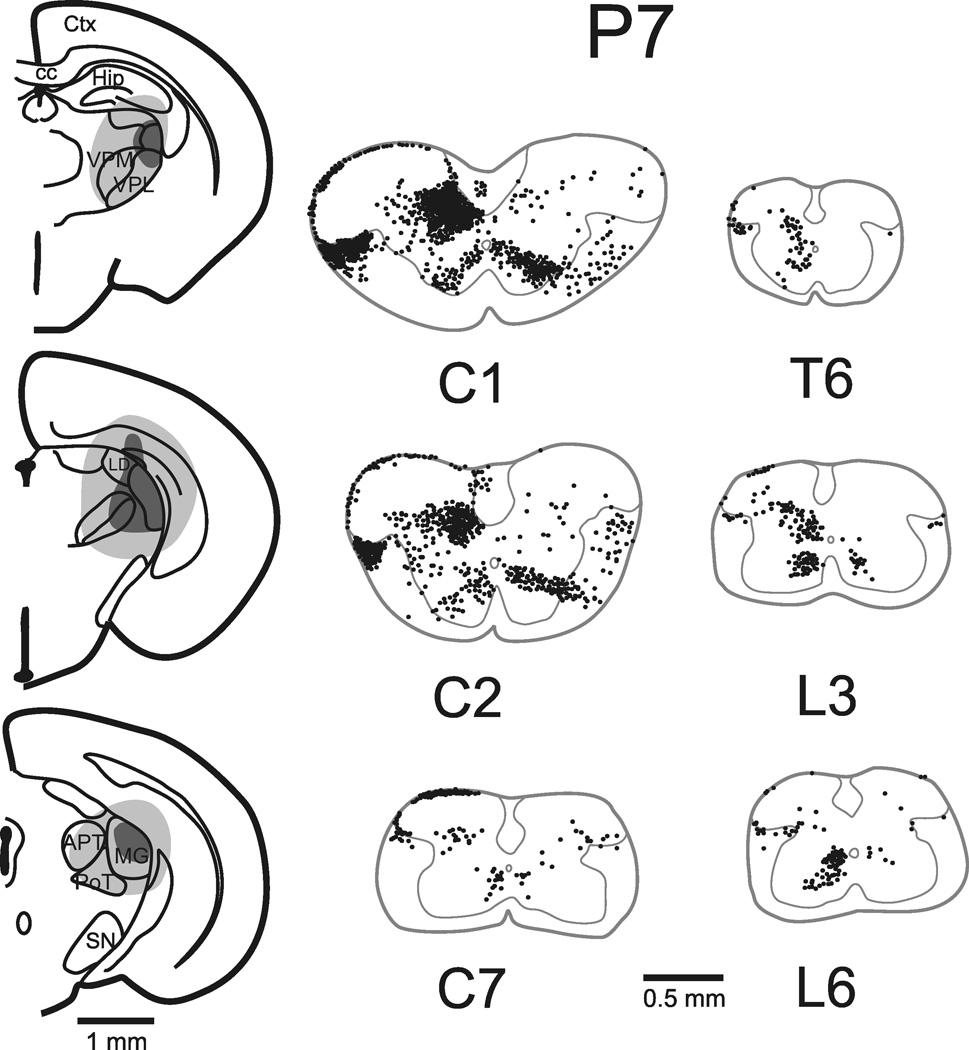
Four mice in each age group: E18, P2, P7 and adult, were selected to assess the quantity and localization of retrogradely labeled spinothalamic tract (STT) neurons. One additional mouse in each group was used to obtain measures of STT cell diameter in sagittal sections. Injections of Fluoro-Gold typically produced necrosis within the core of the injection site and spread in the extracellular space to surrounding areas of the thalamus. Reconstructions of typical injection sites are shown in Figure 1–Figure 3 for adult, P7 and P2 mice. Photomicrographs from an example of an injection in the thalamus of an E18 mouse show the extent of the spread of 50 nL of Fluoro-Gold and the pattern of labeling revealed in the spinal cord (Figure 4). Injection sites that missed the thalamus or that spread into the contralateral thalamus or midbrain were rejected from further analysis. The spread of Fluoro-Gold around the injection site did not always extend to the rostral thalamus and did not always include the midline thalamic nuclei. However, Fluoro-Gold typically extended across the mediolateral extent of the posterior thalamus, and because Fluoro-Gold has been shown to be taken up by axons of passage (Dado et al., 1990), most axons terminating in more rostral parts of the thalamus were expected to take up the tracer in transit. Nevertheless, some thalamic projecting spinal neurons may have been missed, possibly leading to an underestimation of the total number of STT cells.
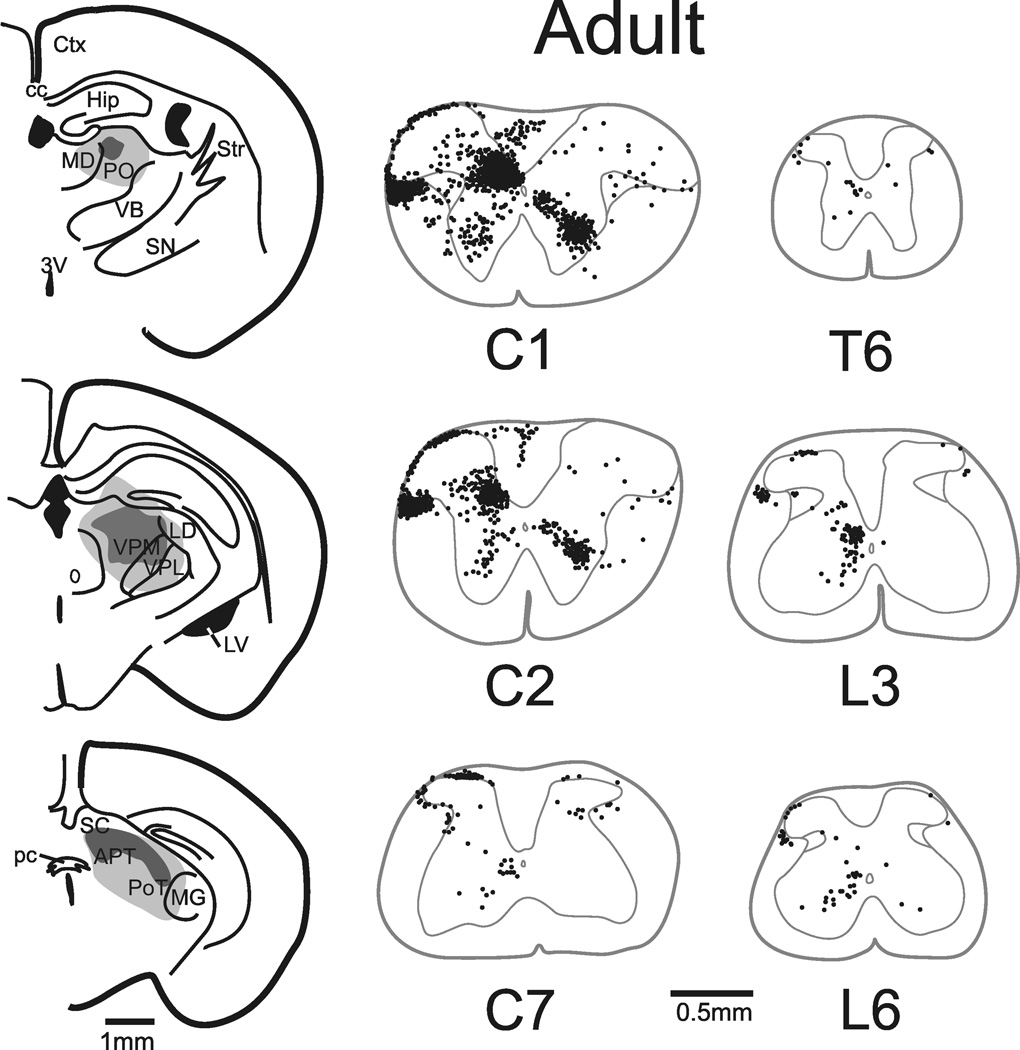
Figure 1.
Cells of origin of the STT in the adult mouse. Left, injection sites in the thalamus. Dark gray regions represent the core of the tracer injection and light gray represents its spread. Right, locations in representative segments of the spinal cord of retrogradely labeled neurons. A single dot was placed at the location of each retrogradely labeled cell in each segment as shown. Each segment was reconstituted from several 50 µm coronal sections into a representative trace. No correction factor was used to produce these reconstructions. The total number of labeled cells in the adult mouse represented in this figure was 3,007 (corrected). The complete illustration of all retrogradely labeled cells throughout the length of the adult spinal cord is shown in Supplementary Figure 1.
Figure 3.
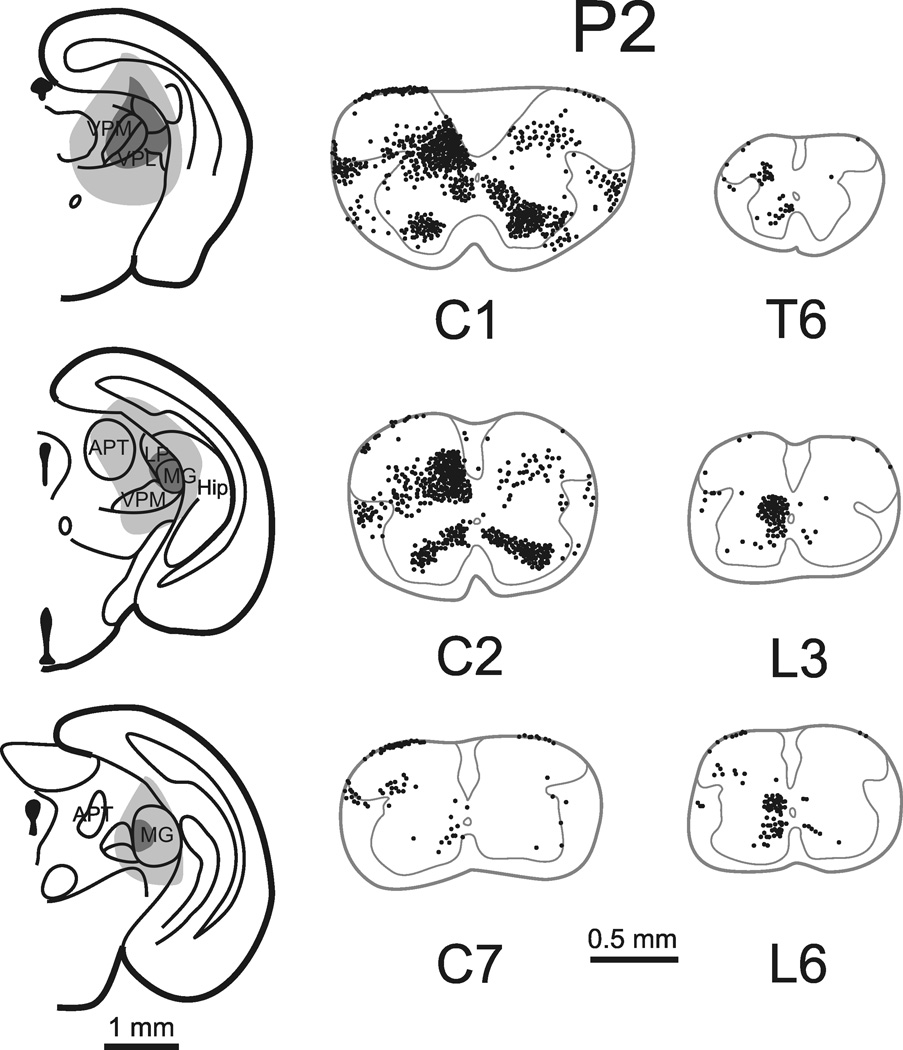
Cells of origin of the STT in a neonatal mouse injected on the day of birth and perfused 48 hours later. Left, site in the thalamus into which Fluoro-Gold was injected. Right, retrogradely labeled STT neurons are shown as in Figure 1. Total number of STT neurons (corrected) in this mouse was 3,047.
Figure 4.
Cells of origin of the STT in an embryonic mouse injected on E17 and perfused 24 hours later. Left, photomicrographs of the site in the thalamus into which Fluoro-Gold was injected (anterior is top). Note a lack of tracer spread to the midbrain indicating that retrogradely labeled neurons must reach to the forebrain. The solid line represents the injection site and the dotted line represents the regions to which tracer spread. Right, retrogradely labeled STT neurons as shown in Figure 1. Total number of STT neurons (corrected) in this mouse was 3,944.
In the adult mouse, retrogradely labeled marginal zone neurons were found predominantly within cervical segments with fewer observed in thoracic and lumbar segments (Figure 1, Supplemental Figure 1). Labeled neurons were almost never observed in the substantia gelatinosa, which together with the marginal zone is referred to as the superficial dorsal horn (SDH). Throughout the spinal cord, 95% of labeled neurons in the SDH were located on the side of the spinal cord contralateral to the injection site. Of retrogradely labeled cells found in the lateral cervical (LCN) and lateral spinal nuclei (LSN) 84% were located on the contralateral dorsolateral funiculus. Retrogradely labeled neurons were heavily concentrated in the nucleus proprius and intermediate basilar nucleus of the upper cervical cord; together with the lateral reticular area these regions make up the deep dorsal horn (DDH). Eighty-seven percent of DDH cells were located on the side of the spinal cord contralateral to the injection site. The ventral horns, including the area around the central canal (VH), contained a substantial proportion of STT neurons which were distributed bilaterally (57% contralateral) within the spinal cord.
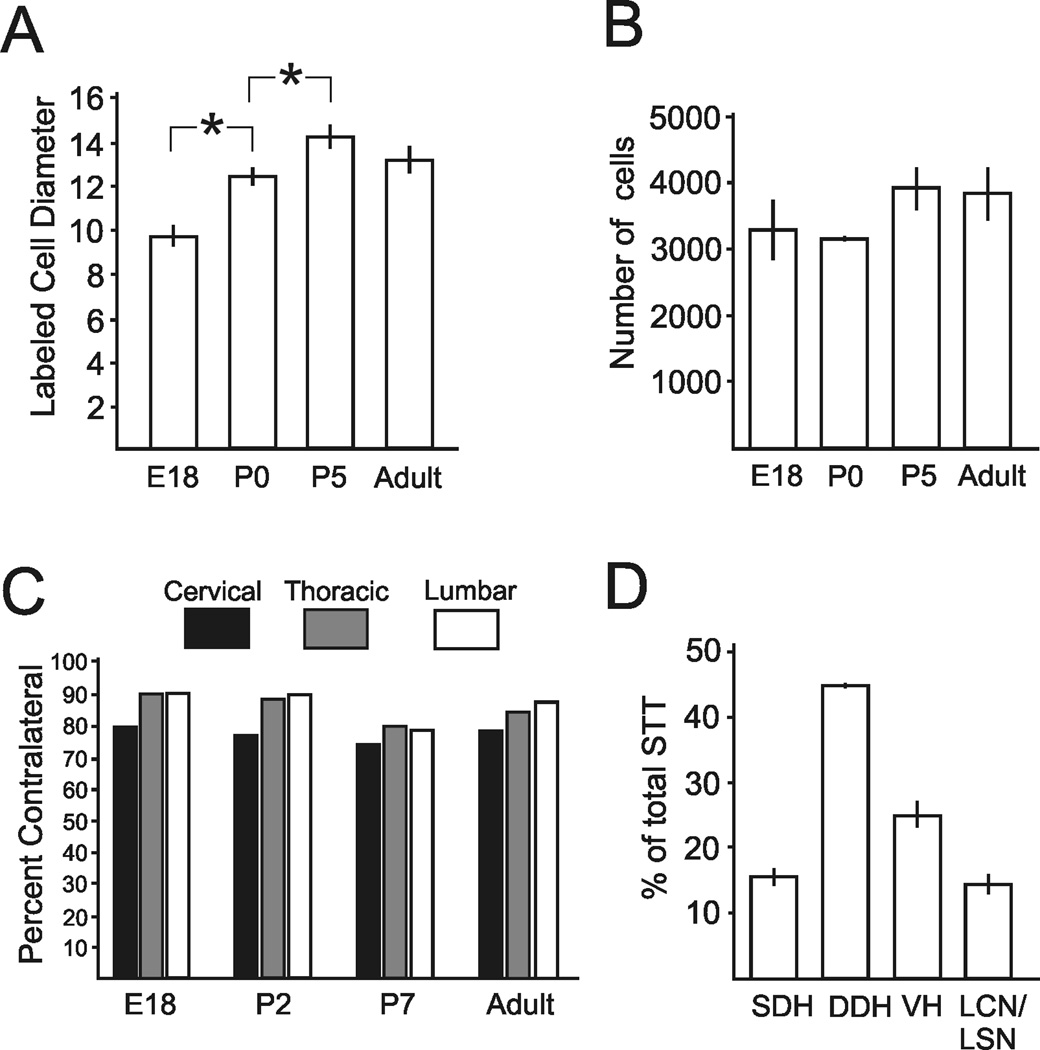
To determine the diameter of labeled neurons for use in calculating the Abercrombie correction factor, labeled neurons from sagittal sections throughout the length of the spinal cord of each age group were examined. Within any of the individual age groups no differences were detected in the mean diameter of neurons between cervical, thoracic or lumbar levels (One-way ANOVA, p> 0.05). Therefore, the measurements of diameter from all levels of the spinal cord were pooled within each age group to test for differences between groups. Photomicrographs of representative sections through the DDH of the lumbar enlargement of each age group are shown for comparison (Figure 5). The mean diameter of retrogradely labeled cells from E18 mice (9.8 ± 0.4 µm) was found to be significantly smaller than for cells at P2 (12.4 ± 0.3 µm; One-way ANOVA, p < 0.001, Dunn’s post-test for multiple comparisons). Furthermore, the diameter of P2 cells was significantly smaller than P7 cells (14.3 ± 0.5 µm; p< 0.001). Adult cells (13.3 ± 0.6 µm) were not statistically different in size from P7 (Figure 6A).
Figure 5.
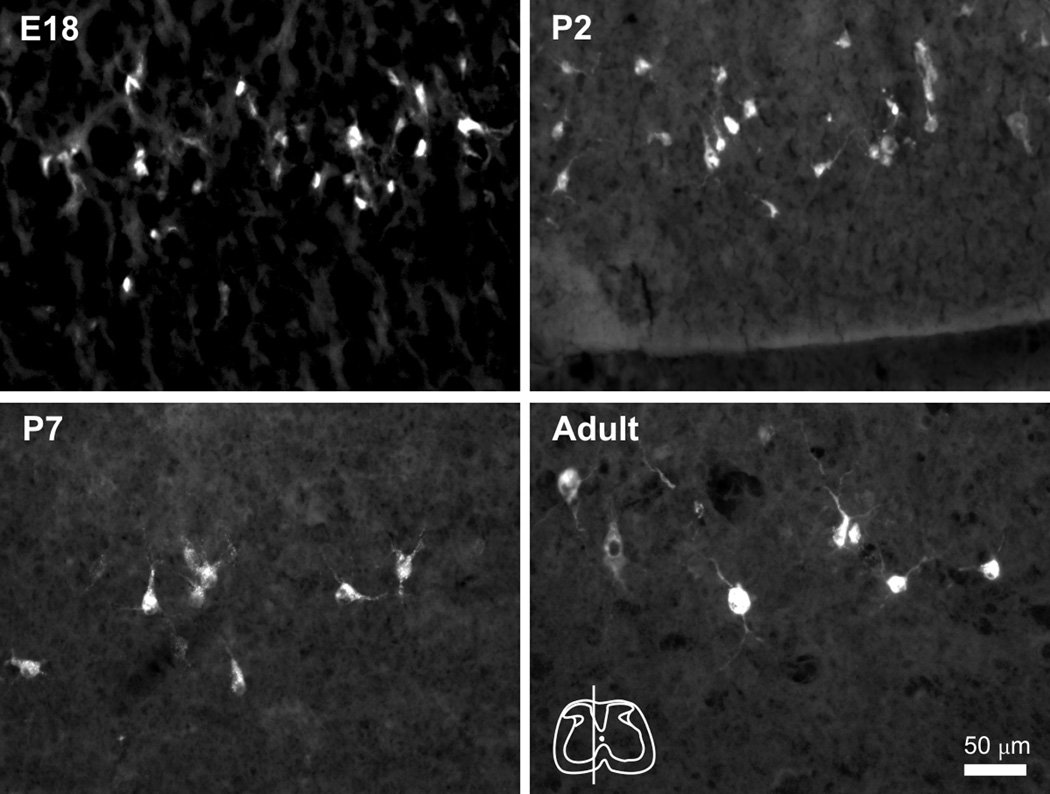
Sagittal sections showing retrogradely labeled cells in the deep dorsal horn of the lumbar enlargement. The diameter of STT neurons was measured to determine the Abercrombie correction factor for each age group. Top left, E18 cells appeared generally spherical but small and with few distinct processes. Top right, P2 cells. Bottom left, P7 cells and processes become larger. Bottom right, Adult cells. Rostral is left. Inset, location of the section through lumbar enlargement of each panel.
Figure 6.
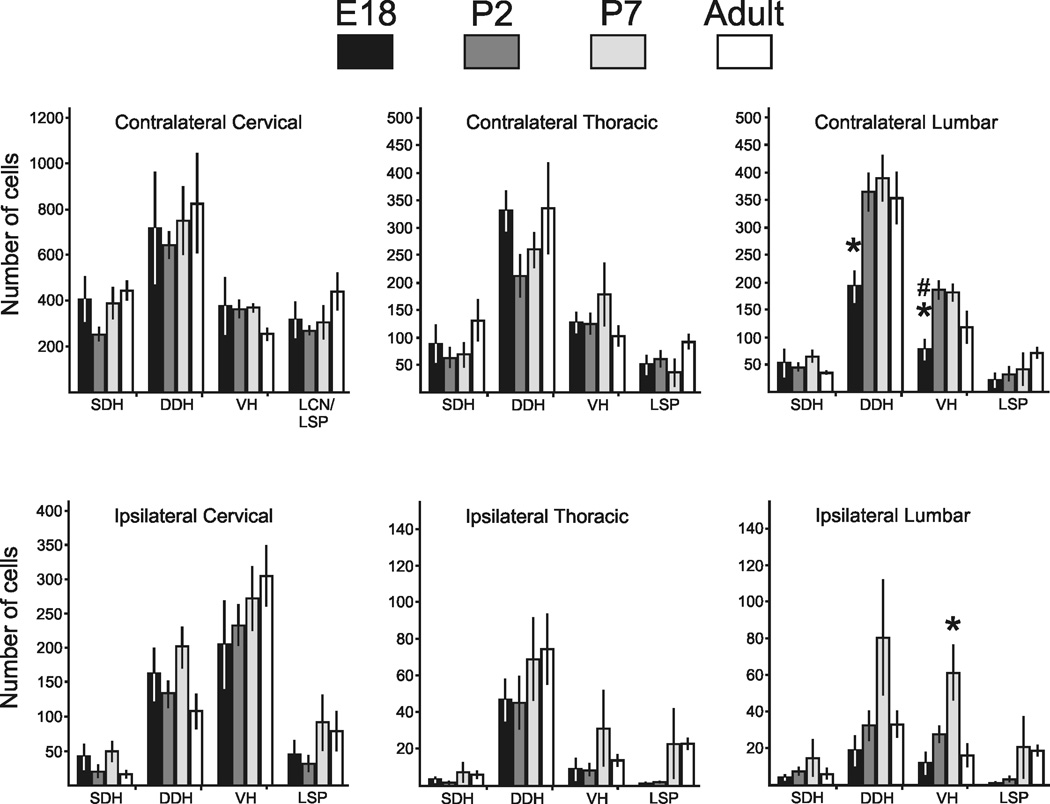
Measurement and quantification of retrogradely labeled cells. A. Diameters of cells in each age group. Somas of STT neurons progressively increase in size during the early postnatal period and reach adult size at P5. B. No differences were detected between the total mean numbers of corrected STT neurons in each age group. C. Percent of retrogradely labeled cells at each age within cervical, thoracic and lumbosacral levels that were located contralateral to the injection site. D. The percentage of retrogradely labeled neurons in each categorized part of the spinal cord calculated throughout the length of the spinal cord and averaged over each age group.
The Abercrombie correction factor was calculated and the total count for each animal was adjusted. The total corrected number of cells throughout the entire spinal cord in each age group did not differ between ages (One-way ANOVA, p = 0.32), indicating that the constituent number of cells contributing to the STT in mice is present before birth (Figure 6B). The corrected mean number of cells making up the STT projecting to one side of the thalamus in mice pooled over all age groups was 3,552 ± 190 STT neurons. In all age groups the cervical cord possessed the largest ipsilateral projection relative to thoracic and lumbosacral cord, and this ratio was stable across development (Figure 6C). The percentage of neurons located throughout the length of the spinal cord in each area of the gray matter was calculated (Figure 6D). Across all age groups, 63% percent of the labeled cells were located within the cervical spinal cord. The remainder was split evenly between the thoracic and lumbar cord.
Retrogradely labeled neurons were categorized by their location in the gray matter as SDH, DDH, LCN/LSN or VH and were counted within each region in every age group. The mean number of cells (corrected) in each category from each age is shown in Figure 7. To determine whether axons from some regions of the spinal cord reached the thalamus before other regions, the number of cells in each category was compared across all age groups. No differences were detected in the number of retrogradely labeled cells within any category of the cervical and thoracic spinal cord across any of the age groups. However, some differences reached significance in lumbar spinal cord. Embryonic mice displayed fewer retrogradely labeled cells in the contralateral DDH than the neonatal and adult groups. Also, fewer neurons were labeled in the embryonic VH than in either neonatal group but not in adult. Finally, P7 mice possessed more labeled neurons in the ipsilateral VH than the embryonic, P2 or adult groups.
Figure 7.
The number (corrected) of retrogradely labeled cells in each region of the spinal cord across all ages. No differences across age groups were detected in the number of cells in any given region within the cervical and thoracic cord. Embryonic mice had fewer cells in the contralateral lumbar DDH than all older mice, and fewer cells in the VH than neonatal mice. Postnatal day 7 mice had a larger number of cells in the ipsilateral ventral horn than all other ages (One-way ANOVA with Student-Neuman-Kuels post-test for multiple comparisons).
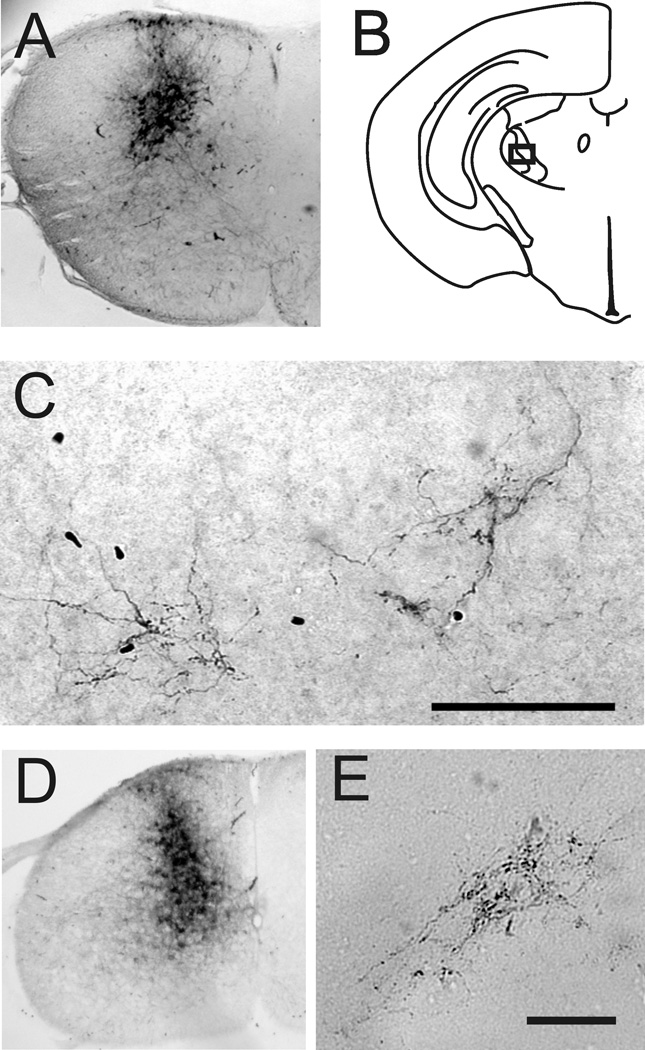
Fluoro-Gold has been shown to be taken up by axons of passage (Dado et al., 1990); therefore we wanted to confirm that STT neurons in neonates possessed axon terminations within the thalamus. A single 30 nL injection of the anterograde tracer biotinylated dextran amine (BDA) was delivered into the dorsal horn of the cervical spinal cord in P2 and P7 neonates (Figure 8). Sections throughout the thalamus were examined for evidence of anterograde labeling. Within the contralateral ventrobasal complex, labeled axon terminal arborizations were observed in both age groups. These arborizations also possessed boutons. Attempted injections of BDA into the dorsal horn of embryos in vivo failed. This was likely because the embryonic spine proved too difficult to immobilize during the injections with our hand-held technique. The data from neonates confirm that axons from spinal cord neurons reach the thalamus before P2 and possess some of the cellular features required for neural transmission.
Figure 8.
Anterograde transport to the thalamus of biotinylated dextran amine (BDA) injected into the cervical spinal cord. A. Upper cervical spinal cord injected on P5 with 30 nL 5% BDA and perfused on P7. B. Illustration of the location of labeled axons terminals in the ventrobasal complex of the thalamus. C. Axon arborizations and boutons from the injection in (A) and located in the box in (B). D. Lower cervical spinal cord of a mouse injected with 30 nL of 5% BDA on the day of birth and perfused 48 hours later. E. Axon arborizations and boutons located in the ventrobasal complex.
DISCUSSION
The spinothalamic tract is a major ascending pathway required for the normal processing of pain, temperature and itch that was previously unexamined in the mouse. The pattern and quantity of STT neurons in the adult, neonatal and embryonic mouse were determined using pressure injection into the thalamus of the retrograde tracer Fluoro-Gold. The main findings from these experiments are 1) that the adult mouse possesses a pattern of STT neurons that appears broadly homologous to the STT previously described in rats (Granum 1986; Kemplay and Webster, 1986; Huang, 1989; Burstein et al., 1990), cats (Carstens and Trevino, 1978; Jones et al., 1987; Craig et al., 1989; Klop et al., 2005) and monkeys (Hayes and Rustioni, 1980; Apkarian and Hodge, 1989; Willis et al., 2001); and 2) that the overall number and pattern of retrogradely labeled neurons when compared between adult, neonatal and embryonic mice, was found not to differ between age groups. Together these results indicate that the mouse STT is similar to that found in other mammals and that the number and organization of the cells of origin, as well as their axon projections are established before birth.
The number of STT neurons projecting to one side of the thalamus in the mouse was approximately 3,500. This compares with 9,500 in rat (Burstein et al., 1990), 12,000 – 14,000 in cat (Klop et al., 2004; 2005) and an estimation of 18,000 in monkey (Apkarian and Hodge, 1989). The estimation in monkey may be inflated because no correction factor was used (Klop et al., 2004). In rats, peak apoptosis of spinal cord interneurons occurs on the day of birth and continues until about P7 (Lowrie and Lawson, 2000); but we found no evidence for a loss of STT neurons in mice during the first postnatal week. A common outbred strain of mouse was used in these studies (CD-1, also known as ICR) and it’s possible that strain differences may affect the total number of STT neurons. Various strains of mice examined under a battery of nociceptive, pruritic and thermal tests exhibited strain dependent thresholds for response (Mogil et al., 1999; Green et al., 2006). It is unclear how genetic variability between these strains produced differences in pain behavior, but it would be interesting if differences in pain behavior were correlated with the quantity or pattern of STT neurons.
Throughout the length of the mouse spinal cord, 80% of STT neurons were labeled on the side contralateral to the injection site. This is consistent with what has been previously reported in rats (Granum 1986; Burstein et al., 1990); although Kemplay and Webster (1986) documented that over 90% of the STT neurons were contralateral. Throughout the length of the spinal cord the percent of STT neurons contralateral to the injection site in monkeys and cats has been reported to be between 80 – 95% (Apkarian and Hodge, 1989; Willis et al., 2001; Klop et al., 2005). Retrogradely labeled neurons located in the superficial and deep dorsal horn accounted for 15% and 45% of the total STT in mouse, respectively. Similar results were seen in rats and monkeys (Apkarian and Hodge, 1989; Burstein et al., 1990). Several studies have found that STT neurons project selectively to various thalamic targets based on their laminar distribution in the spinal cord (Giesler et al., 1979; Craig 2006; Craig and Zhang, 2006; Al-Khater et al., 2008). Therefore, the possibility exists that specific components of the mouse STT could also exhibit different trajectories.
In mice, as in other species, more than half the total number of STT neurons were observed in the upper cervical cord (C1-C3). Functional studies of upper cervical STT neurons performed in cats and monkeys have shown that many of these cells possess large, bilateral receptive fields and respond to tactile and noxious mechanical stimulation (Carstens and Trevino, 1978; Smith et al., 1991). Some upper cervical STT neurons also responded to algogenic chemicals applied to the pericardium or stimulation of the cardiopulmonary sympathetic fibers, suggesting a mechanism for referred pain arising from myocardial ischemia (Chandler et al., 1996; 1998; 2000). A large proportion of upper cervical STT neurons appear to have no mechanical receptive fields and their functions are not clear (Carstens and Trevino, 1978; Smith et al., 1991). In contrast, STT neurons located within the dorsal horn and intermediate gray (laminae I – VI) of spinal segments caudal to C3 nearly all possess mechanical receptive fields and respond to noxious stimuli including chemical and thermal stimuli (Willis and Coggeshall, 2004). Spinothalamic tract neurons located within the ventral horn of rats often respond to joint movement or kneading of the muscle suggesting a possible role in proprioception (Giesler et al., 1976; Menetrey et al., 1984).
Axon terminations of nociceptive rat STT neurons occur predominantly in the ventrobasal complex and the posterior region of the thalamus (Cliffer et al., 1991; Gauriau and Bernard, 2004; Zhang and Giesler, 2005; Zhang et al., 2006). The presence of axon terminations in the thalamus from STT neurons of neonatal mice was confirmed by spinal injection of biotinylated dextran amine. Terminal arborizations with boutons were observed in the ventrobasal complex indicating that neonatal mice posses some of the morphological features necessary for synaptic transmission. The STT should not be considered fully developed at birth however, because the neurons of origin continue to increase in size until one week postnatal. This increase in size may contribute to changes in input resistance and firing properties that have been observed in unidentified dorsal horn neurons during the perinatal period (Walsh et al., 2009). The functional properties of STT neurons during early life have not been studied.
Neurons in the dorsal horn, some of which make up the ascending nociceptive system, receive inputs from unmyelinated and thinly myelinated primary afferent fibers (Light and Perl, 1979; Sugiura et al., 1986). In rats, the central projections of myelinated primary afferent fibers begin to enter the lumbar dorsal horn on E15 and unmyelinated fibers enter subsequently on E18/19 (Fitzgerald 1987a; Mirnics and Koerber, 1995; Jackman and Fitzgerald, 2000). In mice, primary afferent innervation of the dorsal horn occurs in similar waves: first large diameter fibers penetrate the gray matter on embryonic day (E) 14.5 and then small diameter fibers on E15.5 (Ozaki and Snider, 1997). Studies examining the physiology and anatomy of several types of myelinated primary afferent fibers in neonatal mice indicate that some functional properties and adult-like morphology of central projections are present within a day or two after birth (Woodbury et al., 2001; Woodbury and Koerber, 2003; 2007). However, ion channel expression patterns in dorsal root ganglion neurons continue to develop over the neonatal period (Benn et al., 2001; Hjerrling-Leffler et al., 2007). Likewise, electrical and anatomic properties of neonatal primary afferent fibers become increasingly adult-like with age (Mirnics and Koerber, 1997; Jennings and Fitzgerald, 1998; Ritter et al., 2000; Beggs et al., 2002). Despite incomplete and ongoing development, responses similar to those seen in adults can be elicited by mechanical, thermal and chemical stimuli from primary fibers of neonates at birth (Fitzgerald, 1987b).
Action potentials in dorsal horn neurons evoked from stimulation of A-fibers are present at P3, but C-fiber evoked responses are only observed from P10 onward in rats (Fitzgerald 1988; Jennings and Fitzgerald, 1998; Baccei et al., 2003). Over the first several postnatal weeks cutaneous receptive fields decrease in size and mechanical thresholds increase in rat dorsal horn neurons; moreover, withdrawal reflexes are exaggerated and inaccurate in neonatal rats but mature significantly by three weeks of age (Fitzgerald and Jennings, 1999; Fitzgerald, 2005). These studies suggest that continued postnatal refinement of central innervation and the maturation of intrinsic properties of dorsal horn neurons occur after birth. Recently, the intrinsic electrical properties of unidentified neurons in the mouse superficial dorsal horn were shown to become more adult-like during a “critical period” over the second postnatal week (Walsh et al., 2009; but see: Baccei and Fitzgerald, 2005). For example, superficial dorsal horn neurons from mouse embryos produced only single spikes to intracellular current injections but bursts of action potentials to the same stimuli after the critical period (Walsh et al., 2009). Because STT neurons represent the major output of the spinal cord to the forebrain for sensation and localization of noxious stimuli it would be interesting to determine whether they too undergo a functional transformation during the second postnatal week. The data in the present study are consistent with the idea that signals transmitted by nociceptors reach the dorsal horn and may continue to ascend in a nascent form to the thalamus via the STT during early life.
Studies examining nociceptive systems in mice have rapidly increased because of the advantages provided by transgenic models (Mogil, 2009). Such studies have contributed to the characterization of the TRP family of genes which produce receptors in nociceptors responsive to thermal and chemical stimuli and produce deficits in pain behavior when silenced in mice (Caterina et al., 2000; Bautista et al., 2006; 2007). Other work has elucidated the importance of a gene that codes a sodium channel (Nav1.7) in the mouse the expression of which is necessary for normal pain behavior (Nassar et al., 2004). This gene is homologous to a human gene (SCN9A) the absence of which contributes to congenital insensitivity to pain in humans (Cox et al., 2006). Despite these advances, direct therapeutic benefits have been slow to develop. Indeed, one criticism against the use of rodents in studies of pain is the lack of anatomic data establishing the existence of central pain pathways consistent with those in primates (Craig, 2009). Here we demonstrate that ascending pathways from the superficial and deep layers of the spinal cord reach the forebrain in the mouse. Furthermore, because this pathway is extant prior to birth it is an interesting target for study during development. Transcription factors expressed during development that are involved in patterning nociceptive pathways have been identified (Chen et al., 2001; Ding et al., 2004). The transcription factors responsible for patterning the STT are unknown, but could be explored by combining retrograde and molecular identification techniques in neonates (Davidson et al., 2010).
An STT formed before birth suggests the possibility that nociceptive information may directly reach the brain at early stages of development. This finding supports the conclusions of electroencephalographic and hemodynamic imaging studies that have demonstrated activity in the cortex of preterm humans produced by noxious stimuli applied to the skin (Fernandez et al., 2003; Bartocci et al., 2006; Slater et al., 2006). Without direct physiological evidence it is impossible to conclude that STT neurons are responsive to nociceptive stimuli during the neonatal period. Future studies to determine the functional properties of adult and developing mouse STT neurons must be performed to reach conclusions about ascending nociceptive transmission in mouse models. Additionally, anatomic and functional development of thalamo-cortical fibers must be better understood to determine when nociceptive information reaches the cortex. These studies may help to inform decisions about the use of anesthetics and pain relieving drugs during early life.
Supplementary Material
Figure 2.
Cells of origin of the STT in a neonatal mouse injected on P5 and perfused on P7. Left, site in the thalamus into which Fluoro-Gold was injected. Right, retrogradely labeled STT neurons are shown as in Figure 1. Total number of corrected STT neurons in this mouse was 4,252.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke, Grants NS-047399 and NS-059199, and by the Graduate School of the University of Minnesota.
REFERENCES
- Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511:1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol. 1984;85:1–166. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ. Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol. 1989;288:447–473. doi: 10.1002/cne.902880307. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiology and Behavior. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol. 2003;549:231–242. doi: 10.1113/jphysiol.2003.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M. Intrinsic firing properties of developing rat superficial dorsal horn neurons. Neuroreport. 2005;16:1325–1328. doi: 10.1097/01.wnr.0000175612.08560.10. [DOI] [PubMed] [Google Scholar]
- Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Beal JA, Bice TN. Neurogenesis of spinothalamic and spinocerebellar tract neurons in the lumbar spinal cord of the rat. Brain Res Dev Brain Res. 1994;78:49–56. doi: 10.1016/0165-3806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ. Developmental expression of the TTX resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci. 2001;21:6077–6085. doi: 10.1523/JNEUROSCI.21-16-06077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice TN, Beal JA. Quantitative and neurogenic analysis of neurons with supraspinal projections in the superficial dorsal horn of the rat lumbar spinal cord. J Comp Neurol. 1997;388:565–574. doi: 10.1002/(sici)1096-9861(19971201)388:4<565::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Giesler GJ., Jr The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res. 1990;511:329–337. doi: 10.1016/0006-8993(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ., Jr Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol. 1990b;291:329–344. doi: 10.1002/cne.902910302. [DOI] [PubMed] [Google Scholar]
- Carstens E, Trevino DL. Laminar origins of spinothalamic projections in the cat as determined by the retrograde transport of horseradish peroxidase. J Comp Neurol. 1978;182:161–165. [PubMed] [Google Scholar]
- Carstens E, Trevino DL. Anatomical and physiological properties of ipsilaterally projecting spinothalamic tract neurons in the second cervical segment of the cat’s spinal cord. J Comp Neurol. 1978;182:167–184. doi: 10.1002/cne.901820111. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Zhang J, Foreman RD. Vagal, sympathetic and somatic sensory inputs to upper cervical (C1-C3) spinothalamic tract neurons in monkeys. J Neurophysiol. 1996;76:2555–2567. doi: 10.1152/jn.1996.76.4.2555. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Zhang J, Foreman RD. Phrenic nerve inputs to upper cervical (C1-C3) spinothalamic tract neurons in monkeys. Brain Res. 1998;798:93–100. doi: 10.1016/s0006-8993(98)00412-0. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Zhang J, Qin C, Yuan Y, Foreman RD. Intrapericardiac injections of algogenic chemicals excite primate C1-C2 spinothalamic tract neurons. Am J Physiol Regul Integr Comp Physiol. 2000;279:R560–R568. doi: 10.1152/ajpregu.2000.279.2.R560. [DOI] [PubMed] [Google Scholar]
- Craig AD, Jr, Linington AJ, Kniffki KD. Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol. 1989;289:568–585. doi: 10.1002/cne.902890404. [DOI] [PubMed] [Google Scholar]
- Craig AD. Retrograde analyses of spinothalamic projections in the macaque monkey: input to ventral posterior nuclei. J Comp Neurol. 2006;499:965–978. doi: 10.1002/cne.21154. [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol. 2006;499:953–964. doi: 10.1002/cne.21155. [DOI] [PubMed] [Google Scholar]
- Craig AD. A rat is not a monkey is not a human: comment on Mogil (Nature Rev. Neurosci. 10, 283–294 (2009)) Nat Rev Neurosci. 2009;10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- Dado RJ, Burstein R, Cliffer KD, Giesler GJ., Jr Evidence that Fluoro-Gold can be transported avidly through fibers of passage. Brain Res. 1990;533:329–333. doi: 10.1016/0006-8993(90)91358-n. [DOI] [PubMed] [Google Scholar]
- Davidson S, Truong H, Nakagawa Y, Giesler GJ., Jr A microinjection technique for targeting regions of embryonic and neonatal mouse brain in vivo. Brain Res. 2010;1307:43–52. doi: 10.1016/j.brainres.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Blass EM, Hernandez-Reif M, Field T, Diego M, Sanders C. Sucrose attenuates a negative encephalographic response to an aversive stimulus for newborns. J Dev Behav Pediatr. 2003;24:261–266. doi: 10.1097/00004703-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Prenatal growth of fine-diameter primary afferents into the rat spinal cord: a transganglionic tracer study. J Comp Neurol. 1987a;261:98–104. doi: 10.1002/cne.902610108. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hind-limb of the neonatal rat. J Physiol. 1987b;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The development of activity evoked by fine diameter cutaneous fibres in the spinal cord of the newborn rat. Neurosci Lett. 1988;86:161–166. doi: 10.1016/0304-3940(88)90564-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. PNAS. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Menétrey D, Guilbaud G, Besson JM. Lumbar cord neurons at the origin of the spinothalamic tract in rat. Brain Res. 1976;118:320–324. doi: 10.1016/0006-8993(76)90718-6. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Menétrey D, Basbaum AI. Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol. 1979;184:107–126. doi: 10.1002/cne.901840107. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Miller LR, Madsen AM, Katter JT. Evidence for the existence of a lateral cervical nucleus in mice, guinea pigs, and rabbits. J Comp Neurol. 1987;263:106–112. doi: 10.1002/cne.902630109. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Schofield PR, Callister RJ. Altered potassium channel function in the superficial dorsal horn of the spastic mouse. J Physiol. 2007;584:121–136. doi: 10.1113/jphysiol.2007.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum SL. The spinothalamic system of the rat. I. Locations of cells of origin. J Comp Neurol. 1986;247:159–180. doi: 10.1002/cne.902470204. [DOI] [PubMed] [Google Scholar]
- Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–275. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Müller U. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Rustioni A. Spinothalamic and spinomedullary neurons in macaques: a single and double retrograde tracer study. Neuroscience. 1980;5:861–874. doi: 10.1016/0306-4522(80)90155-4. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LYM. Origin of thalamically projecting somatosensory relay neurons in the immature rat. Brain Res. 1989;495:108–114. doi: 10.1016/0006-8993(89)91223-7. [DOI] [PubMed] [Google Scholar]
- Jackman A, Fitzgerald M. Development of peripheral hindlimb and central spinal cord innervations by subpopulations of dorsal root ganglion cells in the embryonic rat. J Comp Neurol. 2000;418:281–298. [PubMed] [Google Scholar]
- Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J Physiol. 1998;509:859–868. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Willis WD. Collaterals of spinothalamic cells in the rat. J Comp Neurol. 1983;215:453–464. doi: 10.1002/cne.902150409. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Holstege G. How many spinothalamic tract cells are there? A retrograde tracing study in cat. Neurosci Lett. 2004;360:121–124. doi: 10.1016/j.neulet.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Holstege G. Segmental and laminar organization of the spinothalamic neurons in cat: evidence for at least fibe separate clusters. J Comp Neurol. 2005;493:580–595. doi: 10.1002/cne.20777. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. Distribution and morphology of spinothalamic tract neurons in the rat. Anat Embyrol (Berl) 1998;197:51–67. doi: 10.1007/s004290050119. [DOI] [PubMed] [Google Scholar]
- Lowrie MB, Lawson SJ. Cell death of spinal interneurones. Prog Neurobiol. 2000;61:543–555. doi: 10.1016/s0301-0082(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131:3.e1–3.e4. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- Menétrey D, de Pommery J, Roudier F. Properties of deep spinothalamic tract cells in the rat, with special reference to ventromedial zone of lumbar dorsal horn. J Neurophysiol. 1984;52:612–624. doi: 10.1152/jn.1984.52.4.612. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Prenatal development of rat primary afferent fibers: II Central projections. J Comp Neurol. 1995;355:601–614. doi: 10.1002/cne.903550409. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Properties of individual embryonic primary afferents and their spinal projections in the rat. J Neurophysiol. 1997;78:1590–1600. doi: 10.1152/jn.1997.78.3.1590. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Snider WD. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J Comp Neurol. 1997;380:215–229. [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang HQ. Atlas of the developing mouse brain. London: Elsevier; 2007. [Google Scholar]
- Ritter AM, Woodbury CJ, Albers K, Davis BM, Koerber HR. Maturation of cutaneous sensory neurons from normal and NGF-overexpressing mice. J Neurophysiol. 2000;83:1722–1732. doi: 10.1152/jn.2000.83.3.1722. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling Q-D, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg WF, Scorr L, Smith LD, Ridgway CG, Stout M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain. 2005;113:347–353. doi: 10.1016/j.pain.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Zhang X, Honda CN, Giesler GJ., Jr Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267–276. doi: 10.1016/s0304-3959(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Zhang X, Bao L. The development and modulation of nociceptive circuitry. Curr Opin Neurobiol. 2006;16:460–466. doi: 10.1016/j.conb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Giesler GJ., Jr Response characteristics of spinothalamic tract neurons that project ot the posterior thalamus in rats. J Neurophysiol. 2005;93:2552–2564. doi: 10.1152/jn.01237.2004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Davidson S, Giesler GJ., Jr Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci. 2006;26:5215–5223. doi: 10.1523/JNEUROSCI.0701-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.