Abstract
Objective
To assess the impact of an elective clinical research course on second- and third-year pharmacy students' knowledge of clinical research methods, training programs, career options, and interest in pursuing postgraduate training.
Design
A 2-credit hour elective course in clinical research was designed that included lectures, discussions, workshops, and in-class presentations related to study design and implementation, protocol synthesis, research evaluation, ethical and legal considerations, data analysis, and professional opportunities involving clinical research. Learner knowledge of these topics was assessed using several methods, including 3 assignments related to research protocol, ethical documentation, and presentation.
Assessment
A survey instrument designed to evaluate the effect the course had on pharmacy students' knowledge of clinical research methods and interest in pursuing postgraduate training in clinical research was administered. Students who completed the elective had a greater level of familiarity with research-related topics, training options, and career opportunities (p < 0.05) and a greater interest in pursuing a career in clinical research (p < 0.05) than did students in a matched control group.
Conclusion
Taking a 2-credit hour elective course in clinical research increased pharmacy students' interest in pursuing a career in clinical research.
Keywords: clinical research, translational research, elective course
INTRODUCTION
Pharmacists are trained to have an extensive range of knowledge related to pharmacotherapeutics. This knowledge base provides pharmacists with the potential to contribute expertise to research teams in several areas related to drug therapy. However, despite a strong foundation to pursue careers as pharmaceutical scientists, the profession may underutilize this opportunity.1-4 When pharmacy faculty members who were working in research intensive settings in 2002 were surveyed, only half of the respondents had formal research training.1 Additionally, the American Association of Colleges of Pharmacy (AACP) reported that only 22 doctor of pharmacy (PharmD)-trained researchers were principal investigators on funded awards from the National Institutes of Health (NIH) in 2008.5 There appears to be a shortage of pharmacy faculty members serving as principal investigators on projects related to clinical and translational research despite their having a foundation to pursue formal research training in the pharmaceutical sciences.
The critical need for pharmacists to expand their training in clinical and translational research was outlined by an American College of Clinical Pharmacy (ACCP) White Paper, and was the focus of a special conference convened by the NIH.1,2 Although the preferred path for pharmacists to be trained as clinical pharmaceutical scientists has been disputed, it is generally accepted that exposing students to research while pursuing a PharmD degree would be beneficial. In fact, a uniform recommendation from the ACCP White Paper and the NIH conference was to increase students' exposure to research with faculty members early in the PharmD curricula.1,2 The impetus behind early exposure to research is to facilitate students' interest in pursuing a career in research. The hope is that early exposure will result in more students pursing research careers to increase the critical mass of pharmacist-trained researchers.
According to the 2007 Accreditation Council for Pharmacy Education (ACPE) Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree, the foundation of pharmacy education has the common goal to prepare graduates with the professional competencies to ensure optimal medication therapy and patient safety. This evolving and increased emphasis on patient care may limit the opportunity for clinical research training in pharmacy curricula. Murphy and colleagues surveyed colleges and schools of pharmacy to identify the extent of research-related courses and experiences provided in PharmD programs.6 Approximately half of the 79 schools that responded reported no required content area related to research methods. Of the schools that responded, 57% offered elective opportunities in research training. However, fewer than 10% of students took advantage of these research opportunities.6 Elective research courses and summer research experiences aimed at increasing the exposure to research for pharmacy students have been reported.7-10 However, to our knowledge, the impact of these elective research experiences on students' interest in pursuing careers as clinical pharmaceutical scientists has not been assessed.
Since 2007, the elective course Introduction to Clinical Research has been offered annually to PharmD students at Purdue University. The goals of this course have been to provide students with concepts and basic application skills relevant to clinical research within the field of pharmacy. This paper describes the implementation, evolution, and assessment of the course. The primary objective was to assess students' knowledge of training programs and career options after graduation, knowledge of basic research methods, and students' interest in pursuing postgraduate training following this elective clinical research course, versus that of students who did not take the course. The ultimate goal was to assess the usefulness of elective research training opportunities in pharmacy curricula.
DESIGN
The 2-credit-hour elective course entitled Introduction to Clinical Research was designed to provide concepts and basic application skills relevant to clinical pharmacy research. The course was offered to second year (P2) and third year (P3) PharmD students at Purdue University in the fall semesters of 2007, 2008, and 2009. This course was designed to facilitate active participation during course sessions and serve the most interested or motivated students in clinical research. Therefore, the desired class size ranged between a minimum of 5 and a maximum of 20 students (serving between 3% of P2 and 5% of P3 students), a number similar for the majority of pharmacy electives at Purdue University.
There were no prerequisites for enrollment in the course. The primary objective was to introduce essential components of clinical research to students to facilitate interest in research-oriented career paths within pharmacy. Therefore, the course focused on exposing students to topics including study design and implementation, protocol synthesis, research evaluation, ethical and legal considerations in research, data analysis, and professional opportunities involving clinical research. The goals of this course were for students to:
(1) Gain familiarity with clinical and translational research within the field of pharmacy;
(2) Develop baseline knowledge in the appropriate design, conduct, ethical considerations, and presentation of a research study and protocol;
(3) Gain introductory skills to develop and evaluate clinical research design and methods;
(4) Develop skills necessary to work productively as a team member;
(5) Relate new insights to future careers involving clinical and translational research.
The course was designed also to help students develop the following general and professional outcome abilities that have been approved by the faculty members of the college of pharmacy.
Critical thinking and decision making
Communication
Self-learning habits
Group interaction and citizenship
Responsible use of values and ethical principles
Fifteen 2-hour class sessions included a mixture of lectures, workshops, and in-class student presentations. The course used a team-teaching approach with 7 faculty members and occasional guest speakers (ranging between 3 and 8 guest speakers per year over the 3 years). This approach provided students with several perspectives on the role of the pharmacist in clinical research and various career paths. The recommended text for the course was “Foundations of Clinical Research, Applications to Practice” by Leslie Portney.11 Reading assignments were given to students prior to most class sessions. In addition, many instructors provided required reading from other sources that students were expected to read to be prepared for upcoming class sessions. Given the number of instructors, a Web-based classroom instructional and management tool (Blackboard Vista, Washington, DC) was used to provide course material, and distribute supplemental teaching materials, assignments, and grading information.
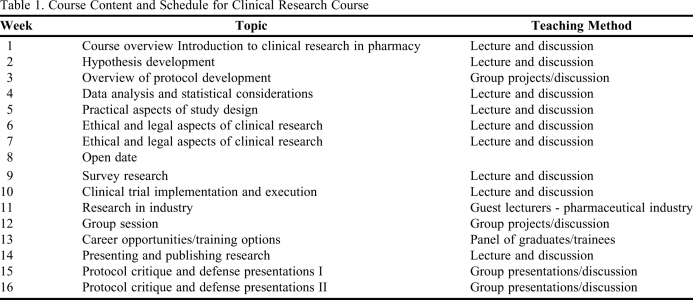
The content of the course is presented in Table 1. The first 7 weeks focused on fundamental topics including study design, hypothesis development, protocol components, ethical considerations, and data analysis. The second half of the course focused on applying these fundamentals to specialty areas such as execution of clinical trials and research in industry, followed by instruction on the dissemination of research results. Throughout the course, instructors presented their career paths to give students perspective on training options in diverse backgrounds. The course concluded with students presenting mock protocol review sessions.
Table 1.
Course Content and Schedule for Clinical Research Course
Points for attendance were awarded, and students were encouraged to engage in class sessions by being assigned a professionalism grade for each session. Based on contributions to class discussions, a numeric grade between 0 and 10 points that corresponded to a scale of learner professional attributes was assigned to each student. These attributes included the demonstration of preparation and comprehension of learning issues. In addition to the professionalism assessment, 4 scheduled quizzes assessed student preparedness. Attendance, professionalism, and quiz points collectively accounted for 30% of the student's final grade. The primary platform to assess learner comprehension of course topics was based on the student's performance on 3 major assignments which comprised 55% of the final grade. These 3 assignments were designed to be completed in groups to enhance the student's ability to function in a group dynamic, considered essential to conducting research. Each group member was assigned the same grade as their peers in the group for each of the 3 assignments, with the exception of the Protection of Human Research Participants Certification Test in the second assignment, which was graded individually. Also, group members were evaluated individually by their peers in their group to reflect a fair assessment of each member's overall contribution to their group work, including dependability, preparation, leadership, collegiality, and quality of work. This latter assessment accounted for 10% of a student's overall grade.
At the beginning of the semester, students were assigned to a group of 3 to 4 members that represented a research team developing a protocol and Institutional Review Board (IRB) application to be defended, based on critiques provided by peer-group presentations in the final 2 class sessions. The team designated 1 member as the principal investigator to oversee the project. Each group was assigned a published clinical research report to guide the assignments. For the first assignment, the group had to develop hypotheses and specific aims based on the published study. From the hypotheses, the group constructed a detailed protocol using the published trial as a guide. A published manuscript was provided because it gave the students some detail regarding the topic background and methodology. Students were encouraged to expand or alter the protocol from the research article to develop a complete protocol, including hypotheses, specific aims, and detailed research methods. The completed protocol was graded by an instructor as the first major assignment. The group was then provided substantial feedback to be incorporated into the second major assignment.
The second major assignment was an extension of the protocol assignment and was designed to expose students to the necessary components for an IRB submission of a proposed clinical study. Each individual was required to pass the Protection of Human Research Participants Certification test through the university's human protection agency. The score on this test was incorporated into each student's grade for this assignment. Students then developed an informed consent statement, project summary statement, and revised protocol from the previous assignment. The entire submission was graded as the second assignment, but feedback was not provided until completion of the third major assignment, after assignments were critically evaluated by a group of peers (as described below).
The third major assignment was an extension of the research protocol and IRB submission assignments. This assignment was designed to assess peer review, defense, and presentation skills of the groups. Each group was assigned to critically review a packet of the first and second assignments from another peer group in the class. During the final 2 class sessions, each group was given 20 minutes to present the critiques of the peer group packet to which they were assigned. The groups were required to provide a detailed handout to guide the audience during the presentation. Following the presentation of the study critique, the group that prepared the original documents was given an opportunity to respond and defend their work. Major assignment 3, therefore, was a compilation of each group's critique (presentation and handout) and their ability to defend the criticism in the original submission of the full application packet.
EVALUATION AND ASSESSMENT
Course Evaluation and Evaluation of Teaching
At the end of the semester, all students were encouraged to complete course and instructor evaluations provided online. All submissions remained anonymous and were not reviewed until final grades were submitted. Due to the number of instructors in the course, 5% of the student's overall grade was reflected by the completion of all the evaluation forms. The course evaluation contained 21 statements based on a 5-point Likert scale, with 5 indicating the learner strongly agreed with the statement, and 1 indicating the learner strongly disagreed.
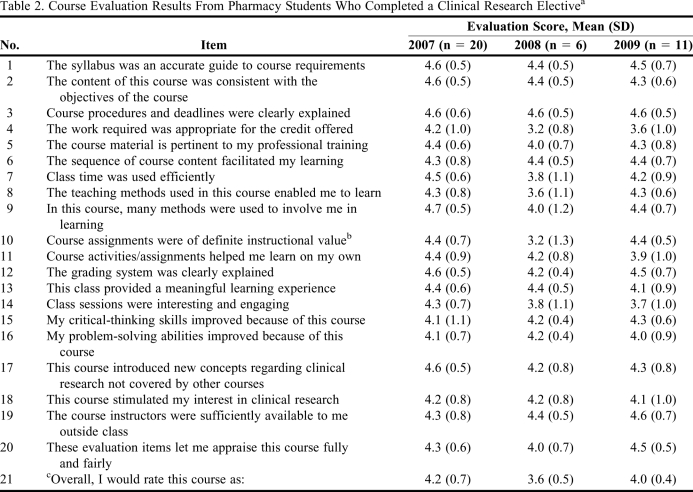
The mean results from the course evaluations in 2007, 2008, and 2009 are provided in Table 2. Overall, the course was well received by the students as indicated by mean course ratings in the good to excellent ranges in both 2007 and 2009. Mean differences among course offerings were small, with most scores > 4 (agree) for all 3 years. Overall, there were no clearly identifiable trends in the categories evaluated, which may be expected because there were only minor changes in content and presentation of the course among the years. Students indicated that the course introduced new concepts regarding clinical research that were not covered in other courses, such as Drug Literature Evaluation (average = 4.3 out of 5.0). Items 15 through 18 of the course evaluation (Table 2) related specifically to the goals of the course, and all had mean scores > 4.0 following all 3 course offerings. Specifically, as presented in Table 2, students indicated they agreed that the course improved their critical-thinking skills (item 15) and problem-solving ability (item 16) while introducing new concepts regarding clinical research (item 17). Further, on average, the students agreed that the course stimulated their interest in clinical research (item 18), which was a primary course goal.
Table 2.
Course Evaluation Results From Pharmacy Students Who Completed a Clinical Research Electivea
The course evaluation used a 5-point Likert scale with 5 = strongly agree; 1 = strongly disagree.
b Overall ANOVA, p < 0.05.
c 5 = excellent; 1 = poor.
Assessment of Learner Knowledge and Interest in Clinical Research
In addition to course and instructor evaluations, a formal survey instrument was used to assess overall knowledge and interest in clinical research among students who completed the introduction to clinical research course, and a sample of professional pharmacy students who did not complete the course. The comparison group was a cohort of students in the professional pharmacy program at Purdue University who had not enrolled in the clinical research elective. The control group had a significantly smaller number of students who had completed a research project and fewer male students. Therefore, a matched control group was developed to account for these significant differences in the baseline characteristics. To develop the matched control group, female students who did not complete a research project were selected at random and omitted from the control group until similar percentages were achieved, as compared to the experimental group.
The survey instrument was designed to evaluate the effect of an introductory course in clinical research on pharmacy students in 2 fundamental areas: (1) knowledge of basic clinical research methods, and (2) interest in pursuing postgraduate training in clinical research. The electronic survey instrument consisted of 20 items (4 included additional subitems for assessment) with additional demographic questions, and was created using Qualtrics software (Qualtrics Labs, Provo, UT). Additional items assessed students' prior research experience and programmatic measures that may have improved their interest in clinical research.
The survey instrument was designed in an iterative process. The first iteration was developed by clinical faculty members with active clinical research programs. The second iteration was developed based on expert feedback on the survey instrument's format to optimize response. This preliminary survey instrument was administered to a convenience sample of students who did not complete the research elective for final modification. The final survey instrument included a standardized measurement tool using a 5-point Likert scale. The anchors varied depending on the fundamental area being assessed. For example, students' knowledge of clinical research methods was assessed by their self-perception of familiarity in 11 areas related to research. The anchors ranged from very familiar to not at all familiar. Student interest in pursuing postgraduate training was assessed by 6 postgraduate options with anchors that ranged from very interested to not at all interested. The same anchors were used to assess students' interest in pursuing a career that involved research.
In October 2009, an electronic invitation describing the project and including the hyperlink to the survey instrument was sent to all P2, P3, and P4 PharmD students at Purdue University. Two reminder e-mails were sent at 1-week intervals to all individuals reminding them to complete the survey instrument. The survey instrument was available for 1 month between October and November 2009. Students enrolled in the 2009 offering of Introduction to Clinical Research were not sent the survey instrument until the final day of the course in December 2009. In addition to the general invitation to participate, students who had completed the clinical research elective previously were sent personalized e-mails requesting their participation. The survey instrument and research protocol were granted exempt status by the IRB at Purdue University.
Statistical comparisons between the groups were performed using GraphPad InStat software (GraphPad Software, Inc, La Jolla, CA). Categorical data were compared using the Fisher exact test, and continuous data were compared using the students' t tests for 2 groups. An analysis of variance was used when comparing more than 2 groups followed by Bonferroni's correction for post hoc analysis, if necessary. For all statistical comparisons, α was set at 0.05.
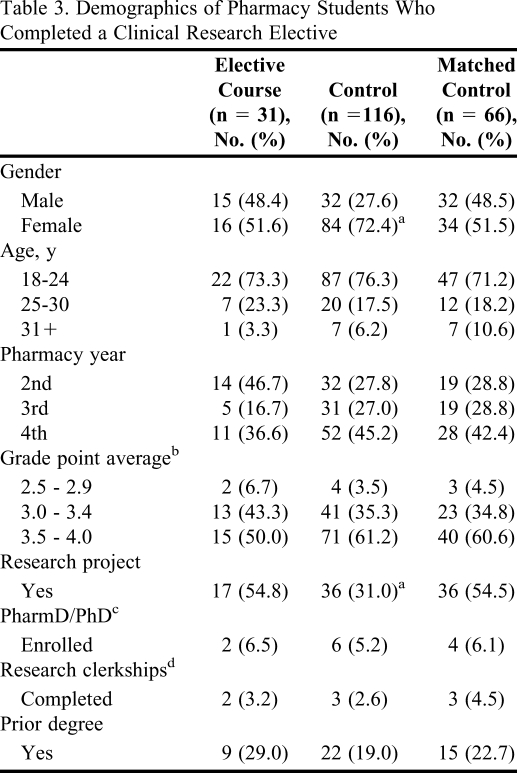
During/over 3 academic years, 37 students completed the clinical research elective, and 31 of these students completed the survey instrument (83.7% response rate). The control group consisted of students in the PharmD program who did not enroll in the clinical research elective. In the control group, 135 students began the survey instrument and 116 completed it, which corresponded to a response rate in the comparator group of 28%. The lower response rate of the control group could have limited the generalizability of the assessment. However, it was not viewed as a significant limitation because the actual number of the control group was 3 times larger than the experimental group (students who enrolled in the course). The demographics for the experimental and control groups are provided in Table 3. The collected demographics were similar between the groups with the exception of a higher percentage of male students enrolled in the clinical research elective versus the control group (p = 0.03). Additionally, a higher percentage of students completed a research project during prepharmacy or in the PharmD program among students who completed the clinical research elective (p = 0.02). Therefore, a matched control group was created by randomly selecting female students who did not complete a research project and omitted them from the analysis. There were no significant differences between the experimental group and the matched control group, as presented in Table 3.
Table 3.
Demographics of Pharmacy Students Who Completed a Clinical Research Elective
a p < 0.05 versus students who completed the clinical research elective.
b Grade point average on a scale of 0 – 4.
c Purdue University offers a combined PharmD/PhD program.
dApplies to students in fourth year only.
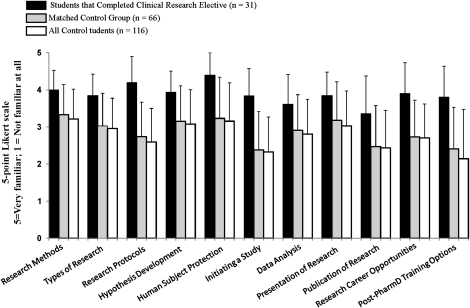
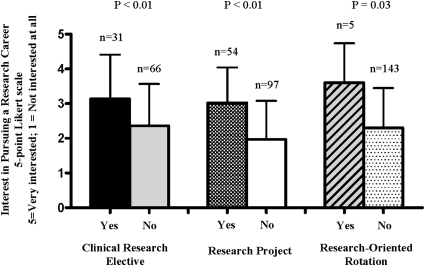
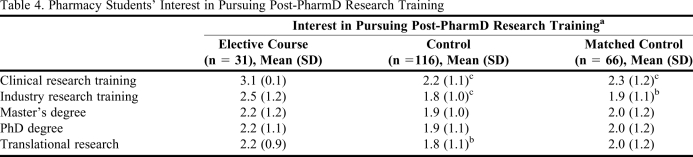
Students who completed the clinical research elective perceived themselves as having a greater level of familiarity with all of the research-related topics, training options, and career opportunities, as compared with students in either the unmatched or matched control groups (p < 0.05 for all comparisons; Figure 1). This corresponded to students who completed the elective having a greater interest in pursuing a career in clinical research as compared to the control students, as displayed in Figure 2 (p < 0.05). The level of interest in pursuing a career in clinical research was similar for students who completed the elective and those who conducted a research project or a clerkship focused in clinical research, as displayed in Figure 2. Furthermore, among the students with these 3 avenues of research exposure (ie, elective, project, clerkship) all groups completing a research experience on average were more interested in pursuing a career in research, compared to the corresponding control groups. Thus, based on survey results, students who completed the elective course, a research project, or a research-related clerkship had the same level of interest in pursuing careers in research. Table 4 breaks down the specific research careers and training opportunities that students who completed the survey instrument were interested in pursuing. Students who completed the elective had the highest mean interest in pursuing training in clinical research and the lowest interest in pursuing a PhD degree. However, interest in all research training options was significantly higher in the students who completed the elective versus control groups.
Figure 1.
Familiarity with research topics was compared between students who completed the research elective (n = 31), and those who did not (n = 116). Students indicated their familiarity with the following research topics. The bar represents the mean (SD) familiarity level, based on a5-point Likert scale with 5 = very familiar; 1 = not at all familiar. All statistical comparisons of the matched control group or entire control group versus students who completed the elective resulted in p < 0.05.
Figure 2.
Interest in pursuing research careers among pharmacy students who completed a clinical research elective versus the matched control group, students who completed a research project with a faculty member versus those who did not, and students who completed a research-oriented clerkship versus those who did not. The bar represents the mean (SD) interest level, based on a 5-point Likert scale with 5 = very interested; 1 = not interested at all.
Table 4.
Pharmacy Students' Interest in Pursuing Post-PharmD Research Training
aAssessed using a 5-point Likert scale on which 5 = very interested and 1 = not interested at all.
b p < 0.05 versus students who completed the clinical research elective.
c p < 0.01 versus students who completed the clinical research elective.
A secondary objective of the survey process was to obtain student feedback on potential measures designed to enhance their interest in pursuing a clinical research career, whether they took the elective or not. Using a 5-point Likert scale, the survey instrument asked that the student individually rate a series of measures that could be initiated during the PharmD curriculum to improve their interest in pursuing postgraduate research training using a 5-point Likert scale (Table 5). One of the highest rated measures was increasing the availability of research-related clerkships (mean score = 3.5 ± 1.2). The measure that students rated lowest was the requirement of a research-related project as part of the pharmacy curriculum (mean score = 2.5 ± 1.2.) At the time the survey instrument was administered, P4 students were required to complete a project for poster presentation, but these projects were not required to be a research project. To ensure that the students working on their required projects were not biased, the results were compiled excluding P4 students. The mean score was similar when the P4 students were excluded from the analysis with a mean of 2.4 (n = 87) in P2 and P3 students.
Table 5.
Student Responses to Measures That Could Be Used to Enhance Their Interest in Research (N = 147)
aThe evaluation used a 5-point Likert scale with 5 = strongly agree; 1 = strongly disagree.
DISCUSSION
The new elective course, Introduction to Clinical Research, was offered first to PharmD students in 2007, with the goal to introduce students to essential components involved in clinical research. The intent of this exposure was to facilitate pharmacy student interest in research-oriented career avenues to increase the critical mass of pharmacy-trained clinical researchers. This report outlines the implementation of the course, evolution over 3 years, and an assessment of its potential impact on the interest of pharmacy students to pursue clinical research careers. Course evaluations completed by students indicate that the course was well received overall, with mean course ratings in the good to excellent ranges. Because of the positive student feedback, and the fact that no clear trends in categories evaluated were observed from year to year, only minor modifications have been implemented to the content or delivery of the course in subsequent years. One notable change implemented following the first year was a restructuring of the point system to decrease the number of points awarded for attendance. Professionalism points were added with the goal to enhance student engagement in and preparation for course topics. In course evaluations, students indicated that the course introduced new concepts regarding clinical research that were not covered in other courses, such as Drug Literature Evaluation. This was a careful consideration during course development because the instructors wanted to ensure that they were complementing topics in other courses and not repeating them.
An overarching goal in designing this course was to foster the development of student knowledge and interest in clinical pharmacy research. Therefore, to assess this goal, a survey instrument was used to assess overall knowledge and interest in clinical research among students who completed the introduction to clinical research course, and a sample of professional pharmacy students who did not complete the course. Students who participated in the elective course perceived themselves as having a stronger knowledge base related to topics in clinical research as compared to students who did not enroll in the course. This corresponded to an interest in pursuing a career in clinical research that was similar to that of students that completed either a research project with a faculty member or a research-oriented clerkship during their professional training.
Courses and training programs developed to increase research skills of pharmacy students have been reported previously.7,8,10 The elective course described in this report was designed based on these previously developed experiences, while minimizing overlap in the current professional curriculum at Purdue University. To our knowledge, this is the first report assessing the interest of students enrolled in an elective research course to pursue careers in clinical research. The data generated from the assessment survey instrument suggest that students who are exposed to clinical research experiences in this elective course or through clerkship experiences have an interest in pursuing a career in research that is greater than that of students who do not receive these educational experiences. This supports the notion that the incorporation of elective training in clinical research in PharmD curricula may increase the pool of pharmacy students pursuing research careers.
This evaluation may be subject to several limitations. Enrollment in this course was voluntary, and students who completed the course may have been interested in research careers prior to completing the elective. This may explain the similar interest level in research careers among students who completed the course and those who were exposed to other voluntary research experiences. Nonetheless, the course was offered on a voluntary basis to provide introductory research topics to a group of motivated students to further stimulate their interest in pursuing research careers. This overarching goal probably was accomplished because on average, students enrolled in the course agreed that the course stimulated their interest in clinical research (data presented as item 18 in Table 2). Therefore, the generalizability of the study's findings is that students who complete research experiences through this elective course or other practical applications have a higher interest in pursuing research careers than those students who do not get this exposure. Moreover, an introductory research elective further stimulated student interest in clinical research. Ultimately, the full impact of this course and other efforts to stimulate students' interest in the pursuit of research-related careers cannot be assessed without knowing the percentage of graduates who actually pursue postgraduate research training and/or research-related careers compared to that of a control group of graduates who did not take the course.
SUMMARY
Students who completed a 2-credit hour elective course in clinical research had a greater interest in pursuing a career path in research than those who did not take the course. This interest was similar to students who completed a research-focused clerkship or a faculty-guided research project. Therefore, offering these experiences in pharmacy curricula to highly motivated students interested in research may be warranted. Future studies are needed to determine the impact of these approaches on the number of pharmacy graduates who enter postgraduate research training programs.
ACKNOWLEDGMENTS
The authors would like to thank all of the instructors and guest lecturers who assisted in implementing and delivering the Introduction to Clinical Research course at Purdue University. Authors Brian R. Overholser and David R. Foster contributed equally to this paper.
REFERENCES
- 1.Fagan SC, Touchette D, Smith JA, et al. The state of science and research in clinical pharmacy. Pharmacotherapy. 2006;26(7):1027–1040. doi: 10.1592/phco.26.7.1027. [DOI] [PubMed] [Google Scholar]
- 2.Figg WD, Chau CH, Okita R, et al. PharmD pathways to biomedical research: the National Institutes of Health special conference on pharmacy research. Pharmacotherapy. 2008;28(7):821–833. doi: 10.1592/phco.28.7.821. [DOI] [PubMed] [Google Scholar]
- 3.Robles JR, Youmans SL, Byrd DC, Polk RE. Perceived barriers to scholarship and research among pharmacy practice faculty: survey report from the AACP Scholarship/Research Faculty Development Task Force. Am J Pharm Educ. 2009;73(1) doi: 10.5688/aj730117. Article 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JA, Olson KL, Sowinski KM. Pharmacy practice research careers. Pharmacotherapy. 2009;29(8):1007–1011. doi: 10.1592/phco.29.8.1007. [DOI] [PubMed] [Google Scholar]
- 5.The Last Word. Acad Pharm Now. 2009;2(2):54–55. [Google Scholar]
- 6.Murphy JE, Slack MK, Boesen KP, Kirking DM. Research-related coursework and research experiences in doctor of pharmacy programs. Am J Pharm Educ. 2007;71(6) doi: 10.5688/aj7106113. Article 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher BA. Design and conduct of clinical research: an elective course. Am J Pharm Educ. 2004;68(2) Article 42. [Google Scholar]
- 8.Johnson JA, Moore MJ, Shin J, Frye RF. A summer research training program to foster PharmD students' interest in research. Am J Pharm Educ. 2008;72(2) doi: 10.5688/aj720223. Article 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draugalis JR, Carter JT, Slack MK. Survey course on research methods: integrating statistical analysis and study design. Am J Pharm Educ. 1998;62(1):17–23. [Google Scholar]
- 10.Smith JA. An introduction to clinical research and drug development for pharmacy and pharmacology graduate students. J Clin Pharmacol. 2002;42(8):867–869. doi: 10.1177/009127002401102777. [DOI] [PubMed] [Google Scholar]
- 11.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]