Abstract
Silver nanoparticles (Ag-NPs) were successfully synthesized in the natural polymeric matrix. Silver nitrate, gelatin, glucose, and sodium hydroxide have been used as silver precursor, stabilizer, reducing agent, and accelerator reagent, respectively. This study investigated the role of NaOH as the accelerator. The resultant products have been confirmed to be Ag-NPs using powder X-ray diffraction (PXRD), UV-vis spectroscopy, and transmission electron microscopy (TEM). The colloidal sols of Ag-NPs obtained at different volumes of NaOH show strong and different surface plasmon resonance (SPR) peaks, which can be explained from the TEM images of Ag-NPs and their particle size distribution. Compared with other synthetic methods, this work is green, rapid, and simple to use. The newly prepared Ag-NPs may have many potential applications in chemical and biological industries.
Keywords: silver nanoparticles, green synthesis, stabilizer, gelatin, glucose, accelerator
1. Introduction
Nowadays, nanomaterials have been improved as a result of truly important recent advances by applying “green” chemistry rules to nanotechnology and materials science. Silver nanoparticles (Ag-NPs) have been widely used during the past few years in various applications, such as biomedicine [1,2], biosensor [3], and catalysis [4]. Many methods can be used to synthesize Ag-NPs, including microemulsion [5], γ-Ray [6], UV-irradiation [7], microwave irradiation [8], and spray pyrolysis [9]. Polymers have also been used as matrices in nanocomposites or as stabilizers to provide stability for the metal nanoparticles against oxidation, agglomeration, and precipitation [10,11]. Preparations of Ag-NPs in various polymers have been published to give well-dispersed Ag-NPs, such as polyvinylpyrrolidine (PVP) [12], polyethylene glycol [13], poly(methyl methacrylate) [14], poly (vinyl alcohol) [15], polyaniline [16], and polyacrylonitrile (PAN) [17]. Natural polymers have also been used because they are non-toxic and biocompatible. For example, natural rubber [18], polysaccharides [19], cellulose [20], chitosan [21], and starch [22] have been used as matrices or stabilizers for preparation of metallic nanoparticles. The present work is on the use of polymers as the stabilizing agent and use of OH as an accelerator in the synthesis of metallic nanoparticles e.g., Ag [23,24] and Cu [25]. Gelatin is a natural biopolymer extracted from the partial hydrolysis of collagen and has good biocompatibility and biodegradability; in recent years, it has been widely used in wound dressings, drug carriers, and tissue scaffolds [26].
The current work describes a green, rapid, and easy method for preparing well-dispersed Ag-NPs in natural polymeric media like gelatin. The morphologies of Ag-NPs and the mechanism of their formation by oxidation of glucose using an accelerator reagent are discussed.
2. Results and Discussion
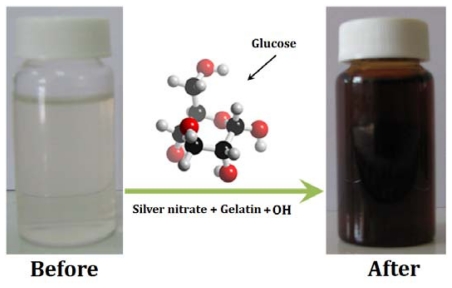
Glucose as an aldehyde can reduce silver ions to metallic silver and through this process oxidizes itself to glucolic acid. The obtained colloidal Ag-NPs were characterized by spectroscopic results. The formation of Ag-NPs was studied using UV-vis spectroscopy, which has proven to be a very useful technique for the analysis of nanoparticle formation over time. The color of the obtained samples at different volumes of NaOH rapidly changed from colorless to dark brown, indicating the formation of Ag-NPs (Figure 1).
Figure 1.
Schematic of the preparation process of Ag-NPs.
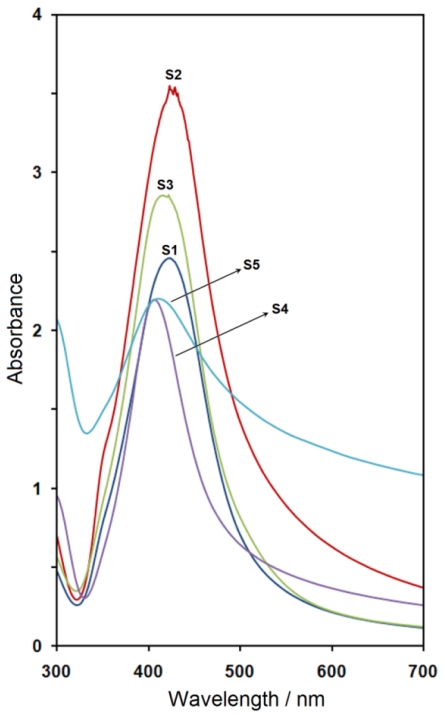
The UV-vis absorption spectra of prepared samples are shown in Figure 2. The obtained Ag-NPs displayed absorption peaks, the characteristic surface plasmon resonance (SPR) band for silver, centered from 407 to 424 nm. The location (λmax) of the SPR peaks and volumes of NaOH with corresponding pH are also summarized in Table 1.
Figure 2.
The UV-visible spectra of Ag-NPs prepared at different volumes of NaOH.
Table 1.
The characteristics of Ag-NPs prepared at different volumes of NaOH.
| Sample | Volume of NaOH (mL) | pH | λmax |
|---|---|---|---|
| S1 | 0.5 | 3.5 | 424 |
| S2 | 1 | 3.3 | 423 |
| S3 | 1.5 | 3.2 | 422 |
| S4 | 5 | 3.7 | 407 |
| S5 | 10 | 4.3 | 412 |
As indicated, the first addition of NaOH led to the broadening of the SPR peak relative to the sample S1. Greater volumes of NaOH (S2) increased the absorbance due to increases in silver concentration [27]. In sample S3, this resulted in a stronger blue-shift in λmax to 422 nm. In sample S4, the increased volume of accelerator led to the SPR peak being considerably blue-shifted to a lower wavelength (407 nm). This blue-shift is related toa decrease in the particle size of Ag-NPs [28] because the SPR band in metal nanoparticles displays the blue-shift based on the decreased size of particles. For S5, the λmax was red-shifted to 412 nm due to the increased size of Ag-NPs [27,29]. These results demonstrated good agreement with the results obtained in the transmission electron miscroscopy (TEM) images of Ag-NPs and their particle size distribution.
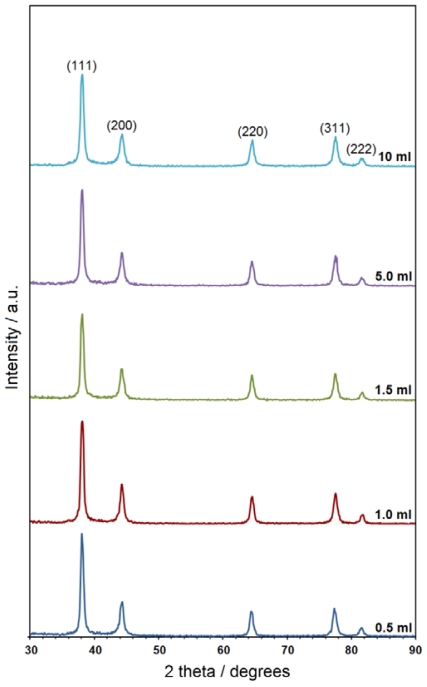
The powder X-ray diffraction (PXRD) patterns of the prepared samples at different volumes of NaOH indicate the formation of the Ag-NPs. As shown in Figure 3, all samples have similar diffraction profiles. The PXRD peaks at 2θ of 35.9°, 42.1°, 62.8°, 75.7° and 82.7° can be attributed to the (111), (200), (220), (311) and (222) crystallographic planes of face-centered cubic (fcc) silver crystals, respectively (Ref. # 01-087-0597). For the all samples, the main crystalline phase was silver; no obvious impurities (e.g., Ag2O [22]) were evident in the PXRD patterns. Moreover, no other peaks related to an impurity, indicating that the silver metal was relatively pure. Gelatin was used as the stabilizer and matrix during this synthesis. The possible chemical equation for preparing the Ag-NPs is:
Figure 3.
The PXRD patterns of Ag-NPs prepared at different volumes of NaOH.
| (1) |
| (2) |
After dispersion of silver ions in the gelatin matrix (Equation 1), gelatin reacted with the Ag+ to form a stable gelatinous complex [Ag(gel)]+, which reacted with OH− to form silver metal due to the reduction of silver ions through the oxidation of glucose to gluconic acid (Equation 2).
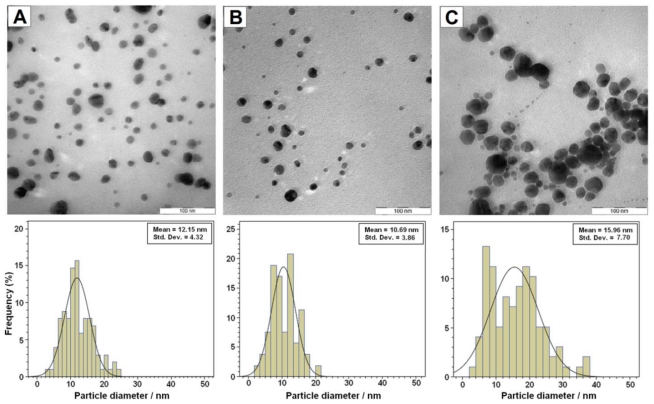
The TEM images demonstrate the formation of Ag-NPs at different volumes of NaOH. Figure 4 shows typical TEM images and the corresponding particle size distribution of the prepared Ag-NPs. The TEM results indicated that the obtained samples retained a narrow particle size distribution and the average size of all prepared Ag-NPs was less than 20 nm, and a smaller size (~10.7 nm) was obtained for S4.
Figure 4.
TEM images of Ag-NPs and their particle size distribution at different volumes of NaOH; 1.5 (A), 5 (B), and 10 mL (C).
3. Experimental Section
3.1. Materials
All chemicals and reagents in this work were of analytical grade and used without any purification. Materials used for the synthesis of Ag-NPs included silver nitrate (Alfa-Aesar, 99.98%), gelatin (Sigma, Type A), D-glucose (BDH-Limited Poole, Analar), and sodium hydroxide (R & M Chemicals, 99.0%). Doubly distilled water was used throughout the experiments.
3.2. Synthesis of Silver Nanoparticles
In the present study, 1% wt. gelatin was added to an aqueous solution of AgNO3 (10 mL, 1 M) in a flask. Different volumes of aqueous solution of NaOH (1 M) were added to prepare various samples (0.5 (S1), 1.0 (S2), 1.5 (S3), 5 (S4) and 10 mL (S5)). These solutions were heated at 60 °C and then 10 mL of aqueous solution of glucose (2 M) was added. The suspension immediately turned brown in all samples, indicating the formation of silver nanoparticles. The reaction was continued for 15 minutes. The obtained suspensions were centrifuged at 15,000 rpm.
3.3. Characterization Methods and Instruments
The prepared Ag-NPs were characterized using ultraviolet–visible (UV-vis) spectroscopy, transmission electron microscopy (TEM) and powder X-ray diffraction (PXRD). The UV-vis spectra were recorded over the range of 300–700 nm with a Lambda 35-Perkin Elmer UV-vis spectrophotometer. TEM observations were conducted using a Hitachi H-7100 electron microscope while the particle size distribution were determined using the UTHSCSA Image Tool version 3.00 program. The structure of produced Ag-NPs has been studied using powder X-ray diffraction (PXRD, Philips, X’pert, Cu Kα). PXRD patterns were recorded at a scan speed of 2°/min. After reactions, the samples were centrifuged using a high-speed centrifuge machine (Avanti J25, Beckman).
4. Conclusions
We have demonstrated a green synthesis of high purity Ag-NPs with glucose reduction of aqueous AgNO3 at different volumes of NaOH as a reaction accelerator. Nanoparticle agglomeration was controlled with the addition of gelatin as a stabilizing agent. PXRD and TEM measurements displayed that the resultant nanoparticles were faced centered cubic (fcc) structures smaller than 20 nm in diameter. Thus, this work provides important advantages namely, simplicity, speed, and eco-friendliness.
Acknowledgements
The authors would like acknowledge technical supports from the staff of the Faculty of Science and the Institute of Bioscience at UPM in this work.
References
- 1.Schultz DA. Plasmon resonant particles for biological detection. Curr. Opin. Biotech. 2003;14:13–22. doi: 10.1016/s0958-1669(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 2.Parak WJ, Gerion D, Pellegrino T, Zanchet D, Micheel C, Williams SC, Boudreau R, Le Gros MA, Larabell CA, Alivisatos AP. Biological applications of colloidal nanocrystals. Nanotechnology. 2003;14:15–27. [Google Scholar]
- 3.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 4.Haneda M, Kintaichi Y, Inaba M, Hamada H. Infrared study of catalytic reduction of nitrogen monoxide by propene over Ag/TiO2-ZrO2. Catal. Today. 1998;42:127–135. [Google Scholar]
- 5.Husein MM, Rodil E, Vera JH. A novel method for the preparation of silver chloride nanoparticles starting from their solid powder using microemulsions. J. Colloid Interf. Sci. 2005;288:457–467. doi: 10.1016/j.jcis.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Huang NM, Radiman S, Lim HN, Khiew PS, Chiu WS, Lee KH, Syahida A, Hashim R, Chia CH. γ-Ray assisted synthesis of silver nanoparticles in chitosan solution and the antibacterial properties. Chem. Eng. J. 2009;155:499–507. [Google Scholar]
- 7.Darroudi M, Ahmad MB, Shameli K, Abdullah AH, Ibrahim NA. Synthesis and characterization of UV-irradiated silver/montmorillonite nanocomposites. Solid State Sci. 2009;11:1621–1624. [Google Scholar]
- 8.Yin H, Yamamoto T, Wada Y, Yanagida S. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater. Chem. Phys. 2004;83:66–70. [Google Scholar]
- 9.Kim H-S, Lee K-H, Kim S-G. Growth of monodisperse silver nanoparticles in polymer matrix by spray pyrolysis. Aerosol. Sci. Tech. 2006;40:536–544. [Google Scholar]
- 10.Lin C-A, Tsai H-C, Tung C-C. Preparation of silver nanoparticles/pseudo-thermoplastic polyvinyl alcohol (PT-PVA) films by the synchronous chemical reduction method. Polym.-Plast. Technol. 2009;48:1171–1175. [Google Scholar]
- 11.Faraha AA, Alvarez-Puebla RA, Fenniri H. Chemically stable silver nanoparticle-crosslinked polymer microspheres. J. Colloid Interf. Sci. 2008;319:572–576. doi: 10.1016/j.jcis.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M, Gu M, Jin Y, Jin G. Optical properties of silver-dispersed PVP thin film. Mater. Res. Bull. 2001;36:853–859. [Google Scholar]
- 13.Luo C, Zhang Y, Zeng X, Zeng Y, Wang Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interf. Sci. 2005;288:444–448. doi: 10.1016/j.jcis.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Khanna PK. In situ synthesis of silver nano-particles in polymethylmethacrylate. Mater. Chem. Phys. 2007;104:367–372. [Google Scholar]
- 15.Zhou Y, Yu SH, Wang CY, Li XG, Zhu YR, Chen ZY. A novel ultraviolet irradiation photo-reduction technique for preparation of single crystal Ag nanorods and Ag dendrites. Adv. Mater. 1999;11:850–852. [Google Scholar]
- 16.Khanna PK, Singh N, Charan S, Viswanath AK. Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism. Mater. Chem. Phys. 2005;92:214–219. [Google Scholar]
- 17.Zhang Z, Zhang L, Wang S, Chen W, Lei Y. A convenient route to polyacrylonitrile/silver nanoparticle composite by simultaneous polymerization–reduction approach. Polymer. 2001;42:8315–8318. [Google Scholar]
- 18.Abu Bakar NHH, Ismail J, Abu Bakar M. Synthesis and characterization of silver nanoparticles in natural rubber. Mater. Chem. Phys. 2007;104:276–283. [Google Scholar]
- 19.Huang NM, Lim HN, Radiman S, Khiew PS, Chiu WS, Hashim R, Chia CH. Sucrose ester micellar-mediated synthesis of Ag nanoparticles and the antibacterial properties. Colloid Surface A. 2010;35:69–76. [Google Scholar]
- 20.Cai J, Kimura S, Wada M, Kuga S. Nanoporous cellulose as metal nanoparticles support. Biomacromolecules. 2009;10:87–94. doi: 10.1021/bm800919e. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Yuan Q, Yang X. Preparation and characterization of metal-chitosan nanocomposites. Colloid Surface B. 2004;39:31–37. doi: 10.1016/j.colsurfb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 23.Singh M, Sinha I, Mandal RK. Role of pH in the green synthesis of silver nanoparticles. Mater. Lett. 2009;63:425–427. [Google Scholar]
- 24.Singh M, Sinha I, Singh AK, Mandal RK. Formation of fractal aggregates during green synthesis of silver nanoparticles. J. Nanopart. Res. 2010 doi: 10.1007/s11051-010-0001-8. [DOI] [Google Scholar]
- 25.Singh M, Sinha I, Premkumar M, Singh AK, Mandal RK. Structural and surface plasmon behavior of Cu nanoparticles using different stabilizers. Colloid Surface A. 2010;359:88–94. [Google Scholar]
- 26.Xu X, Zhou M. Antimicrobial gelatin nanofibers containing silver nanoparticles. Fiber. Polym. 2008;9:685–690. [Google Scholar]
- 27.Bohren CF, Huffman DR. Absorption and Scattering of Light by Small Particles. John Wiley & Sons Inc; New York, NY, USA: 1998. [Google Scholar]
- 28.Heath JR. Size dependent surface plasmon resonances in bare silver particles. Phys. Rev. B. 1989;40:9982–9985. doi: 10.1103/physrevb.40.9982. [DOI] [PubMed] [Google Scholar]
- 29.Aragon SR, Elwenspoek M. Mie scattering from thin spherical bubbles. J. Chem. Phys. 1982;77:3406–3413. [Google Scholar]