Abstract
Our previous study indicated that both 17β-estradiol (E2), known to be an endogenous estrogen, and bisphenol A (BPA), known to be a xenoestrogen, could positively influence the proliferation or differentiation of neural stem/progenitor cells (NS/PCs). The aim of the present study was to identify the signal transduction pathways for estrogenic activities promoting proliferation and differentiation of NS/PCs via well known nuclear estrogen receptors (ERs) or putative membrane-associated ERs. NS/PCs were cultured from the telencephalon of 15-day-old rat embryos. In order to confirm the involvement of nuclear ERs for estrogenic activities, their specific antagonist, ICI-182,780, was used. The presence of putative membrane-associated ER was functionally examined as to whether E2 can activate rapid intracellular signaling mechanism. In order to confirm the involvement of membrane-associated ERs for estrogenic activities, a cell-impermeable E2, bovine serum albumin-conjugated E2 (E2-BSA) was used. We showed that E2 could rapidly activate extracellular signal-regulated kinases 1/2 (ERK 1/2), which was not inhibited by ICI-182,780. ICI-182,780 abrogated the stimulatory effect of these estrogens (E2 and BPA) on the proliferation of NS/PCs, but not their effect on the differentiation of the NS/PCs into oligodendroglia. Furthermore, E2-BSA mimicked the activity of differentiation from NS/PCs into oligodendroglia, but not the activity of proliferation. Our study suggests that (1) the estrogen induced proliferation of NS/PCs is mediated via nuclear ERs; (2) the oligodendroglial generation from NS/PCs is likely to be stimulated via putative membrane-associated ERs.
Keywords: 17β-estradiol, bisphenol A, estrogen, neural stem cell, oligodendrocytes, proliferation, differeciation
1. Introduction
Embryonic neural stem cell (NSC)-transplantation or endogenous NSC-activation is a recent challenging approach for the treatment of neurological diseases or injuries to the central nervous system (CNS) [1–3]. NSCs comprise most of the progenitor cells (PCs) in the CNS [4,5] and are capable of self-renewal and multipotent differentiation into the three cell types of the CNS (neurons, astrocytes, and oligodendrocytes). The NSC-based therapies should be of note as the stem cell microenvironment or niche is important as various external signals have been shown to play a role in regulating their proliferation and differentiation [6]. Estrogens (female estrogenic hormones such as 17β-estradiol (E2) and estrogen-like endocrine-disrupting chemicals such as bisphenol A (BPA), etc.) are candidates for extrinsic regulators [7–10]. Our previous study showed that both E2 and BPA influence the fate of neural stem/progenitor cells (NS/PCs) when the cells are poorly supplied with mitogens or differentiation factors [10]. The aim of the present study was to investigate the underlying molecular mechanisms of estrogen actions on the proliferation and differentiation of NS/PCs.
Many estrogenic actions are known to be mediated via nuclear estrogen receptors (ERs), ERα and ERβ. Nuclear ERs function as ligand-activated transcription factors to regulate the expression of estrogen-responsive genes (ERE) [11]. In addition to nuclear ERs, membrane-associated ERs have recently been suggested to exist and to function, because some estrogenic effects occur within seconds to minutes, which is a finding that cannot be explained by a nuclear ER-mediated mechanism [12,13]. Investigations of nonclassical estrogen signaling suggest that ERα and ERβ, or ER-like proteins, are likely to be the membrane ERs in each target cells, and GPR-30, the orphan G protein-coupled receptors (GPCR)-like protein, was suggested to be one of the candidates of ER-like proteins [14,15]. Thus, to put it another way, the present study is aimed at identifying the signal transduction pathways for estrogenic activities promoting proliferation and differentiation of NS/PCs via well known nuclear ERs or putative membrane-associated ERs.
We have already confirmed that NS/PCs express both types of nuclear ERs [10], while even the existence of membrane-associated ERs has not yet been determined on NS/PCs. In various cells, the existence of membrane-associated ERs can be functionally confirmed [12,13]. Many reports have shown that E2 activates extracellular signal-regulated kinases (ERK)1/2, components of the mitogen-activated protein kinase (MAPK) signaling cascade, via membrane-associated ER, and that the activation of ERK1/2 is likely to be a key event to discriminate signal cascades dependent on ERs from those depending on non-nuclear ERs [16–19]. In the present study, we thus examined whether ERK 1/2-activation is shown in our NS/PCs after the treatment by estrogens in order to investigate the existence of membrane-associated ERs.
In order to confirm the participation of nuclear ERs on the estrogen actions, we investigated the protective efficacy of ICI-182,780 on the proliferation and differentiation of NS/PCs. ICI-182,780, a 7α–alkylsulfinyl analog of E2 [20], is able to bind to both ERα and ERβ with an affinity comparable to that of E2; and micromolar concentrations of this antagonist have been used to inhibit estrogen function [21]. In order to confirm the participation of putative membrane-associated ERs on the estrogen actions, we investigated the effect of a cell-impermeable E2, bovine serum albumin-conjugated E2 (E2-BSA) [17], on the proliferation or oligodendroglial differentiation of cultured NS/PCs.
2. Materials and Methods
2.1. Reagents
E2, BPA, and β-estradiol 6-(O-carboxymethyl)oxime: bovine serum albumin (E2-BSA), U0126 were purchased from Sigma (St. Louis, MO, USA). ICI-182,780 was obtained from Tocris (Ellisville, MO, USA). E2-BSA was separated from free E2 by use of a centrifugal filter, Microcon YM-30 (Millipore, Billerica, MA, USA). The E2-BSA used in this study was composed of BSA conjugated with 33 molecules of E2, i.e., 3 × 10−10 M E2-BSA was equivalent to 10−8 M E2.
2.2. Primary Cultures
As previously described in detail [10], NS/PCs were prepared from the telencephalon of Wistar rats (Nippon SLC, Shizuoka, Japan) at E15, propagated by the neurosphere method, and then plated on poly-L-ornithine-coated plates or dishes for the experiments. The proliferation medium consisted of Dulbecco's modified Eagle's minimum essential medium nutrient mixture F-12 HAM (DMEM/F12) supplemented with insulin (25 μg/mL), apo-transferrin (100 μg/mL), progesterone (20 nM), putrescine (100 μM), sodium selenite (30 nM), penicillin (100 U/mL), and streptomycin (100 μg/mL). The required amount of FGF-2 (R&D Systems, Minneapolis, MN, USA) was added to the medium every day to achieve a final concentration of 10 ng/mL.
2.3. Western Immunoblot Analysis
Cells were plated on 6-well plates (1 × 105 cells/cm2), and cultured for three days in the proliferation medium, for an additional day in FGF-2-free medium, then incubated with test drugs for various times, and the cell extracts were prepared as previously described [22]. The antibodies and their sources were the following: rabbit antibody against MAPK 1/2 (Erk1/2-CT) which recognizes the C-terminal 35 amino acids of the rat 44 kDa MAPK1/ERK1 and 42 kDa MAPK2/ERK2, from Upstate (Lake Placid, NY, USA); rabbit antibodies against phospho-p44/42 MAPK (Thr202/Tyr204) which recognizes the phosphorylated ERK1/2 (pERK1/2) from Cell Signaling (Danvers, MA, USA); and alkaline phosphatase-conjugated anti-rabbit IgG (H + L), from Promega (Madison, WI, USA).
2.4. BrdU Labeling
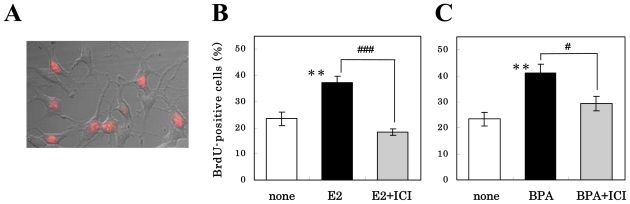
Cells were plated on micro slide/cover glasses (Matsunami Glass Ind. Ltd., Osaka, Japan) in 24-well plates (6 × 104 cells/well) and cultured for three days in the proliferation medium, for an additional day in FGF-2-free medium containing the test compound, and then for 2 h in the presence of 10 μM 5-bromo-2′-deoxyuridine (BrdU; Sigma). The number of BrdU-positive cells was determined by an immunocytochemical technique using anti-BrdU mouse antibody (Sigma) and rhodamine-labeled anti-mouse IgG antibodies (Chemicon, Temecula, CA, USA), as described previously [10]. The total number of cells and the number of BrdU-positive cells (Figure 2A) were counted in seven arbitrarily selected fields of each well. For each treatment, 4–8 wells were analyzed; and the experiments were repeated more than three times. For statistical analysis Student’s t-test was used.
Figure 2.
Effect of E2 or BPA, with or without ICI-182,780 pretreatment. on cell proliferation of NS/PCs, by measuring the incorporation of the thymidine analogue BrdU. The BrdU-positive cells were immunocytochemically examined (A), and their percentage in the presence of E2 (B) or BPA (C) with or without ICI-182,780 pretreatment is shown on the ordinate. The ratios of the value for the estrogen-treated cells to that value for the control cells (ethanol-treated cells) were calculated, and are shown on the ordinate. Values are presented as the mean ± SEM (n = 4–8, different cultures). Significant differences in values between the estrogen-treated and non-treated cells (**P < 0.01; Student’s t test) and in those indicated by the brackets are shown (#P < 0.05, ###P < 0.001; Student’s t test).
2.5. MTT Assay
As described previously [10], cells were plated (2 × 104 cells/well) in 96-well plates and cultured for three days in the proliferation medium, for an additional day in FGF-2-free medium containing the test compound, and then for 4 h in the presence of 0.5 mg/mL of 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma). After the crystals had been dissolved in HCl/isopropanol, the absorbance at 570 nm was determined by using a micro plate reader (Bio-Rad, model 550). The cell number compared with that of the ethanol-treated control group was presented as a fold-increase. The total MTT converted to formazan by the cells (i.e., total cell number) in all of the wells, had an absorbance that ranged from 0.2 to 0.75 at 570 nm.
2.6. Immunocytochemistry
Cells were plated on micro slide/cover glasses that had been placed in 24-well plates filled with the proliferation medium (4 × 104 cells/well), and cultured for three days. They were then reacted for another five days with the test compound prepared in FGF-2-free medium. Immunocytochemical analysis was performed as described previously [10] using anti-nestin mouse antibody, anti-NG2 rabbit antibody (Chemicon), anti-βIII tubulin (Tuj-1) mouse antibody, anti-glial fibrillary acidic protein (GFAP) mouse antibody, anti-2′3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) mouse antibody (Sigma), and rhodamine-labeled anti-mouse IgG antibody (Promega) or rhodamine-labeled anti-rabbit IgG antibodies (Chemicon). The total number of cells and the number of immunoreactive cells were counted in seven arbitrarily selected fields of each well. For each treatment, 4–8 different wells were analyzed, and the experiments were repeated more than three times. For statistical analysis, Student’s t-test was used.
3. Results and Discussion
3.1. Effect of Estrogens on the Phosphorylation of MAPK/ERK of NS/PCs
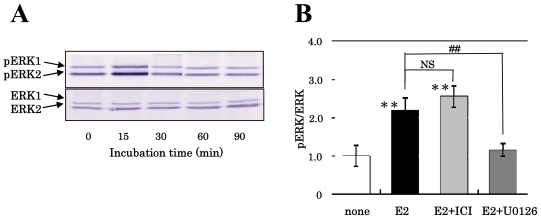
In order to functionally confirm the existence of membrane-associated ERs on NS/PCs, we assessed the effect of estrogens on the activation (phosphorylation) of ERK1/2 in the cultured NS/PCs. Our previous study showed that 2 ERK isoforms (ERK1 and ERK2) are present in NS/PCs [22]. As shown in Figure 1A, pERK1/2 increased transiently 15 min after the exposure of the cells to E2 at a concentration of 10−8 M, and declined to the control level 30 min after exposure, presenting the possibility that functional membrane-associated ERs were expressed on the NS/PCs and responded to E2 exposure.
Figure 1.
Effect of E2 on the phosphorylation of ERK of NS/PCs. (A) Cells were treated with 10−8 M E2 for the indicated times. The immunoreactive bands of pERK1/2 and ERK1/2 were scanned for intensity. (B) Cells were treated for 15 min with 10−8 M E2 after pretreatment for 1 h with 10−6 M ICI-182,780 or 30 min with 10−5 M U0126. The immunoreactive bands of pERK and ERK were scanned for intensity, and the ratios of pERK2/ERK2 are shown in the panel. Significant differences in values between the estrogen-treated and non-treated cells (**P < 0.01; Student’s t test) and in those indicated by the brackets are shown (##P < 0.01; Student’s t test, NS; not significant).
When cells were pretreated with ICI-182,780, the activation of ERK2 induced by E2 was not blocked (Figure 1B) as previously indicated [23,24]. The reason why we analyzed the ratio of phosphorylated ERK2 (pERK2) per total ERK2 in Figure 1B is that only the ERK2 isoform has been suggested to be attributable to neural development [25] although ERK1 and ERK2 exhibit 84 % sequence homology and are coordinately activated through the MAPK cascade. The pretreatment with U0126, a selective inhibitor of MAPK kinase (MEK), inhibited E2-induced phosphorylation of ERK2 (Figure 1B), indicating the phosphorylation of ERK1/2 elicited by estrogen to be MEK dependent. These results suggested the possibility that our NS/PCs express membrane ERs.
3.2. Mechanism of Action of Estrogens on Proliferation of NS/PCs
We previously showed that the administration of E2 or BPA to the NS/PCs stimulated their proliferation in the absence, but not in the presence, of FGF-2 [10]. In order to investigate whether the stimulatory effects of estrogens on the proliferation of NS/PCs were mediated through nuclear ERs, we pretreated cells for 1 h with 10−6 M ICI-182,780 before exposure to 10−8 M E2 or 10−5 M BPA for 24 h, and then determined the percentages of the cells in the S phase of the cell cycle by using the BrdU labeling method. As shown in Figure 2B and 2C, E2 and BPA, respectively, significantly increased the number of BrdU-positive cells compared with their number among the control cells; and pretreatment with ICI-182,780 significantly inhibited their effect. ICI-182,780 alone did not significantly decrease the BrdU-positive cells (not shown). These results suggest that the estrogens had the ability to stimulate proliferation of NS/PCs via nuclear ERs.
We also checked the effect of the pretreatment with ICI-182,780 on the E2- or BPA-induced proliferation by use of the MTT assay. As shown in Figure 3A, compared with the MTT signals (indicating viable cells) from the non-treated control group, those from the E2-treated group were significantly greater; and pretreatment with ICI-182,780 significantly suppressed this effect. The cell number was unchanged when the cells were treated with ICI-182,780 alone (not shown). Figure 3B shows that similar results were obtained with BPA. These results confirmed those evaluated by the BrdU labeling experiment that the nuclear ERs participated in the stimulatory effect of estrogens on NS/PC proliferation.
Figure 3.
Effect of E2, BPA or E2-BSA, with or without ICI-182,780 pretreatment on cell proliferation of NS/PCs, by measuring the MTT assay. NS/PCs were pretreated or not with 10−6 M ICI-182,780 for 1 h, treated with E2 (A), BPA (B) or E2-BSA (C) for 24 h, and then subjected to the MTT assay. Values are presented as the mean ± SEM (n = 4–8, different cultures). Significant differences in values between the estrogen-treated and non-treated cells (***P < 0.001; Student’s t test) and in those indicated by the brackets are shown (##P < 0.01, ###P < 0.001; Student’s t test; NS, not significant).
In order to investigate the participation of putative membrane-associated ERs on the estrogen actions, we cultured NS/PCs for 24 h in FGF-2-free medium containing 3 × 10−10 M E2-BSA (equivalent to 10−8 M E2), a membrane-impermeable E2 conjugate, instead of E2. As shown in Figure 3C, E2-BSA did not increase the MTT signal intensity, thus suggesting that the membrane-associated ERs did not participate in the proliferation of NS/PCs. As expected, ICI-182,780 did not affect the cell number in the presence of E2-BSA.
Previous reports have shown the proliferative effect of E2 [7,8] and BPA [26] on cultured embryonic NSCs, though the action mechanisms involved in signal transduction were unknown. This is the first report suggesting a role for nuclear ERs in estrogen-induced proliferation of NS/PCs. It is now established that agonist-bound ERα has a strong positive effect on cell proliferation of several cell models including breast cancer cells [27]. Our NS/PCs expressed predominantly ERβ mRNA rather than the ERα mRNA that was predominant in neurons [10]. It will be necessary to clarify which ER has the most impact on proliferation of NS/PCs.
3.3. Mechanism of Action of Estrogens on Differentiation of NS/PCs
We previously showed that E2- or BPA-treatment increased the ratio of the oligodendrocytes generated from the NS/PCs to total cells; however, this ratio did not change when the cells were stimulated with platelet-derived growth factor (PDGF) or with neurotrophin-3 [10]. We tested the action mechanisms of these estrogens on the differentiation of NS/PCs into oligodendroglia. NS/PCs were pretreated for 1 h with or without 10−6 M ICI-182,780, and cultured for five days in FGF-2-free medium containing 10−8 M E2. Figure 4 shows that the proportion of nestin-positive (immature neural stem cells), Tuj-1-positive (neurons) and GFAP-positive (astrocytes) cells did not change significantly, but the percentage of CNPase-positive (oligodendrocytes) cells was significantly increased by E2-treatment as previously shown [10]; and that the pretreatment of the cells with ICI-182,780, a classical ER antagonist, did not affect the percentage of these positive cells.
Figure 4.
Effect of E2-treatment and/or ICI-182,780-pretreatment on the differentiation of NS/PCs. The cells were examined by immunocytochemistry using anti-nestin, anti-Tuj-1, anti-GFAP or anti-CNPase antibodies (Scale bar = 50 μm) (A). The percentage of cells positive for each marker was calculated, and is shown on the ordinate (B). Their values were expressed as the mean ± SEM (n = 4–16). Significance, *p < 0.05, **p < 0.01 versus control; Student’s t test.
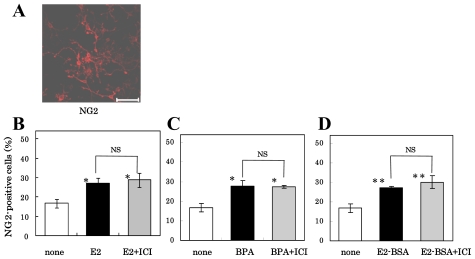
We then pretreated NS/PCs for 1 h with or without 10−6 M ICI-182,780 and cultured for five days in FGF-2-free medium containing 10−8 M E2, 10−5 M BPA or 3 × 10−10 M E2-BSA. As shown in Figure 5, the percentage of NG2-positive oligodendrocyte precursor cells in the NS/PC cultures was significantly increased by BPA or E2-BSA as well as E2 (closed bar) compared with the percentage in control cultures (open bar), and the pretreatment of the cells with ICI-182,780 did not reverse the differentiation-inducing effect of E2, BPA or E2-BSA (shaded bar). ICI-182,780 alone did not affect the percentage of the NG-2-positive cells (data not shown).
Figure 5.
Effect of E2, BPA, or E2-BSA with or without ICI-182,780-pretreatment on the oligodendroglial differentiation of NS/PCs. The cells were examined by immunocytochemistry using anti-NG2 antibodies (Scale bar = 50 μm) (A), and NG2-positive cells in the various cultures was determined. Their values in the presence of E2 (B), BPA (C) or E2-BSA (D) are expressed as the mean ± SEM (n = 4–16) on the ordinate. Significance, *p < 0.05, **p < 0.01 versus control; Student’s t test. NS, not significant.
These results suggested that estrogen-induced glial generation from NS/PCs was mediated, not through nuclear ERs, but probably through membrane-associated ERs. Recently evidence was presented that STX, a synthetic selective ER modulator, and G1, the GPR-30 agonist, are beneficial tools as non-classical ER agonist [28]. Both STX and G1 do not bind to or activate the nuclear ERs, but activate MAPK pathway. It will be necessary to investigate the effect of these compounds on NS/PCs in near future.
4. Conclusions
This study demonstrates that the estrogen-induced proliferation of NS/PCs was mediated via nuclear ERs, and suggests that the oligodendroglial generation from NS/PCs was stimulated by putative membrane-associated ERs.
References
- 1.Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp. Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Imitola J, Khoury SJ. Neural stem cells and the future treatment of neurological diseases: Raising the standard. Methods Mol. Biol. 2008;438:9–16. doi: 10.1007/978-1-59745-133-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 4.Lovell-Badge R. The future for stem cell research. Nature. 2001;414:88–91. doi: 10.1038/35102150. [DOI] [PubMed] [Google Scholar]
- 5.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 6.Watt FM, Hogan BLM. Out of eden: Stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 7.Brännvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol. Cell. Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 8.Brännvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur. J. Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- 9.McEven BS. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, Furukawa Y. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed. Res. 2008;29:163–170. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- 11.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: Many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 12.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones: A focus on rapid, nongenomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 13.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: Where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 14.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, R⊘nnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J. Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptors coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 16.Björnström L, Sjöberg M. Mechanism of estrogen receptor signaling convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 17.Bouskine A, Nebout M, Mograbi B, Brücker-Davis F, Roger C, Fenichel P. Estrogens promote human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology. 2008;149:565–573. doi: 10.1210/en.2007-1318. [DOI] [PubMed] [Google Scholar]
- 18.Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen facilitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J. Neuroci. 2008;28:6473–6482. doi: 10.1523/JNEUROSCI.0514-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelsohn EM, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J. Clin. Inv. 2010;120:2277–2279. doi: 10.1172/JCI43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 21.Howell A, DeFriend DJ, Robertson JF, Blamey RW, Anderson L, Anderson E, Sutcliffe FA, Walton P. Pharmacokinetics, pharmacological and anti-tumour effects of the specific anti-oestrogen ICI 182,780 in women with advanced breast cancer. Br. J. Cancer. 1996;74:300–308. doi: 10.1038/bjc.1996.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa Y, Urano T, Minamimura M, Nakajima M, Okuyama S, Furukawa S. 4-Methylcatechol-induced heme oxygenase-1 exerts a protective effect against oxidative stress in cultured neural stem/progenitor cells via PI3 kinase/Akt pathway. Biomed. Res. 2010;31:45–52. doi: 10.2220/biomedres.31.45. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez R, Jalali C, de Lacalle S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J. Neurosci. 2004;24:982–990. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao L, O’Neill K, Brinton RD. Estrogenic agonist activity of ICI 182,780 (faslodex) in hippocampal neurons: Implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J. Pharm. Exp. Ther. 2006;319:1124–1132. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 25.Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo C, Wada K, Masuda T, Yonemura T, Shibuya A, Fujimoto Y, Nakajima A, Niwa H, Kamisaki Y. Nonylphenol induces the death of neural stem cells due to activation of the caspase cascade and regulation of the cell cycle. J. Neurochem. 2003;88:1416–1423. doi: 10.1046/j.1471-4159.2003.02270.x. [DOI] [PubMed] [Google Scholar]
- 27.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 28.Lebesque D, Traub M, Butte-Smith MD, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;30:12950–12957. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]