Abstract
Diffuse traumatic axonal injury (TAI) is a type of traumatic brain injury (TBI) characterized predominantly by white matter damage. While TAI is associated with cerebral atrophy, the relationship between gray matter volumes and TAI of afferent or efferent axonal pathways remains unknown. Moreover, it is unclear if deficits in cognition are associated with post-traumatic brain volumes in particular regions. The goal of this study was to determine the relationship between markers of TAI and volumes of cortical and subcortical structures, while also assessing the relationship between cognitive outcomes and regional brain volumes. High-resolution magnetic resonance imaging scans were performed in 24 patients with TAI within 1 week of injury and were repeated 8 months later. Diffusion tensor imaging (DTI) tractography was used to reconstruct prominent white matter tracts and calculate their fractional anisotropy (FA) and mean diffusivity (MD) values. Regional brain volumes were computed using semi-automated morphometric analysis. Pearson's correlation coefficients were used to assess associations between brain volumes, white matter integrity (i.e., FA and MD), and neuropsychological outcomes. Post-traumatic volumes of many gray matter structures were associated with chronic damage to related white matter tracts, and less strongly associated with measures of white matter integrity in the acute scans. For example, left and right hippocampal volumes correlated with FA in the fornix body (r = 0.600, p = 0.001; r = 0.714, p < 0.001, respectively). In addition, regional brain volumes were associated with deficits in corresponding neuropsychological domains. Our results suggest that TAI may be a primary mechanism of post-traumatic atrophy, and provide support for regional morphometry as a biomarker for cognitive outcome after injury.
Key words: atrophy, diffuse axonal injury, diffusion tensor imaging, traumatic brain injury, volumetric magnetic resonance imaging
Introduction
Traumatic brain injury (TBI) is a major cause of disability in the United States, with an estimated 3.2 million adults or 1.1% of the total U.S. population living with long-term disability as a result of their injury (Zaloshnja et al., 2008). This conservative estimate is likely to increase in coming years due to a variety of factors, such as increased detection of TBI using recent advances in neuroimaging, an increased number of soldiers returning home after having suffered blast injuries in military combat zones (Howe, 2009), and heightened public awareness of cognitive impairments in former professional and amateur athletes (McKee et al., 2009).
TBI is associated with a variety of disturbances in cognition, including deficits in learning, working memory, attention, information processing speed, and executive function (Evans et al., 2003; Mathias and Wheaton, 2007; Niogi et al., 2008; Scheid et al., 2006). While the exact mechanism of TBI-related functional impairment is unknown, it is likely that some neuropsychological sequelae result from focal trauma to particular cortical or subcortical brain regions, resulting in characteristic impairments dependent on the area of involvement. However, not all brain trauma results in focal lesions. Diffuse traumatic axonal injury (TAI) is a common mechanism of injury in brain trauma, and is estimated to be the predominant mechanism of injury in up to 50% of TBI patients (Meythaler et al., 2001). It occurs as a consequence of rotational or acceleration-deceleration forces, such as those suffered in a motor vehicle collision, which place shear stress on axons, and may result in reversible injury or complete axotomy (Smith et al., 2003). In this setting, it is likely that cognitive deficits are the manifestation of underlying disruptions of critical white matter pathways between the cortex and deep gray matter structures.
Cerebral atrophy is a common consequence of TBI (Ariza et al., 2006; Bigler, 2001; Bigler et al., 2006; Ding et al., 2008; Gale et al., 2005; MacKenzie et al., 2002; Sidaros et al., 2009; Tomaiuolo et al., 2004, 2005; Warner et al., 2010; Xu et al., 2010), and it is postulated that volume loss occurs due to direct injury to neuronal cell bodies, ultimately resulting in cytotoxic or apoptotic cell death (Povlishock and Katz, 2005). However, it is also possible that loss of cerebral volume may be the result of TAI with secondary wallerian degeneration and delayed neuronal cell death rather than primary somal injury (Ding et al., 2008). In rodent models, it has been shown that axotomy results in neuronal cell death and atrophy (Bonatz et al., 2000; Giehl and Tetzlaff, 1996; Villegas-Perez et al., 1993), and in humans recent evidence indicates that TAI, in the absence of focal trauma, results in substantial atrophy of cortical and subcortical gray matter (Warner et al., 2010). While this suggests that axonal injury may be a mechanism of atrophy after TBI, the relationship between white matter integrity and post-traumatic brain volumes remains unclear.
The goals of this investigation were: (1) to determine the association between cerebral volume and acute and chronic diffusion tensor imaging (DTI) markers of white matter integrity, and (2) to assess the relationship between regional brain volumes and neuropsychological outcomes. We hypothesized that regional gray matter volumes would be correlated with markers of axonal integrity in efferent or afferent fibers, providing supportive evidence for TAI as a primary mechanism of post-traumatic atrophy. We also hypothesized that the volume of gray matter structures known to contribute to particular cognitive domains would be associated with follow-up neuropsychological scores (i.e., hippocampal volume would correlate with measures of learning and memory).
Methods
Subjects
This study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center in Dallas, Texas. All TBI patients were referred from the Department of Neurological Surgery at Parkland Memorial Hospital between September 2005 and October 2008, and were enrolled according to the following inclusion criteria: (1) injury mechanism consistent with TAI (involving high-velocity rotational or acceleration-deceleration forces); (2) Marshall grade I or II on admission computed tomography (CT) scan; (3) age 16–75 years; (4) hemodynamically stable for transfer to scanner; and (5) English or Spanish speaking. Exclusion criteria included penetrating head injury; any focal, mixed, or high-density lesion >10 mL in volume on admission CT (including contusion, extra-axial hematoma, and intraparenchymal hemorrhages); previous hospitalization for TBI; injury requiring craniectomy or craniotomy; bilaterally absent pupillary responses; reported history of pre-existing neurological disease or disabling substance abuse; or any contraindication to MRI. Written informed consent was obtained from all study participants or their legally authorized representative.
A total of 46 patients were enrolled and scanned on a GE Signa Excite 3T magnet (GE Healthcare, Milwaukee, WI). Fourteen patients did not return for follow-up MRI (3 expired after the acute scan), 7 patients had significant MRI motion artifact rendering their images unanalyzable, and the diffusion tensor images were lost from one patient, resulting in 24 patients with complete data. Initial MRI scans were obtained within 1 week (range 1–9 days) of injury, and follow-up MRI scans were performed approximately 8 months (range 6–14 months) later.
MRI image acquisition
MRI scans were acquired on a GE Signa Excite 3T scanner, and no major scanner upgrades were performed during the study period. The DTI images were obtained with a single-shot, spin-echo, echo-planar imaging sequence with a field of view of 240 mm, a slice thickness/gap of 3.0/0 mm, 45 slices, echo time of 75.5 msec, a flip angle of 90°, number of excitations of 2, a matrix of 128 × 128, and an acquisition time of 9 min. Diffusion-sensitizing gradients were applied using a b value of 1000 s/mm2 per axis, with 19 noncolinear directions, and 3 b0 images. Voxel size was 2 × 2 × 3 mm3, which was interpolated by the scanner to 1 × 1 × 3 mm3. Three-dimensional T1-weighted structural images were obtained using a fast-spoiled gradient-recalled acquisition in the steady state (GRASS) sequence, with a field of view of 240 mm, a slice thickness/gap of 1.3/0 mm, approximately 130 slices, an echo time of 2.4 msec, a flip angle of 25°, number of excitations of 2, a matrix of 256 × 92, and a 6-min acquisition time.
DTI tractography preprocessing and analysis
Preprocessing for DTI included brain extraction and eddy-current correction using FSL (http://www.fmrib.ox.ac.uk/fsl) software applications. DTI Studio was used for diffusion map generation, fiber tractography, and fiber-tract quantification.
Tractography was performed using the fiber assignment by continuous tracking algorithm (Mori et al., 1999). Fibers were reconstructed through voxels with fractional anisotropy (FA) values above 0.25 and turning angles <60°. Fiber tracts were selected by using the OR, AND, NOT, and CUT ROI operations, that select and exclude fibers that pass through multiple regions of interest on the color map, consistent with Wang and associates (2008), and following the guidelines of Wakana and colleagues (Wakana et al., 2004).
The reconstructed tracts were categorized as either (1) interhemispheric commissural, (2) limbic, or (3) association fibers. Interhemispheric commissural fibers consisted of white matter tracts of the corpus callosum (CC). Since the CC is a large fiber bundle that connects various parts of the brain, we reconstructed the genu and the splenium, in addition to assessing the entire corpus callosum. Limbic fibers included white matter tracts of the fornix body, bilateral fornix crus, bilateral perforant pathway, and cingulum. Association fibers included bilateral uncinate fasciculi, and the inferior fronto-occipital fasciculi.
To reconstruct the CC, ROI operation AND was used to extract fibers that run between two sagittal slices, each 4 mm lateral to the midsagittal slice. To parcellate the CC, the anterior and posterior bundles of the CC were defined to be the first coronal slices where the red color of the CC in a diffusion color map disappeared. Each section of the CC was tracked using rectangular ROIs with the width of one-quarter of the length of the entire CC plus one slice.
For the fornix body, ROI operation CUT was used on the axial slice two slices above the slice containing the anterior commissure. The boundary of the fornix body was determined to be the most posterior coronal slice where the fornix appears as a unitary bundle.
The fornix crus was also reconstructed using the CUT operation. The first ROI was set on the axial slice where the fornix and stria terminalis form distinct bundles. The second ROI was 15 mm inferior to the first ROI, or in cases for which the fornix crus did not extend this far, the second ROI was set 15 mm anterior to the coronal slice in the middle of the fornix crus.
The perforant pathway was reconstructed using three ROIs on coronal slices. Slice #1 was located at the center of the splenium of the CC. Slice #2 was located at the anterior edge of the pons using ROI operation OR. Slice #3 was placed midway between the first two slices using ROI operation AND. The same procedure was followed to track the fibers on the contralateral hemisphere.
The cingulum was reconstructed similarly to the perforant pathway, for which the first and second slices were placed coronally on the inner curve of the genu and posterior edge of the splenium of the CC, respectively, using ROI operation OR. The third slice was set midway between the first two slices. The ROI operation AND was used to track both the left or right cingulum independently.
For the uncinate fasciculus, the first ROI was placed on the most posterior coronal slice that did not contain the temporal stem. The second ROI encircled uncinate fasciculus fibers of the frontal lobe using operation AND.
For the inferior fronto-occipital fasciculus, the first ROI was placed on the coronal slice tangential to the posterior cingulum. To track the fibers in the left hemisphere, the second ROI was placed on the coronal slice passing through the inner curve of the genu of the CC, where the CC remained as one whole red area. For the fibers in the right hemisphere, the second slice was adjusted to the difference of the first slice between the left and right inferior fronto-occipital fasciculi to correct for symmetry.
Measures of white matter structural integrity recorded for each reconstructed tract included FA and mean diffusivity (MD). Two independent raters were used for white matter tract reconstruction, and interrater reliability was assessed using intraclass correlation coefficients (ICC). Values for FA and MD ranged from 0.91 to 1.00 across all structures, suggesting that they were reliably constructed.
Morphometric image analysis
Image files in DICOM format were transferred to a Macintosh workstation for analysis with FreeSurfer image analysis suite (v 4.5.0; Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA). FreeSurfer is a semi-automated brain morphometry tool that allows for high-resolution quantification of subcortical and cortical brain regions derived from atlas-defined coordinates. It has been described in detail in prior publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002, 1999). FreeSurfer analyses were conducted by three independent raters, and interrater reliability was determined by having each rater independently analyze 20 brains with a mean ICC of 0.97. Given the modest sample size in this study, we limited our analysis to structures previously determined to undergo significant volume loss after TAI (Warner et al., 2010). Subcortical structures included the left and right amygdala, hippocampus, and thalamus, in addition to the CC. Bilateral cortical regions consisted of the inferior parietal, superior parietal, superior frontal, and precuneus cortices.
Neuropsychological assessment
Outcome assessment was performed at the time of follow-up MRI by one of three study coordinators with specific expertise and training in interviewing patients with TBI. The raters were blinded from imaging results. General functional outcome was measured using the Glasgow Outcome Scale–Extended (GOSE), a structured interview assessing overall functional recovery after TBI. Scores range from 1 to 8, with higher scores reflecting better functional outcomes. Inter-rater reliability was assessed by auditing 20% of scoring sheets every 3 months. Reproducibility was greater than 99%.
Neuropsychological outcome was assessed using a test battery assessing information processing speed, learning and memory, and executive functions, three cognitive domains commonly impacted by TBI (Brooks et al., 1986; Levin, 1990; Mathias and Wheaton, 2007; McAllister, 2002). A previously unpublished principal components analysis of the test battery using a completely independent sample of 52 patients with moderate to severe TBI approximately 6 months post-injury revealed that the battery was comprised of a three-factor structure explaining 76% of the total variance in performance (eigenvalues >1). The three-factor solution was utilized in the present study to create three composite neurocognitive measures for the purpose of minimizing the effects of multiple comparisons, which are inherent in these types of studies. The first composite measure assessed executive function with the Trail Making Test B [TMT-B; (Reitan, 1992)], Controlled Word Association Test [COWAT; (Benton and Hamsher, 1983)], Digit Span Backwards Test (Wechsler, 1997), and Stroop reading and Stroop color-naming conditions (Dodrill, 1978).
The second composite measure assessed verbal learning and memory, and included variables from the California Verbal Learning Test-II (CVLT-II; Delis et al., 2000). Learning was measured using the total number of items learned across five trials, and memory was assessed using short- and long-delay free recall trials.
The third composite measure assessed information processing speed, and included the Digit Symbol Coding and Symbol Search subtests from the Wechsler Adult Intelligence Scale-III [WAIS-III; (Wechsler, 1997)], and Trail Making Test A [TMT-A; (Reitan, 1992)]. Demographically corrected scores were used where applicable.
Statistical analysis
Pearson correlation coefficients were used to determine (1) the association between acute and chronic FA and MD values and regional brain volumes, and (2) the association between neuropsychological outcomes and regional brain volumes. To correct for effects of multiple comparisons, false-discovery rates (FDR) of 0.10 and 0.05 were utilized to determine statistical significance. Principal components regression (PCR) was utilized to assess the ability of regional brain volumes to predict particular neuropsychological outcomes. Subcortical brain regions were subjected to a principal components reduction, creating three components explaining >75% of cumulative variance: (1) left and right hippocampus and amygdala, (2) left and right thalamus, and (3) CC. Cortical data were also reduced to create three components accounting for >75% of cumulative variance: (1) left and right superior frontal and superior parietal cortices, (2) left and right inferior parietal cortex, and (3) left and right precuneus cortex. Subcortical and cortical components were then entered into linear regression models for the prediction of each of the three derived neuropsychological outcome measures. Statistical significance for PCR analysis was set at p < 0.05.
Results
Demographic and outcome characteristics
Demographic features and outcome characteristics for the patients enrolled in this study are shown in Table 1. Most patients suffered severe TBI with substantial injury to other organ systems, as reflected by a median Injury Severity Score (ISS) of 29. Many regained a high level of general functioning at the time of follow-up, with a median GOSE score of 7.
Table 1.
Demographic and Neuropsychological Characteristics of the Study Cohort (n = 24)
| Median | Mean | SD | |
|---|---|---|---|
| Age (years) | 23 | 27.2 | 11.4 |
| Gender (% male) | 70.8 | ||
| GCS score | 4.5 | 6.4 | 4.2 |
| Head AIS | 4.0 | 4.0 | 0.8 |
| ISS | 29.0 | 28.5 | 12.3 |
| Days in the ICU | 3.0 | 6.7 | 6.8 |
| Days in hospital | 11.0 | 12.9 | 10.0 |
| Time to initial MRI (days) | 1.0 | 2.2 | 2.4 |
| Time to follow-up MRI (months) | 7.8 | 7.7 | 1.9 |
| GOSE score | 7.0 | 6.4 | 1.9 |
| Digit Symbol T score | 41.7 | 40.6 | 9.8 |
| Symbol Search T score | 46.7 | 46.5 | 11.5 |
| TMT-A T score | 47.0 | 46.2 | 17.1 |
| TMT-B T score | 49.5 | 47.5 | 16.1 |
| COWAT T score | 37.0 | 37.4 | 11.9 |
| Stroop I T score | 45.5 | 40.3 | 16.5 |
| Stroop II T score | 54.5 | 48.7 | 16.2 |
| Stroop Interference T score | 60.0 | 54.4 | 12.1 |
| CVLT-II Total Learning T score | 46.0 | 44.7 | 18.1 |
| CVLT-II Short-delay recall T score | 45.0 | 42.4 | 17.3 |
| CVLT-II Long-delay recall T Score | 45.0 | 41.4 | 18.0 |
GCS, Glasgow Coma Scale; head AIS, head Abbreviated Injury Scale score; ISS, Injury Severity Scale; ICU, intensive care unit; GOSE, Glasgow Outcome Scale–Extended; TMT-A, Trail Making Test A; TMT-B, Trail Making Test B; COWAT, Controlled Oral Word Association Test; Stroop I, reading condition; Stroop II, color-naming condition; CVLT-II, California Verbal Learning Test-II; SD, standard deviation.
Acute DTI and chronic brain volumes
Twenty-one out of 210 comparisons between acute FA values and chronic brain volumes were significant at 0.10 FDR. No comparison survived 0.05 FDR. Of note, post-traumatic volume of the left thalamus was associated with FA in the composite CC and in the splenium of the CC. Volume of the CC was also associated with FA in the splenium. Right amygdala volume was associated with FA in the fornix body and right uncinate fasciculus, and both the left and right precuneus were associated with FA in the splenium of the CC, right perforant pathway, right uncinate fasciculus, and right inferior frontal-occipital fasciculus. With regard to acute MD values and regional brain volumes, no correlation survived correction at 0.05 or 0.10 FDR.
Chronic DTI and chronic brain volumes
One hundred and fourteen out of 210 comparisons between chronic FA measures and chronic brain volumes survived 0.10 FDR, with 66 of those correlations maintaining significance at 0.05 FDR (Table 2). All but three of the correlations between regional brain volumes and acute FA maintained significance in comparisons with chronic FA, and most correlation coefficients grew in magnitude between the time of acute and chronic MRI. Of note, hippocampal volumes were correlated with FA in white matter tracts of the limbic system, including the fornix body, fornix crus, perforant pathway, and cingulum bundle. In addition, the volume of each cortical region was positively correlated with FA in the CC, as well as in other prominent white matter bundles.
Table 2.
Pearson's Correlations between Chronic Fractional Anisotropy Values and Chronic Brain Volumes
| |
|
Subcortical structures |
Cortical regions |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyg L | Amyg R | Hippo L | Hippo R | Thal L | Thal R | CC | IPar L | IPar R | SPar L | SPar R | SFtl L | SFtl R | Prcn L | Prcn R | ||
| Inter- hemispheric commissural | CCt | 0.47* | 0.44* | 0.57** | 0.55** | 0.57** | 0.26 | 0.44* | 0.35 | 0.42* | 0.44* | 0.43* | 0.42* | 0.39 | 0.54** | 0.57** |
| CCg | 0.46* | 0.56** | 0.46* | 0.56** | 0.39 | 0.34 | 0.42* | 0.41* | 0.50** | 0.41* | 0.46* | 0.28 | 0.43* | 0.56** | 0.53** | |
| CCs | 0.44* | 0.40* | 0.58** | 0.57** | 0.61** | 0.29 | 0.45* | 0.33 | 0.38 | 0.39 | 0.34 | 0.46* | 0.33 | 0.49** | 0.56** | |
| Limbic | FxB | 0.50** | 0.61** | 0.60** | 0.71** | 0.68** | 0.48* | 0.44* | 0.30 | 0.32 | 0.48* | 0.32 | 0.53** | 0.34 | 0.58** | 0.70** |
| FxCL | 0.41* | 0.52** | 0.37 | 0.57** | 0.43* | 0.32 | 0.42* | 0.34 | 0.35 | 0.51** | 0.37 | 0.28 | 0.16 | 0.42* | 0.44* | |
| FxCR | 0.40 | 0.57** | 0.59** | 0.66** | 0.43* | 0.43* | 0.51** | 0.35 | 0.55** | 0.57** | 0.40 | 0.42* | 0.28 | 0.64** | 0.72** | |
| PPL | 0.30 | 0.35 | 0.48* | 0.49** | 0.40* | 0.25 | 0.05 | 0.24 | 0.26 | 0.41* | 0.19 | 0.30 | −0.07 | 0.30 | 0.43* | |
| PPR | 0.54** | 0.68** | 0.62** | 0.61** | 0.31 | 0.43* | 0.31 | 0.15 | 0.19 | 0.43* | 0.28 | 0.40* | 0.11 | 0.40* | 0.49** | |
| CIL | 0.36 | 0.36 | 0.49** | 0.54** | 0.40* | 0.32 | 0.35 | 0.16 | 0.21 | 0.30 | 0.29 | 0.13 | 0.19 | 0.35 | 0.27 | |
| CIR | 0.45* | 0.41 | 0.52** | 0.58** | 0.50** | 0.42* | 0.38 | 0.31 | 0.22 | 0.28 | 0.27 | 0.17 | 0.17 | 0.36 | 0.26 | |
| Association fibers | UNCL | 0.22 | 0.38 | 0.18 | 0.34 | 0.28 | 0.23 | 0.57** | 0.17 | 0.16 | 0.32 | 0.24 | 0.27 | 0.23 | 0.28 | 0.39 |
| UNCR | 0.26 | 0.51* | 0.69** | 0.71** | 0.32 | 0.35 | 0.18 | 0.17 | 0.34 | 0.35 | 0.28 | 0.25 | 0.14 | 0.56** | 0.57** | |
| IFOL | 0.57** | 0.59** | 0.49** | 0.49** | 0.39 | 0.47* | 0.21 | 0.34 | 0.34 | 0.59** | 0.43* | 0.41* | 0.18 | 0.32 | 0.50** | |
| IFOR | 0.54** | 0.57** | 0.55** | 0.53** | 0.42* | 0.50** | 0.50** | 0.53** | 0.57** | 0.54** | 0.46* | 0.47* | 0.36 | 0.55** | 0.69** | |
Significant at 0.10 FDR; **significant at 0.05 FDR.
CCt, corpus callosum total; CCg, corpus callosum genu; CCs, corpus callosum splenium; FxB, fornix body; FxCL, fornix crus left; FxCR, fornix crus right; PPL, perforant pathway left; PPR, perforant pathway right; CIL, cingulum left; CIR, cingulum right; UNCL, uncinate fasciculus left; UNCR, uncinate fasciculus right; IFOL, inferior frontal-occipital fasciculus left; IFOR, inferior frontal-occipital fasciculus right; Amyg, amygdala; Hippo, hippocampus; Thal, thalamus; CC, corpus callosum; IPar, inferior parietal lobule; SPar, superior parietal lobule; SFtl, superior frontal lobule; Prcn, precuneus cortex; FDR, false-discovery rate.
With regard to chronic MD values, two correlations survived 0.10 FDR. MD in the fornix body correlated with volume of the right hemisphere precuneus (r = −0.671, p < 0.001), and MD in the right uncinate fasciculus also correlated with volume of the right hemisphere precuneus (r = −0.626, p = 0.001). No correlation survived 0.05 FDR.
Neuropsychological outcome and chronic brain volumes
Performance on tests of particular cognitive domains was spatially associated with chronic brain volumes (Table 3). For example, bilateral thalamus volumes were associated with tests of processing speed such as the Digit Symbol Coding test, Symbol Search test, and TMT-A. Amygdala and hippocampal volumes, as well as volumes of many cortical regions, were associated with learning and memory performance. The superior frontal, superior parietal, and precuneus cortices were all positively correlated with measures of executive function, as was the thalamus.
Table 3.
Pearson's Correlations between Neuropsychological Outcomes and Chronic Brain Volumes
| |
|
Subcortical structures |
Cortical regions |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyg L | Amyg R | Hippo L | Hippo R | ThalL | Thal R | CC | IPar L | IPar R | SPar L | SPar R | SFtl L | SFtl R | Prcn L | Prcn R | ||
| Processing speed | DigSym | 0.37 | 0.43* | 0.34 | 0.31 | 0.51** | 0.62** | 0.35 | 0.42* | 0.37 | 0.47* | 0.52** | 0.58** | 0.62** | 0.45* | 0.54** |
| SymSrch | 0.40 | 0.44* | 0.39 | 0.36 | 0.58** | 0.62** | 0.31 | 0.35 | 0.23 | 0.39 | 0.38 | 0.57** | 0.57** | 0.31 | 0.47* | |
| TMT-A | 0.46* | 0.41* | 0.23 | 0.33 | 0.58** | 0.54** | 0.14 | 0.35 | 0.37 | 0.45* | 0.40* | 0.51** | 0.37 | 0.25 | 0.31 | |
| Executive function | TMT-B | 0.39 | 0.42* | 0.32 | 0.35 | 0.44* | 0.54** | 0.33 | 0.28 | 0.20 | 0.37 | 0.43* | 0.54** | 0.53** | 0.27 | 0.43* |
| COWAT | 0.44* | 0.27 | 0.34 | 0.18 | 0.26 | 0.40 | 0.42* | 0.28 | 0.27 | 0.18 | 0.39 | 0.32 | 0.39 | 0.16 | 0.21 | |
| Stroop-I | 0.41* | 0.29 | 0.45* | 0.17 | 0.23 | 0.44* | 0.39 | 0.33 | 0.18 | 0.27 | 0.45* | 0.42* | 0.47* | 0.25 | 0.42* | |
| Stroop-II | 0.32 | 0.35 | 0.38 | 0.34 | 0.32 | 0.57** | 0.40 | 0.35 | 0.14 | 0.20 | 0.35 | 0.36 | 0.24 | 0.18 | 0.33 | |
| DigBack | 0.19 | 0.18 | 0.17 | 0.19 | −0.09 | −0.02 | 0.38 | −0.10 | −0.03 | −0.15 | −0.03 | 0.04 | 0.10 | 0.32 | 0.28 | |
| Learning and memory | CVLT-II | 0.57** | 0.52* | 0.56** | 0.40 | 0.51* | 0.43 | 0.61** | 0.49* | 0.45* | 0.47* | 0.62** | 0.55** | 0.62** | 0.64** | 0.69** |
| CVLT-Sh | 0.48* | 0.51* | 0.61** | 0.50* | 0.57** | 0.47* | 0.67** | 0.53** | 0.60** | 0.47* | 0.62** | 0.54** | 0.61** | 0.66** | 0.68** | |
| CVLT-Ln | 0.43* | 0.55** | 0.64** | 0.53** | 0.59** | 0.53** | 0.64** | 0.50* | 0.55** | 0.51* | 0.58** | 0.50* | 0.57** | 0.65** | 0.70** | |
Significant at 0.10 FDR; **significant at 0.05 FDR.
DigSym, digit symbol; SymSrch, symbol search; TMT-A, Trail Making Test A; TMT-B, Trail Making Test B; COWAT, Controlled Oral Word Association Test; Stroop-I, reading condition; Stroop-II, color-naming condition; DigBack, digit span backwards; CVLT-II, California Verbal Learning Test-II total learning; CVLT-Sh, California Verbal Learning Test-II short delay recall; CVLT-Ln, California Verbal Learning Test-II long delay recall; Amyg, amygdala; Hippo, hippocampus; Thal, thalamus; CC, corpus callosum; IPar, inferior parietal lobule; SPar, superior parietal lobule; SFtl, superior frontal lobule; Prcn, precuneus cortex; FDR, false-discovery rate.
In PCR analysis, subcortical brain volumes were suggestive of performance scores on tests of learning and memory and processing speed, but not executive function (Table 4). The greatest contributor to model strength for processing speed prediction was the thalamus bilaterally. All subcortical components contributed significantly to learning and memory performance.
Table 4.
Principal Components Regression for Prediction of Learning and Memory, Processing Speed, and Executive Function Scores from Subcortical Brain Volumesa
|
Overall model statistics with all subcortical components as predictors | |||||
|---|---|---|---|---|---|
| Model # | Predicted | Model parameters | Sum of squares | Mean square | F-value (p value) |
| 1 | L&M | Regression | 9.55 | 3.18 | 5.88 (0.006)* |
| Residual | 9.20 | 0.54 | |||
| 2 | PS | Regression | 14.67 | 4.89 | 5.46 (0.007)* |
| Residual | 17.91 | 0.90 | |||
| 3 | Exec | Regression | 11.96 | 3.99 | 2.63 (0.078) |
| Residual | 30.32 | 1.52 | |||
|
Contribution of input variables to model strength | ||||
|---|---|---|---|---|
| |
|
Standardized beta coefficients (p value) |
||
| Input variable | Subcortical regions | L&M | PS | Exec |
| Component 1 | Amygdala, hippocampus | 0.38 (0.040)* | 0.23 (0.175) | 0.24 (0.217) |
| Component 2 | Thalamus | 0.38 (0.041)* | 0.63 (0.001)* | 0.38 (0.060) |
| Component 3 | Corpus callosum | 0.50 (0.009)* | 0.08 (0.658) | 0.29 (0.145) |
Significant at p < 0.05.
Principal components regression was conducted using three components derived from subcortical brain volumes to predict each of the three composite cognitive measures (top rows), and to determine which subcortical components contributed the most to neurocognitive outcome (bottom rows).
L&M, learning and memory; PS, processing speed; Exec, executive function.
Cortical brain volumes were likewise suggestive of learning and memory and processing speed performance, but not performance on executive function tasks (Table 5). The precuneus cortex and the superior frontal and parietal cortices were the most influential predictors of learning and memory performance, while only the superior frontal and parietal cortices contributed significantly to information processing speed scores. Although the cumulative model for prediction of executive function performance from cortical components was not statistically significant, the superior frontal and superior parietal cortex contributed significantly.
Table 5.
Principal Components Regression for Prediction of Learning and Memory, Processing Speed, and Executive Function Scores from Cortical Brain Volumesa
|
Overall model statistics with all cortical components as predictors | |||||
|---|---|---|---|---|---|
| Model # | Predicted | Model parameters | Sum of squares | Mean square | F-value (p value) |
| 1 | L&M | Regression | 9.70 | 3.23 | 6.07 (0.005)* |
| Residual | 9.06 | 0.53 | |||
| 2 | PS | Regression | 10.82 | 3.61 | 3.31 (0.041)* |
| Residual | 21.77 | 1.09 | |||
| 3 | Exec | Regression | 9.86 | 3.29 | 2.03 (0.142) |
| Residual | 32.41 | 1.62 | |||
|
Contribution of input variables to model strength | ||||
|---|---|---|---|---|
| |
|
Standardized beta coefficients (p value) |
||
| Input variable | Cortical regions | L&M | PS | Exec |
| Component 1 | Sup frontal and Parietal | 0.40 (0.032)* | 0.52 (0.010)* | 0.45 (0.033)* |
| Component 2 | Inf parietal | 0.28 (0.119) | 0.16 (0.403) | 0.10 (0.610) |
| Component 3 | Precuneus | 0.52 (0.006)* | 0.20 (0.289) | 0.15 (0.461) |
Significant at p < 0.05.
Principal components regression was conducted using three components derived from cortical brain volumes to predict each of the three composite cognitive measures (top rows), and to determine which cortical components contributed the most to neurocognitive outcome (bottom rows).
L&M, learning and memory; PS, processing speed; Exec, executive function; Sup, superior; Inf, inferior.
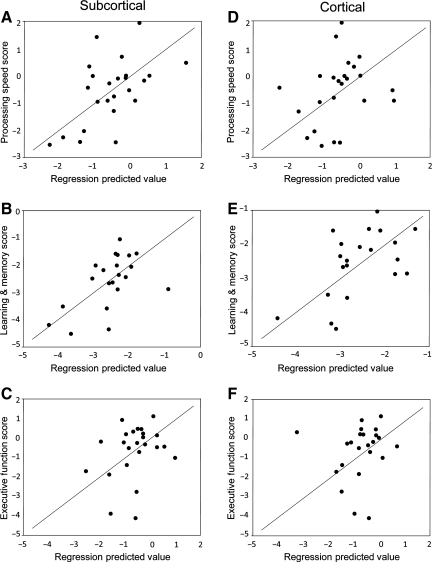
Prediction plots for neuropsychological outcomes using all subcortical and cortical volume components are displayed in Figure 1. Subcortical and cortical models for prediction of learning and memory performance were nearly equal in strength; however, subcortical brain volumes were more predictive of information processing speed performance than the model for cortical volumes. Cumulative models for predicting executive function scores using subcortical and cortical components were not significant.
FIG. 1.
Plots of standardized predicted processing speed (A and D), learning and memory (B and E), and executive function (C and F) scores, from subcortical (A–C) and cortical brain volumes (D–F), against actual performance scores using principal components regression (see text for details). Reference lines (y = x) have been added to enhance interpretation.
Discussion
In this investigation we examined the relationship between measures of white matter integrity and regional brain volumes after TAI, and found that the degree of compromise of selected white matter tracts was correlated with post-traumatic volumes in related gray matter structures. To our knowledge this is the first study to demonstrate a spatial relationship in vivo between axonal injury and brain volumes in humans with TBI. In addition, this investigation demonstrated that post-traumatic brain volumes were predictive of performance in neuropsychological domains conventionally associated with those subcortical and cortical regions.
DTI is a relatively novel MRI modality that allows for non-invasive quantification of microstructural alterations in white matter structures based on the directionality and degree of water diffusion through brain tissue (Basser et al., 1994; Metting et al., 2007; Schiff, 2006). In DTI tractography, the entire length of white matter structures can be reconstructed in three dimensions using fiber propagation algorithms, allowing for enhanced quantification of key diffusion variables such as FA and MD (Mori and van Zijl, 2002). In TBI, recent evidence suggests that tractography-based quantification of diffuse axonal injuries is superior to fluid-attenuated inversion recovery (FLAIR) imaging for prediction of long-term functional outcomes (Wang et al., 2008). However, the association between fiber integrity and post-traumatic brain volumes has yet to be assessed in vivo. Recent histopathological data indicate that loss of frontotemporal axons in patients with TAI is accompanied by loss of neurons in overlying gray matter (Maxwell et al., 2010). Moreover, recent neuroimaging data have shown that TAI results in atrophy that is regionally specific rather than global (Warner et al., 2010), and volume loss after TBI is most prominent in regions susceptible to the consequences of TAI (Sidaros et al., 2009). Previous work by our group has shown that acute axonal lesions as identified by subcortical white matter hyperintensities on FLAIR imaging are predictive of global atrophy (Ding et al., 2008), and the current study provides the first evidence for a more specific spatial relationship between gray matter volume and the compromise of efferent and afferent white matter fibers after trauma.
The strength of the correlations between post-traumatic brain volumes and DTI-derived FA values were substantially stronger for comparisons with chronic than with acute MRI data. This suggests that changes in tract-based FA values continue beyond the acute time period, and potential gray matter changes after TAI are not fully explained by the integrity of white matter at the acute stage. While complete mechanical disruption of the axonal cytoskeleton (primary axotomy) may occur within hours to days in the most severe cases of injury, current evidence based on animal models suggests that TAI progresses for days to months after trauma, with progressive axonal cytoskeletal disorganization, protein accumulation, and eventual axonal disconnection [secondary axotomy; (Povlishock and Katz, 2005; Smith et al., 2003)]. It is our working hypothesis that both primary and secondary axotomy may progress to widespread wallerian degeneration, contributing to delayed neuronal cell death and potential gray matter atrophy.
It is also possible that some axonal lesions identified acutely after injury may recover over time. A recent longitudinal investigation of 22 patients with severe TBI found that while FA values were lower in patients in the subacute period after injury (8 weeks) than in healthy controls, patient FA values actually increased over the 12-month study period in the posterior limb of the internal capsule and in the centrum semiovale, but decreased in the posterior aspect of the corpus callosum (Sidaros et al., 2008). Further, the authors hypothesized that increases in FA may represent areas of late axonal recovery or even axonal regrowth. Hence it is probable that some axonal injuries are reversible and resolve within weeks or months after injury, while axonal injuries persisting chronically may represent areas of irreversible damage that may progress to axonal degeneration, neuronal death, and subsequent gray matter volume loss. Future studies with multiple sequential time points for MRI acquisition will be essential in enhancing our understanding of the progression and recovery of acute axonal injuries after head trauma. In addition, it should be noted that correlations between regional brain volumes and FA were much stronger than associations between volume and MD, suggesting that FA may be a more sensitive biomarker for the long-term structural consequences of TAI. This finding is consistent with prior studies in which researchers noted that while both FA and MD are altered after TAI, the directionality of water diffusion is more closely associated with clinical outcome than the magnitude of diffusion (Bazarian et al., 2007; Benson et al., 2007; Huisman et al., 2004).
Functional deficits are common after TBI, yet few studies have assessed the spatial relationship between regional brain volumes and particular cognitive outcomes after trauma. In their analysis of 9 adults after TBI, Gale and colleagues (2005) found that poor attention was correlated with decreased gray matter concentration in several locations in the right frontal, temporal, and parietal lobes, and in the left cingulate and frontal cortices. In pediatric TBI, a cross-sectional study of 16 children with moderate to severe TBI found that poor working memory was associated with decreased thickness in the inferior temporal and superior and inferior parietal cortices (Merkley et al., 2008). Our results demonstrate that regional brain volumes after trauma are associated with particular neuropsychological outcomes in adults with TAI. As expected, thalamic volumes were correlated with measures of information processing speed, and amygdala and hippocampal volumes were associated with learning and memory performance. In regression analysis, performance in both cognitive domains was significantly predicted by subcortical brain volumes, with thalamic volume contributing most to assessment of information processing speed. Interestingly, composite cortical brain volumes were also predictive of learning and memory and information processing speed performance, suggesting that many cognitive tasks are dependent on functional connections between deep gray matter structures and the cerebral cortex, and that these may be significantly altered after TAI. Among cortical structures, the superior frontal, superior parietal, and precuneus cortices were all positively correlated with measures of executive function, with the greatest predictive value seen in the superior frontal and superior parietal cortices. These findings suggest that brain morphometry may hold value as a potential biomarker for post-traumatic neuropsychological functioning. However, further characterization of such relationships in larger studies over longer time periods will be required in order to determine its clinical significance.
There are several limitations to the present investigation. While our results demonstrate a strong association between disruptions in white matter integrity and gray matter volume, we were unable to ascertain causality. Moreover, given the small sample size of the investigation, white matter and volume relationships were only assessed for a limited number of brain regions. Larger studies exploring a greater number of regions and over a longer study period are required to more fully understand the relationships between white matter integrity and brain morphometry changes after trauma. In addition, we were unable to obtain pre-injury neuropsychological assessments, and it is probable that post-injury outcomes depend largely on premorbid cognitive function. Hence, the apparent differences in neuropsychological function after trauma may be indicative of baseline cognitive status, as well as changes in functional status after injury. Finally, this investigation utilized only two time points for MRI, and future investigations with multiple scan points will be crucial in determining the progression of axonal injuries and gray matter volume changes in real time.
In summary, the current investigation provides in vivo evidence for a spatial relationship between metrics of acute and chronic white matter integrity after brain trauma and volume of corresponding gray matter structures. In addition, post-traumatic brain volumes may be suggestive of particular neuropsychological outcomes, providing support for regional morphometry as a biomarker for cognitive outcome after injury. Finally, our results suggest that TAI may be a primary mechanism of post-traumatic atrophy; however, future studies with larger sample sizes are needed to establish causality.
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation (Clinical Research Fellowship to M.W.); the National Institutes of Health–National Institute of Child Health and Human Development (R01 HD48179 to R.D.-A and, U01 HD42652 to R.D.-A.); and the United States Department of Education Grant (H133 A020526 to R.D.-A.).
Author Disclosure Statement
No competing financial interests exist.
References
- Ariza M. Serra-Grabulosa J.M. Junque C. Ramirez B. Mataro M. Poca A. Bargallo N. Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Mattiello J. LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Benson R.R. Meda S.A. Vasudevan S. Kou Z. Govindarajan K.A. Hanks R.A. Millis S.R. Makki M. Latif Z. Coplin W. Meythaler J. Haacke E.M. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J. Neurotrauma. 2007;24:446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Benton A.L. Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City: 1983. [Google Scholar]
- Bigler E.D. Quantitative magnetic resonance imaging in traumatic brain injury. J. Head Trauma Rehabil. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Ryser D.K. Gandhi P. Kimball J. Wilde E.A. Day-of-injury computerized tomography, rehabilitation status, and development of cerebral atrophy in persons with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2006;85:793–806. doi: 10.1097/01.phm.0000237873.26250.e1. [DOI] [PubMed] [Google Scholar]
- Bonatz H. Rohrig S. Mestres P. Meyer M. Giehl K.M. An axotomy model for the induction of death of rat and mouse corticospinal neurons in vivo. J. Neurosci. Methods. 2000;100:105–115. doi: 10.1016/s0165-0270(00)00238-7. [DOI] [PubMed] [Google Scholar]
- Brooks N. Campsie L. Symington C. Beattie A. McKinlay W. The five year outcome of severe blunt head injury: a relative's view. J. Neurol. Neurosurg. Psychiatry. 1986;49:764–770. doi: 10.1136/jnnp.49.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. Fischl B. Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis D. Joel H. Kaplan E. Ober B. California Verbal Learning Test. Psychological Corporation; San Antonio: 2000. [Google Scholar]
- Ding K. Marquez de la Plata C. Wang J.Y. Mumphrey M. Moore C. Harper C. Madden C.J. McColl R. Whittemore A. Devous M.D. Diaz-Arrastia R. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J. Neurotrauma. 2008;25:1433–1440. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodrill C.B. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Evans S.A. Airey M.C. Chell S.M. Connelly J.B. Rigby A.S. Tennant A. Disability in young adults following major trauma: 5 year follow up of survivors. BMC Public Health. 2003;3:8. doi: 10.1186/1471-2458-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. Salat D.H. Busa E. Albert M. Dieterich M. Haselgrove C. van der Kouwe A. Killiany R. Kennedy D. Klaveness S. Montillo A. Makris N. Rosen B. Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B. Sereno M.I. Dale A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gale S.D. Baxter L. Roundy N. Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl K.M. Tetzlaff W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur. J. Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Howe L.L. Giving context to post-deployment post-concussive-like symptoms: blast-related potential mild traumatic brain injury and comorbidities. Clin. Neuropsychol. 2009;23:1315–1337. doi: 10.1080/13854040903266928. [DOI] [PubMed] [Google Scholar]
- Huisman T.A. Schwamm L.H. Schaefer P.W. Koroshetz W.J. Shetty-Alva N. Ozsunar Y. Wu O. Sorensen A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- Levin H.S. Memory deficit after closed head injury. J. Clin. Exp. Neuropsychol. 1990;12:129–153. doi: 10.1080/01688639008400960. [DOI] [PubMed] [Google Scholar]
- MacKenzie J.D. Siddiqi F. Babb J.S. Bagley L.J. Mannon L.J. Sinson G.P. Grossman R.I. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am. J. Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Mathias J.L. Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: a meta-analytic review. Neuropsychology. 2007;21:212–223. doi: 10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L. MacKinnon M.A. Stewart J.E. Graham D.I. Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow outcome score. Brain. 2010;133:139–160. doi: 10.1093/brain/awp264. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Evaluation and treatment of neurobehavioral complications of traumatic brain injury—have we made any progress? NeuroRehabilitation. 2002;17:263–264. [PubMed] [Google Scholar]
- McKee A.C. Cantu R.C. Nowinski C.J. Hedley-Whyte E.T. Gavett B.E. Budson A.E. Santini V.E. Lee H.S. Kubilus C.A. Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley T.L. Bigler E.D. Wilde E.A. McCauley S.R. Hunter J.V. Levin H.S. Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. J. Neurotrauma. 2008;25:1343–1345. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metting Z. Rodiger L.A. De Keyser J. van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet Neurol. 2007;6:699–710. doi: 10.1016/S1474-4422(07)70191-6. [DOI] [PubMed] [Google Scholar]
- Meythaler J.M. Peduzzi J.D. Eleftheriou E. Novack T.A. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Mori S. van Zijl P.C. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori S. Crain B.J. Chacko V.P. van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C.E. Kolster R. Lee H. Suh M. Zimmerman R.D. Manley G.T. McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Reitan R. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; Tucson: 1992. [Google Scholar]
- Scheid R. Walther K. Guthke T. Preul C. von Cramon D.Y. Cognitive sequelae of diffuse axonal injury. Arch. Neurol. 2006;63:418–424. doi: 10.1001/archneur.63.3.418. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Multimodal neuroimaging approaches to disorders of consciousness. J. Head Trauma Rehabil. 2006;21:388–397. doi: 10.1097/00001199-200609000-00003. [DOI] [PubMed] [Google Scholar]
- Sidaros A. Engberg A.W. Sidaros K. Liptrot M.G. Herning M. Petersen P. Paulson O.B. Jernigan T.L. Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Sidaros A. Skimminge A. Liptrot M.G. Sidaros K. Engberg A.W. Herning M. Paulson O.B. Jernigan T.L. Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44:1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Meaney D.F. Shull W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F. Carlesimo G.A. Di Paola M. Petrides M. Fera F. Bonanni R. Formisano R. Pasqualetti P. Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F. Worsley K.J. Lerch J. Di Paola M. Carlesimo G.A. Bonanni R. Caltagirone C. Paus T. Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. J. Neurotrauma. 2005;22:76–82. doi: 10.1089/neu.2005.22.76. [DOI] [PubMed] [Google Scholar]
- Villegas-Perez M.P. Vidal-Sanz M. Rasminsky M. Bray G.M. Aguayo A.J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J. Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Wakana S. Jiang H. Nagae-Poetscher L.M. van Zijl P.C. Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wang J.Y. Bakhadirov K. Devous M.D., Sr. Abdi H. McColl R. Moore C. Marquez de la Plata C.D. Ding K. Whittemore A. Babcock E. Rickbeil T. Dobervich J. Kroll D. Dao B. Mohindra N. Madden C.J. Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Warner M.A. Youn T.S. Davis T. Chandra A. Marquez de la Plata C. Moore C. Harper C. Madden C.J. Spence J. McColl R. Devous M. King R.D. Diaz-Arrastia R. Regionally selective atrophy after traumatic axonal injury. Arch. Neurol. 2010. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Wechsler D. Wechsler Adult Intelligence Scale (WAIS-III) The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Xu Y. McArthur D.L. Alger J.R. Etchepare M. Hovda D.A. Glenn T.C. Huang S. Dinov I. Vespa P.M. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J. Cereb. Blood Flow Metab. 2010;30:883–894. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E. Miller T. Langlois J.A. Selassie A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]