Abstract
Background
Nonalcoholic fatty liver disease (NAFLD), the most common cause of liver disease in children, is associated with obesity and insulin resistance. However, the relationship between NAFLD and cardiovascular risk factors in children is not fully understood. The objective of this study was to determine the association between NAFLD and the presence of metabolic syndrome in overweight and obese children.
Methods and Results
This case-control study of 150 overweight children with biopsy-proven NAFLD and 150 overweight children without NAFLD compared rates of metabolic syndrome using Adult Treatment Panel III criteria. Cases and controls were well matched in age, sex, and severity of obesity. Children with NAFLD had significantly higher fasting glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, triglycerides, systolic blood pressure, and diastolic blood pressure than overweight and obese children without NAFLD. Subjects with NAFLD also had significantly lower high-density lipoprotein cholesterol than controls. After adjustment for age, sex, race, ethnicity, body mass index, and hyperinsulinemia, children with metabolic syndrome had 5.0 (95% confidence interval, 2.6 to 9.7) times the odds of having NAFLD as overweight and obese children without metabolic syndrome.
Conclusions
NAFLD in overweight and obese children is strongly associated with multiple cardiovascular risk factors. The identification of NAFLD in a child should prompt global counseling to address nutrition, physical activity, and avoidance of smoking to prevent the development of cardiovascular disease and type 2 diabetes.
Keywords: blood pressure, lipids, liver, obesity, pediatrics
Nonalcoholic fatty liver disease (NAFLD), characterized by the accumulation of large droplets of triglycerides within hepatocytes in the absence of chronic alcohol consumption, is the most common cause of pediatric liver disease.1 In adults, increasing evidence supports the hypothesis that fat accumulation within organs such as the liver is a major step in the development of insulin resistance. The specific clinical entity of NAFLD is increasingly diagnosed and has been proposed to be a component of metabolic syndrome.2–5
The metabolic syndrome is a clustering of risk factors for the development of cardiovascular disease and type 2 diabetes mellitus.6 The key components of metabolic syndrome are central obesity, atherogenic dyslipidemia, impaired glucose tolerance, and elevated blood pressure.7 The underlying pathophysiology is believed to be resistance to insulin-mediated glucose disposal and compensatory hyperinsulinemia. Most children with features of metabolic syndrome are obese.8 However, previous studies suggest that only 10% to 30% of overweight or obese children fulfill the criteria for metabolic syndrome.9–12 Because the severity of obesity does not sufficiently explain why some children do or do not have metabolic syndrome, it is important to identify other factors that explain this difference. The ectopic deposition of fat in the liver may be 1 such factor.13
In children, NAFLD has been consistently associated with obesity, insulin resistance, and hypertriglyceridemia. Thus, it is logical to anticipate a relationship with metabolic syndrome. However, pediatric data in children with NAFLD are limited regarding metabolic syndrome. Therefore, the objective of this study was to determine the association between NAFLD and the presence of metabolic syndrome in overweight and obese children. Our hypothesis was that metabolic syndrome increases the risk of NAFLD among overweight and obese children.
Methods
Study Design
We performed a case-control study of overweight and obese children with and without NAFLD. An overweight and obese control group was used because the vast majority of children with clinical NAFLD also are overweight or obese.14 On the basis of the known differences in the rates of pediatric NAFLD by gender,1,14,15 we choose a priori to stipulate a 1:1 matching of boys and girls in both groups. To minimize ambiguity in assignment as a case or control, inclusion required a clinical determination of the presence or absence of NAFLD. Subjects were ascertained from a prospective clinical research database of children 5 to 17 years of age referred to a pediatric gastroenterology clinic for obesity and/or suspected NAFLD. The parent(s) of all subjects provided written informed consent. Written assent was obtained for all subjects ≥7 years of age. The protocol was approved by the institutional review boards of the University of California, San Diego and Rady Children's Hospital San Diego.
Cases and Controls
Cases were children with biopsy-proven NAFLD. The diagnosis of NAFLD was based on liver biopsy with ≥5% hepatocytes containing macrovesicular fat16 and exclusion of other causes of chronic liver disease, including hepatitis B (hepatitis B surface antigen), hepatitis C (hepatitis C antibody), α-1 antitrypsin deficiency (serum α-1 antitrypsin level and histology), autoimmune hepatitis (antinuclear antibody, anti-smooth muscle antibody, and histology), Wilson's disease (serum ceruloplasmin), drug toxicity, total parenteral nutrition, and chronic alcohol intake (clinical history).
The control group comprised overweight and obese children evaluated for weight management who were classified as not having NAFLD. The absence of NAFLD was defined by the combination of having normal alanine aminotransferase and aspartate aminotransferase and the absence of hepatomegaly.17–19 To improve the negative predictive value of serum aminotransferase activity, we used a more restrictive threshold than many prior pediatric studies; thus, a value of <30 U/L was defined as normal.20 With this combination of strategies, ≈80% to 90% of controls were expected to be accurately classified as not having NAFLD.21–23
Clinical Data Collection
Age and sex were recorded. Each child's race and ethnicity were self-identified by the parent(s). Physical examination included measurements of height, weight, waist circumference, systolic blood pressure, and diastolic blood pressure. After 5 minutes of seated rest, blood pressure was measured twice from the right arm of the seated child with an automated sphygmomanometer with 1 minute of rest between measurements. The average of the 2 measures was recorded. Cuff sizes were selected so that the cuff bladder encircled at least 80% of the mid-upper arm per standard protocol.24 Subjects were instructed to fast overnight for 12 hours before phlebotomy. Fasting laboratory assays included glucose, insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, alanine aminotransferase, and aspartate aminotransferase. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Subjects were classified as overweight (BMI between the 85th and 94th percentiles) or obese (BMI above the 95th percentile).25
Definitions for Cardiovascular Risk Factors and Metabolic Syndrome
Metabolic syndrome was defined as having ≥3 of the 5 factors with the following cut points: abdominal obesity (waist circumference >102 cm in boys and 88 cm in girls), high triglycerides (≥1.68 mmol/L [150 mg/dL]), low HDL cholesterol (<1.03 mmol/L [40 mg/dL] for boys and <1.29 mmol/L [50 mg/dL] for girls), elevated blood pressure (systolic blood pressure ≥135 mm Hg, diastolic blood pressure ≥85 mm Hg), and impaired fasting glucose (≥5.6 mmol/L [100 mg/dL]).7 In addition to the components of metabolic syndrome, hypercholesterolemia was assessed. The thresholds used were total cholesterol ≥5.17 mmol/L (200 mg/dL) and LDL cholesterol ≥3.36 mmol/L (130 mg/dL). Hyperinsulinemia was defined as fasting insulin ≥20 U.26 Insulin resistance was calculated from the homeostasis model assessment of insulin resistance defined as follows: fasting insulin (μU/mL)×[fasting glucose (mmol/L)/22.5].27
Data Analysis
Data are expressed as mean±SD or medians with interquartile ranges. Continuous variables were analyzed with Student's t test; the Mann–Whitney U test was used for nonparametric measures. The Pearson χ2 test was used to test for differences in proportions. For waist circumference, we tested the difference between groups with a 3-way ANCOVA adjusted for age and sex. To examine the association between NAFLD and metabolic syndrome, a series of logistic regression models were tested. The first model was a univariate analysis with subsequent models controlling for sequential demographic and then biological confounders. These models adjusted for age and sex (model 2), race and ethnicity (model 3), BMI (model 4), and finally hyperinsulinemia (model 5). Significance was defined at α=0.05. Analyses were performed with SAS statistical software (version 9.0, SAS Institute, Cary, NC).
In addition, sensitivity analyses were performed to test the effect of misclassification of controls on the outcome of metabolic syndrome. A Monte Carlo simulation was performed with 1000 replications per model. We ran each replication with random assignment of 10% and then 20% of the non-NAFLD group and put them into the NAFLD group. To assess the best- and worst-case possibilities, another sensitivity analysis determined the odds ratio for NAFLD when a fixed portion of controls (20%) either uniformly with or without metabolic syndrome were reclassified as cases.
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Demographics and Clinical Features
By design, there were equal numbers of boys and girls in the groups with (n=150) and without (n=150) NAFLD. As shown in Table 1, the groups also were similar in age and severity of obesity. The mean age for all subjects was 12.7 years. Consistent with the epidemiology of pediatric obesity and pediatric NAFLD, the distribution of race and ethnicity was significantly (P<0.001) different between groups, with more Hispanic and Asian children in the fatty liver group and more white and black children in the control group. All children were overweight, with 96% of subjects classified as obese. Moreover, a majority of subjects in each group (NAFLD, 57%; controls, 54%) had a BMI above the 99th percentile. The distribution of total adiposity is shown in Figure 1 and of central adiposity is shown in Figure 2. There were no significant differences in mean or median values between cases and controls for BMI z score or waist circumference.
Table 1.
Characteristics of Study Population by Liver Status
| Characteristic | Normal Liver (n=150) | NAFLD (n=150) | P |
|---|---|---|---|
| Age, mean (SD), y | 12.8 (2.9) | 12.5 (2.6) | 0.25 |
| Sex, n (%) | … | ||
| Boys | 75 (50) | 75 (50) | |
| Girls | 75 (50) | 75 (50) | |
| Race/ethnicity, n (%) | <0.001 | ||
| Black, non-Hispanic | 31 (21) | 6 (4) | |
| Hispanic | 67 (45) | 99 (66) | |
| White, non-Hispanic | 45 (30) | 30 (20) | |
| Other, non-Hispanic | 7 (5) | 15 (10) | |
| Weight, mean (SD), kg | 89.3 (36.0) | 85.2 (23.0) | 0.24 |
| Height, mean (SD), cm | 157.5 (14.1) | 157.7 (12.0) | 0.85 |
| BMI, kg/m2 | |||
| Mean (SD) | 35.0 (10.2) | 33.8 (6.5) | 0.24 |
| z score, mean (SD) | 2.36 (0.39) | 2.36 (0.38) | 0.55 |
| Percentile, mean (SD) | 98.7 (1.3) | 98.5 (2.2) | 0.55 |
| Alanine aminotransferase, mean (SD), U/L | 20 (5) | 87 (47) | <0.001 |
| Aspartate aminotransferase, mean (SD), U/L | 25 (8) | 58 (31) | <0.001 |
Figure 1.
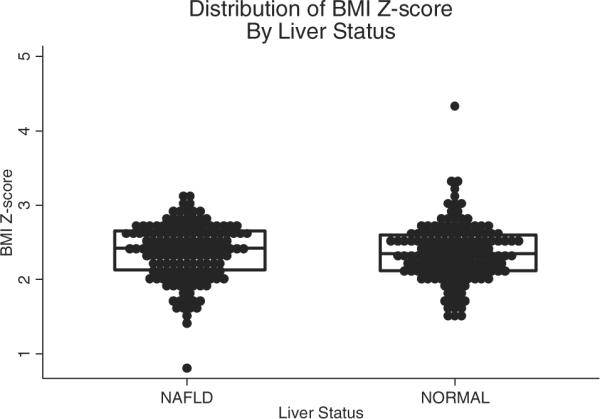
Distribution of BMI z score among obese youth with and without NAFLD. Lines and box represent the medians and interquartile ranges.
Figure 2.
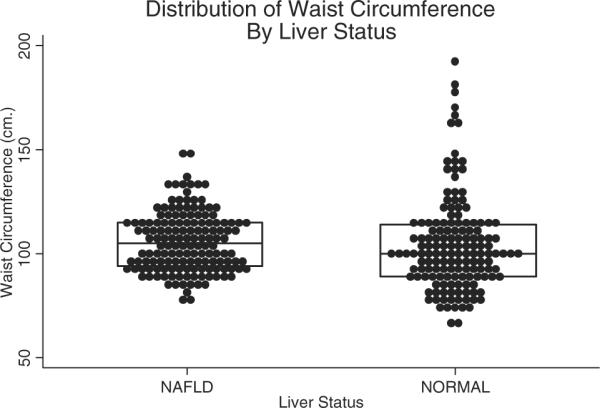
Distribution of waist circumference among obese youth with and without NAFLD. Lines and boxes represent the median and interquartile ranges.
Cardiovascular Risk Factors: Absolute Values
Waist circumference was further evaluated with adjustments for age and sex. Again, there was no significant difference between children with and without NAFLD (Table 2). However, there were significant differences between groups for all of the other metabolic factors. Specifically, children with NAFLD had higher glucose, insulin, systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, and triglycerides than obese children without NAFLD. Subjects with NAFLD also had significantly lower HDL cholesterol than controls.
Table 2.
Cardiovascular Risk Factor values by Liver Status in Obese Children
| Factor | Normal Liver (n=150) | NAFLD (n=150) | P |
|---|---|---|---|
| Abdominal obesity | |||
| Waist circumference, mean (SD), cm | 104 (23) | 106 (23) | 0.127* |
| Dyslipidemia, mean (SD), mmol/L | |||
| Total cholesterol | 4.34 (0.78) | 4.68 (0.83) | <0.001 |
| LDL cholesterol | 2.64 (0.7) | 2.87 (0.77) | 0.01 |
| HDL cholesterol | 1.11 (0.26) | 1.01 (0.28) | 0.003 |
| Triglycerides, median (IQR)† | 1.01 (0.57) | 1.64 (0.85) | <0.001 |
| Hypertension, mean (SD), mm Hg | |||
| Systolic blood pressure | 117 (13) | 123 (12) | 0.001 |
| Diastolic blood pressure | 66 (8) | 68 (8) | 0.02 |
| Insulin resistance | |||
| Glucose, mean (SD), mmol/L | 4.83 (0.49) | 5.1 (1.22) | 0.03 |
| Insulin, median (IQR), pmol/L† | 120 (114) | 180 (138) | <0.001 |
| HOMA-IR, median (IQR)† | 4.3 (4.5) | 6.6 (5.4) | <0.001 |
IQR indicates interquartile range; HOMA, homeostasis model assessment of insulin resistance.
Probability value of difference between groups when waist is controlled for age and sex.
Variables that were not normally distributed were tested with the Mann-Whitney U test. Their medians and interquartile ranges (IQRs) were reported.
Metabolic Syndrome and Liver Status
As shown in Table 3, when dichotomous cut points were applied, children with NAFLD had a significantly higher frequency of central obesity, dyslipidemia, hypercholesterolemia, elevated blood pressure, and impaired fasting glucose. All children with NAFLD had at least 1 metabolic syndrome factor. As shown in Figure 3, the distribution of metabolic syndrome features in children with NAFLD was significantly (P<0.001) shifted to the right, with more features present than in obese children without NAFLD. Moreover, children with NAFLD were significantly (P<0.001) more likely (50% versus 15%) to have metabolic syndrome than obese children without NAFLD.
Table 3.
Prevalence of Metabolic Factors by Liver Status in Obese Children
| Factor | Normal Liver (n=150) | NAFLD (n=150) | P |
|---|---|---|---|
| Abdominal obesity | |||
| Waist circumference >102 cm boys, >88 cm girls, % | 60 | 74 | 0.010 |
| Dyslipidemia | |||
| Total cholesterol ≥5.17 mmol/L, % | 14 | 26 | 0.009 |
| LDL ≥3.36 mmol/L, % | 13 | 23 | 0.016 |
| HDL <1.03 mmol/L boys, <1.29 mmol/L girls, % | 55 | 72 | 0.002 |
| Triglycerides ≥1.68 mmol/L, % | 12 | 46 | <0.001 |
| Elevated blood pressure | |||
| SBP ≥130 and/or DBP 85 mm Hg, % | 16 | 32 | 0.001 |
| Impaired fasting glucose | |||
| Glucose ≥5.6 mmol/L, % | 3 | 19 | <0.001 |
| Metabolic syndrome, % | 15 | 50 | <0.001 |
SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Figure 3.
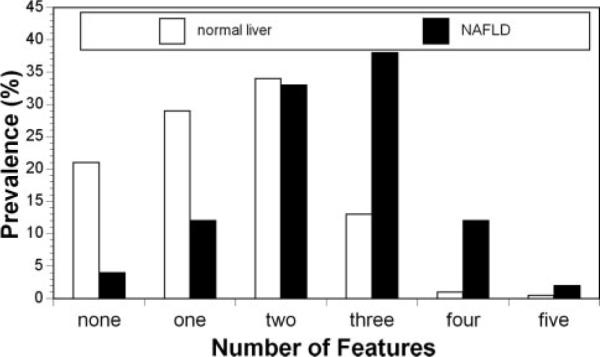
Distribution of features of metabolic syndrome in obese youth with and without NAFLD. Children with NAFLD have significantly (P<0.001) more features.
The unadjusted odds ratio for children with metabolic syndrome having NAFLD was 5.5 (95% confidence interval [CI], 3.2 to 9.5; Table 4). After adjustment for age, sex, race, ethnicity, BMI, and hyperinsulinemia, children with metabolic syndrome had 5.0 (95% CI, 2.6 to 9.7) times the odds of having NAFLD as overweight and obese children without metabolic syndrome. In the fully adjusted model, the Monte Carlo simulation to control for misclassification using random assignment yielded an odds ratios of 4.22 (95% CI, 4.18 to 4.25) for a 10% misclassification rate and 3.75 (95% CI, 3.70 to 3.79) for a 20% misclassification. In the sensitivity analysis for the extreme scenarios in which 20% of controls were falsely labeled as not having NAFLD and all of these children had [162.17 (95% CI, 27.19 to ∞) or did not have [3.01; 95% CI, 1.75 to 5.18) metabolic syndrome, the odds ratios remain significant (P<0.001).
Table 4.
Logistic Regression Model for NAFLD in Obese Children With and Without Metabolic Syndrome
| Model | Odds Ratio | 95% CI | P |
|---|---|---|---|
| 1 | 5.5 | 3.2–9.5 | <0.001 |
| 2 | 7.4 | 4.1–13.6 | <0.001 |
| 3 | 6.8 | 3.6–12.6 | <0.001 |
| 4 | 6.8 | 3.7–12.7 | <0.001 |
| 5 | 5.0 | 2.6–9.7 | <0.001 |
Children with metabolic syndrome vs the reference group of obese children without metabolic syndrome.
Model 1, NAFLD, unadjusted. Model 2, model 1 with correction for age and sex. Model 3, model 2 with correction for race and ethnicity, Model 4, model 3 with correction for BMI. Model 5, model 4 with correction for hyperinsulinemia.
Discussion
We performed a case-control study in a large clinical sample of overweight and obese children and adolescents with and without NAFLD. Children with biopsy-proven NAFLD had higher absolute values of cardiovascular risk markers and were more likely to exceed dichotomous thresholds for increased cardiovascular risk. In addition, after controlling for established demographic and biological risk factors, we found that overweight and obese children with metabolic syndrome had greater odds of having NAFLD than overweight and obese children without metabolic syndrome.
These data substantially extend the knowledge base of metabolic syndrome in children with NAFLD. In a cross-sectional analysis of adolescents in the Korean National Health and Nutrition Examination Survey, the odds of having abnormal serum alanine aminotransferase activity were increased by the presence of central obesity, elevated serum triglycerides, or low HDL cholesterol levels.28 In a substudy of 72 obese adolescents seen at the Yale Pediatric Obesity Clinic, 14 of 23 subjects with fatty liver detected by magnetic resonance imaging met modified adolescent criteria for metabolic syndrome.22 In contrast, only 11 of 49 subjects with a hepatic fat fraction <5.5% met the modified criteria for metabolic syndrome. The present study demonstrates that obese children and adolescents with a definitive diagnosis of NAFLD have a more severe cardiovascular risk profile than age-, sex-, and BMI-matched peers. In adults, hepatic steatosis has been shown to be associated with hepatic insulin resistance and features of metabolic syndrome independently of the amount of visceral adipose tissue.29,30 Taken together, these data illustrate that fat accumulation in the liver may play a more important role than obesity itself in determining the risk for “weight-related” metabolic comorbidities. Thus, in children and adolescents, NAFLD may serve as a marker to stratify the cardiovascular risk of overweight and obese patients.
In a study of Japanese university students ≈8 years older than our study population, those with liver ultrasonography consistent with fatty liver had increased arterial stiffness as measured by ankle brachial pulse-wave velocity compared with students without evidence of fatty liver.31 Similarly, in young obese Italian children, those with liver ultrasonography consistent with fatty liver had greater carotid artery intima-media thickness than those with normal liver ultrasonography.32 The belief that NAFLD is associated with cardiovascular disease is further supported by epidemiological data on cardiovascular morbidity in adults. Among 129 adults with biopsy-proven NAFLD, survival was lower than in a matched reference population mainly because of higher mortality from cardiovascular disease.33 Targher and colleagues34 observed that adults with type 2 diabetes and ultrasound evidence of NAFLD had a significantly increased incidence of cardiovascular events compared with controls with diabetes and without NAFLD. Thus, we believe that children with NAFLD are likely to be a high-risk group for future cardiovascular events, but it remains to be determined whether NAFLD or metabolic syndrome confers additional risk beyond traditional cardiovascular risk factors.
The identification of NAFLD in a child should prompt consideration of cardiovascular health. Global counseling should address nutrition, physical activity, and avoidance of smoking. There are insufficient data on the use of medications in this population. However, there are many potential targets for medication, and future studies need to address the desirability of addressing individual problems such as hypertension pharmacologically versus targeting a broader issue such as obesity or insulin resistance. Many children with NAFLD, if unable to achieve sufficient improvement via lifestyle modification, will likely receive pharmacotherapy for such comorbidities as hypertension or dyslipidemia. However, it is not clear whether these treatments have a beneficial, neutral, or deleterious effect on the natural history of NAFLD.
Fatty liver also may prove to be a mediator of metabolic syndrome. In the liver, fatty acids may be either oxidized or esterified to form triglycerides, which are then either stored in the cytosol or secreted in very-low-density lipoprotein.35 The intracellular triglyceride storage depot likely serves as a temporary buffer for potentially toxic free fatty acids when their delivery to the liver exceeds its oxidative and very-low-density lipoprotein secretory capacity.36 The expanded liver fatty acid pool leads to increased mitochondrial and peroxisomal β-oxidation, which produces reactive oxygen species. This may, in turn, promote both a local proinflammatory state leading to progressive liver injury and the release of proinflammatory cytokines, which stimulate production of suppressors of cytokine signaling proteins. An increase in suppressors of cytokine signaling-3 in the liver leads to persistent hyperinsulinemia, which further exacerbates insulin resistance.37 Hyperinsulinemia stimulates the transcription factor sterol regulatory element-binding protein-1c,38 which leads to activation of lipogenic genes and a decrease in fatty oxidation. Additional overproduction of fatty acid and continued lipotoxicity result in further insulin resistance, creating a vicious cycle. Fatty liver also leads to hepatic insulin resistance by stimulating gluconeogenesis and activating protein kinase C and Jun N-terminal kinase 1, which may interfere with tyrosine phosphorylation of IRS-1 and IRS-2 and impair the ability of insulin to activate glycogen synthase.39 Thus, fatty liver contributes to the dysregulation of both glucose and lipid metabolism.
The development of NAFLD in children may precede the development of type 2 diabetes mellitus. At the time of diagnosis, ≈8% to 10% of children with NAFLD have diabetes.5,40 In contrast, by the time type 2 diabetes mellitus is diagnosed in children, approximately one half have suspected fatty liver because of elevated alanine aminotransferase.41 Hepatocyte triglyceride storage causes acquired insulin signaling defects, leading to insulin resistance, glucose intolerance, and type 2 diabetes mellitus.42 The high rates of overweight, insulin-resistant children with NAFLD meeting the criteria for metabolic syndrome suggest that a large number of these children will go on to develop diabetes. This belief is supported by a cohort study from Sweden of adults with biopsy-proven NAFLD who had a 9% prevalence of diabetes at baseline.33 After nearly 14 years of follow-up, the majority (78%) of these patients developed impaired glucose tolerance or diabetes.
The strengths of the present study were the large series of biopsy-proven pediatric NAFLD and the use of a control group that was well matched for age, gender, and severity of obesity. The control group met a clinically accepted standard for the absence of NAFLD. The limitations of the study included the likely misclassification of some subjects having NAFLD as normal controls. We conducted sensitivity analyses to account for misclassification rates of 10% and 20% and found that the association between metabolic syndrome and NAFLD remained strong. The cross-sectional nature of the study allowed only association rather than causation. Moreover, these data cannot be used to determine the timing of development of metabolic syndrome in children with NAFLD. Although subjects were well matched for gender and age, it is possible that some difference in insulin sensitivity between cases and controls could have been attributable to differences in Tanner stage, which was not assessed. In addition, it is not clear to what extent these data are generalizable to overweight black children because they are known to have high rates of diabetes yet low rates of NAFLD.1,43 Finally, many issues regarding the definition and significance of metabolic syndrome in children and adolescents remain unresolved. The true risk of pediatric NAFLD for cardiovascular events can be determined only by long-term longitudinal cohort studies with detailed baseline characterization and clinically relevant end points.
Conclusions
NAFLD in overweight and obese children was strongly associated with metabolic syndrome. The association was independent of both BMI and hyperinsulinemia. For children with NAFLD, a goal must be the prevention of end-stage liver disease. In addition, children with NAFLD may be at a higher risk for cardiovascular disease than children without NAFLD. These data should be used to increase awareness of this subset of overweight and obese children and to guide future studies aimed at elucidating natural history and treatment. Such studies will help to further define the role of fatty liver as a marker or possible mediator of risk for cardiovascular events.
CLINICAL PERSPECTIVE.
There are >10 million obese children in the United States. Obese children differ in their risk for cardiovascular disease, which may be underappreciated in part because specific comorbidities are typically the focus of different disciplines such as cardiology, endocrinology, gastroenterology, or nephrology. When working with an obese child, one should consider how any single abnormality may interact with another. Metabolic syndrome is a clustering of risk factors for the development of cardiovascular disease and type 2 diabetes mellitus. Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease in children. Both metabolic syndrome and NAFLD are associated with obesity. We performed a case-control study in a large clinical sample of overweight and obese children and adolescents with and without NAFLD. NAFLD was strongly associated with metabolic syndrome. The association was independent of both body mass index and hyperinsulinemia. Thus, when one detects features of metabolic syndrome in an obese child, one must be aware of the risk for NAFLD. Similarly, the presence of NAFLD may serve to stratify risk. The identification of NAFLD in a child should prompt consideration of cardiovascular health. Global counseling should address nutrition, physical activity, and avoidance of smoking. In an integrated model of disease management, therapeutic goals for NAFLD should include not only the prevention of end-stage liver disease but also the prevention of cardiovascular disease and diabetes.
Acknowledgments
Sources of Funding This work was supported in part by grants from the Rest Haven Foundation (Dr Schwimmer), National Institute of Diabetes, Digestive and Kidney Diseases (R21-DK71486)(Dr Schwimmer), M01 RR000827 from the National Center for Research Resources of the National Institutes of Health for the General Clinical Research Center at UCSD, and the National Heart, Lung and Blood Institute (K-23 HL086946) (Dr Cook).
Footnotes
Disclosures None.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353–358. doi: 10.1016/s0261-5614(99)80015-6. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 6.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, the American Heart Association and National Heart, Lung, and Blood Institute Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom E, Hernell O, Persson LA, Vessby B. Insulin resistance syndrome in adolescents. Metabolism. 1996;45:908–914. doi: 10.1016/s0026-0495(96)90168-7. [DOI] [PubMed] [Google Scholar]
- 9.Csabi G, Torok K, Jeges S, Molnar D. Presence of metabolic cardiovascular syndrome in obese children. Eur J Pediatr. 2000;159:91–94. doi: 10.1007/pl00013812. [DOI] [PubMed] [Google Scholar]
- 10.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 11.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 13.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–648. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 15.Schwimmer JB, McGreal N, Deutsch R, Finegold M, Lavine JE. The influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 17.Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, Freemark M, Gruters A, Hershkovitz E, Iughetti L, Krude H, Latzer Y, Lustig RH, Pescovitz OH, Pinhas-Hamiel O, Rogol AD, Shalitin S, Sultan C, Stein D, Vardi P, Werther GA, Zadik Z, Zuckerman-Levin N, Hochberg Z, the Obesity Consensus Working Group Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 18.Barlow S, the Expert Committee Expert Committee recommendations on the assessment, prevention, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein M, Mogren J, Mogren C, Cox S, Jennings R. Undetected hepatomegaly in obese children by primary care physicians: a pitfall in the diagnosis of pediatric nonalcoholic fatty liver disease. Clin Pediatr. 2005;44:135–141. doi: 10.1177/000992280504400205. [DOI] [PubMed] [Google Scholar]
- 20.Van der Poorten D, Kenny DT, Butler T, George J. Liver disease in adolescents: a cohort study of high-risk individuals. Hepatology. 2007;46:1750–1758. doi: 10.1002/hep.21918. [DOI] [PubMed] [Google Scholar]
- 21.Radetti G, Kleon W, Stuefer J, Pittschieler K. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Pediatr. 2006;95:833–837. doi: 10.1080/08035250500449890. [DOI] [PubMed] [Google Scholar]
- 22.Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papa-demetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M, Seyal AA, Caprio S. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer JB. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 2007;27:312–318. doi: 10.1055/s-2007-985075. [DOI] [PubMed] [Google Scholar]
- 24.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern EB. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 25.Committee on Prevention of Obesity in Children and Youth . Preventing Childhood Obesity Health in the Balance. National Academies Press; Washington, DC: 2005. [PubMed] [Google Scholar]
- 26.Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, Bazzarre T. Cardiovascular health in childhood: a statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106:143–160. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–1051. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 29.Seppala-Lindroos A, Vehkavaara S, Hakkinen A-M, Goto T, West-erbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 30.Tiikkainen M, Tamminen M, Hakkinen A-M, Bergholm R, Vehkavaara S, Halavaara J, Teramo K, Rissanen A, Yki-Jarvinen H. Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Obesity Res. 2002;10:859–867. doi: 10.1038/oby.2002.118. [DOI] [PubMed] [Google Scholar]
- 31.Shiotani A, Motoyama M, Matsuda T, Miyanishi T. Brachial-ankle pulse wave velocity in Japanese university students. Intern Med. 2005;44:696–701. doi: 10.2169/internalmedicine.44.696. [DOI] [PubMed] [Google Scholar]
- 32.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423–427. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 33.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 35.Gibbons GF, Bartlett SM, Sparks CE, Sparks JD. Extracellular fatty acids are not utilized directly for the synthesis of very-low-density lipoprotein in primary cultures of rat hepatocytes. Biochem J. 1992;287:749–753. doi: 10.1042/bj2870749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 37.Ueki K, Kondo T, Tseng Y-H, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101:10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferré P, Foretz M, Azzout-Marniche D, Bécard D, Foufelle F. Sterol-regulatory-element-binding protein 1c mediates insulin action on hepatic gene expression. Biochem Soc Trans. 2001;29:547–552. doi: 10.1042/bst0290547. [DOI] [PubMed] [Google Scholar]
- 39.Samuel VT, Liu Z-X, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in nonalcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 40.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 41.Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr. 2005;41:94–98. doi: 10.1097/01.mpg.0000164698.03164.e5. [DOI] [PubMed] [Google Scholar]
- 42.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]


