Abstract
Diabetic gastroparesis is a disorder that occurs in both type 1 and type 2 diabetes. It is associated with considerable morbidity among these patients and resultant economic burden on the health system. It is primarily a disease seen in middle aged women though the increased predisposition in women still remains unexplained. Patients often present with nausea, vomiting, bloating, early satiety, and abdominal pain. The pathogenesis of this complex disorder is still not well understood but involves abnormalities in multiple interacting cell types including the extrinsic nervous system, enteric nervous system, Interstitial Cells of Cajal (ICC), smooth muscles and immune cells. The primary diagnostic test remains gastric scintigraphy though other modalities such as breath test, capsule, ultrasound, MRI and SPECT imaging show promise as alternative diagnostic modalities. The mainstay of treatment for diabetic gastroparesis has been antiemetics, prokinetics, nutritional support and pain control. In recent years, gastric stimulation has been used in refractory cases with nausea and vomiting. As we better understand the pathophysiology, newer treatment modalities are emerging which aim to correct the underlying defect. In this review we highlight what has been learned about diabetic gastroparesis in the past 5 years. We review the epidemiology, pathogenesis, diagnosis and treatment of diabetic gastroparesis focusing on the areas that are still controversial and those that require more studies. We also focus on advances in our understanding of the cellular changes that underlie development of diabetic gastroparesis highlighting new opportunities for targeted therapy.
Keywords: Diabetic gastroparesis, Interstitial Cells of Cajal (ICC), Heme oxygenase 1, neuronal nitric oxide synthase (nNOS)
Introduction
Diabetic gastroparesis is a well established complication of diabetes, first reported in 1958.1 Gastroparesis is defined as a syndrome characterized by abnormal gastric function resulting in delayed gastric emptying in the absence of mechanical obstruction.2 What was first thought to be a rare complication that occurs only in type 1 diabetes is now known to occur in both type 1 and type 2 diabetes3 and to occur more frequently than previously assumed.3 However, the increasing availability of non- invasive tools to measure gastric emptying has not only increased our ability to diagnose the disease, but has also uncovered significant gaps in our understanding of the pathophysiology of the disease, the relationship between slow gastric emptying and severity of symptoms, and the effectiveness of current therapies. The relatively recent realization of our poor understanding of this disease has resulted in a number of human and animal studies aimed at addressing these gaps in our knowledge. This review is aimed at summarizing what has been learned in the past 5 years, highlighting areas that are still controversial and suggesting areas for future studies.
Epidemiology of diabetic gastroparesis
Gastroparesis is increasingly recognized as a significant health problem. The number of hospitalizations for gastroparesis increased by nearly 158% from 1995 to 2004.4 Patients admitted with gastroparesis require more procedures, have a longer hospital stay and incur higher charges than the mean.4 Diabetic gastroparesis was initially described by Kassander in 1958 as “gastroparesis diabeticorum” in patients with type 1 diabetes with gastric retention. Though diabetic gastroparesis has traditionally been associated with advanced type 1 diabetes with poor glycemic control, it is increasingly being recognized in patients with type 2 diabetes. In a tertiary referral study of diabetic patients undergoing gastric scintigraphy for upper gastrointestinal symptoms, approximately equal number of patients had type 1 and type 2 diabetes.5 There are several population based studies which now show an increased occurrence of symptoms suggestive of upper gastrointestinal dysmotility in patients with type 2 diabetes compared with non diabetic controls.3, 6, 7 In a population based survey of 423 diabetic patients (94.8% type 2 diabetes), a significantly higher incidence of upper GI symptoms in diabetic patients were reported.3
Given the definition of gastroparesis, the diagnosis of gastroparesis requires a test to measure the rate of gastric emptying. The prevalence of delayed gastric emptying in patients with diabetes has been reported to be between 28 to 65%.8, 9 However, with the increased availability of gastric emptying tests, it is now well established that there are subsets of patients with delayed gastric emptying and no symptoms,10 accelerated gastric emptying and identical symptoms to patients with delayed gastric emptying, and symptoms with normal gastric emptying.5 From a clinical perspective, the term diabetic gastroparesis is therefore better limited to the combination of a delay in gastric emptying of solids in the absence of obstruction and upper gastrointestinal symptoms including nausea, vomiting, bloating, and early satiety.11 Pain is often overlooked but can be a predominant symptom in a subset of patients with gastroparesis.12 A recent community based study from Olmsted County in the United States using delayed gastric emptying and typical symptoms as criteria for diagnosis showed a cumulative incidence of 4.8% in type1 diabetes and 1% in type 2 diabetes as compared to 0.1% in controls.13
The demographics of diabetic gastroparesis, a disease affecting predominantly young females of child bearing age, makes diabetic gastroparesis a disease associated with considerable morbidity and societal impact.13, 14 In a single center study of patients with gastroparesis seen over 6 years, the mean age of onset was 34 years and 82% of the patients were women.11 The reasons for the female preponderance of gastroparesis remain largely unknown. Gastric emptying is overall slower in diabetic females as compared to diabetic males.9 Some studies have shown that gastric emptying is also slower in normal females.15 This raises the possibility that females are closer to the threshold where a decrease in gastric emptying becomes problematic. However, a large randomized controlled study failed to show any alteration in gastric transit in post menopausal females receiving estrogen and progesterone supplementation.16 Recently, differences in neuronal nitric oxide synthase (nNOS) dimerization between females and males has been proposed as an alternative reason for the striking gender difference.17 While diabetic gastroparesis is associated with considerable morbidity, increased mortality on the other hand is usually not due to diabetic gastroparesis but is rather related to cardiovascular and renal disease that often co-exist.18
Pathogenesis
Our understanding, at a cellular level, of the pathogenesis of diabetic gastroparesis has taken a marked step forward in the past 5 years. Additionally, at the patient level, increased knowledge has resulted in the need to re-evaluate long held tenets. An increasingly controversial area is the relationship between symptoms and gastric emptying. Most recent studies show that the correlation between symptoms and gastric emptying is poor.5, 8, 9, 19 These data have been used to suggest that gastric emptying be no longer used to define diabetic gastroparesis. However, given the increased understanding of the complexity of gastric emptying and of the association between defined changes at a cellular level and changes in gastric emptying, it is possible that the poor correlation reflects the non-specific nature of the symptoms and the inability of current tests to measure different aspects of gastric emptying. Gastric emptying requires coordinated actions of the proximal stomach, which serves as a reservoir that delivers food to the gastric body and antrum which in turn triturate and mix food, the pylorus which regulates the size of the food particles that leave the stomach, the proximal small intestine which feeds back to regulate gastric emptying and hormones and peptides that are released during normal digestion.20 This complex process requires the interplay of the extrinsic nervous system, enteric nervous system, smooth muscle, Interstitial Cells of Cajal (ICC) and immune cells.(Fig. 1A) As new tests are introduced that can separate out segmental as well as global function, it is likely that more will be learned about which, if any, symptom, is associated with which aspect of gastric emptying.
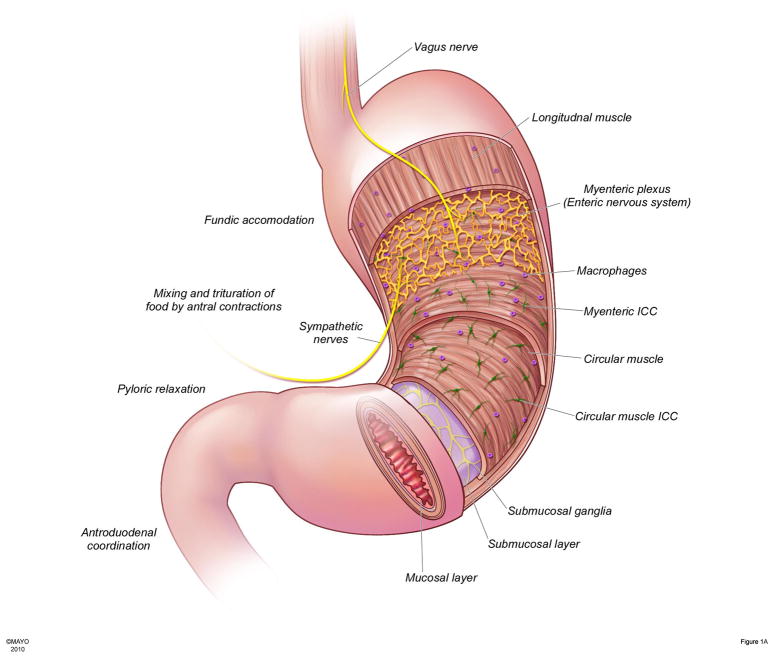
Figure 1.
Figure 1A: Anatomy of the human stomach as it relates to normal gastric physiology in the postprandial state
Normal gastric function requires interplay of several cell types including extrinsic nerves, enteric nervous system, immune cells, interstitial cells of Cajal (ICC) and smooth muscle. The fundus is the receptive organ in the stomach and accommodates a meal without increasing intragastric pressure. Vagal efferents through release of NO and other neurotransmitters are primarily responsible for receptive relaxation of the fundus. ICC generate slow waves which are rapidly propagated to smooth muscle circumferentially and slowly propagated longitudinally resulting in circumferential contractions that sweep the body and antrum. Smooth muscle cells secrete insulin like growth factor (IGF-1) which exerts protective effects on ICC. Macrophages are normally resident in the stomach. In conditions associated with increased oxidative stress, a subclass of macrophages express antioxidant enzymes such as heme oxygenase-1. Heme oxygenase-1 protects against the deleterious effects of increased oxidative stress through generation of products such as carbon monoxide (CO). Antral contractions triturate the food particles against a closed pylorus till they reach a size <3mm at which point they pass through the pylorus into the duodenum. Gastric emptying is also regulated by feedback form the duodenum through enterogastric reflexes and release of hormones such as cholecystokinin.
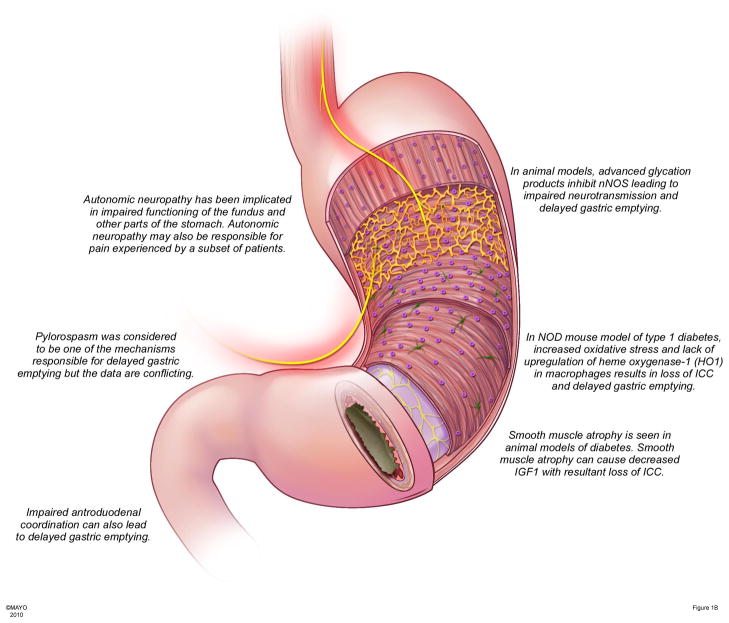
Figure 1B - Pathophysiological changes in diabetic gastroparesis
Autonomic neuropathy leads to disordered motility and function of the stomach and may underlie the pain experienced by patients. In diabetes, advanced glycation products formed due to increased reactive oxygen species can lead to loss of nNOS, impaired neurotransmission and delayed gastric emptying. Increased oxidative stress seen in diabetes is usually offset by upregulation of anti-oxidant enzymes in macrophages such as heme oxygenase-1. Loss of upregulation leads to damage and loss of ICC resulting in delayed gastric emptying. In addition smooth muscle atrophy leads to loss of IGF-1, a survival factor for ICC.
Another controversial area is the role of hyperglycemia in gastric emptying. Acute changes in blood glucose are well documented to alter gastric emptying.21 An acute increase in blood glucose decreases fundic tone, decreases the contractility of the mid and distal stomach and also alters small bowel contractile activity. Hyperglycemia has been shown to stimulate localized pyloric contraction and inhibit antral contraction resulting in delayed gastric emptying.22 Acute hyperglycemia can induce gastric myoelectric disturbances particularly tachygastria.23 In addition, hyperglycemia can also attenuate the effect of prokinetics, reducing their efficacy.24, 25 Induction of acute hypoglycemia accelerates gastric emptying.26 In contrast to these relatively clear effects, the effect of chronic hyperglycemia on gastric emptying is much less clear. There appear to be differences between chronic hyperglycemia and gastric emptying in type 1 and type 2 diabetics with varied effects of hyperglycemia on gastric emptying reported for type 2 diabetes.27, 28 The presence or absence of autonomic neuropathy can also markedly alter the effect of hyperglycemia on gastric emptying.29, 30
Changes in gastric emptying also play an important role in blood glucose homeostasis. The rate of gastric emptying is a major determinant of the initial postprandial glycemic response both in healthy individuals31 and patients with type 1 or type 2 diabetes32 and delayed gastric emptying can cause postprandial hypoglycemia in insulin treated individuals.33 Hence gastric emptying is an important consideration when developing new therapies to improve glycemia control in patients with diabetes. Another consideration is the absorption kinetics of drugs which can be influenced by changes in gastric emptying.
Autonomic neuropathy was one of the first abnormalities associated with diabetic gastroparesis. Sham feeding test, used to evaluate the integrity of the vagus nerve, shows blunted pancreatic polypeptide response as well as reduced gastric secretion in response to sham feeding in patients with diabetic gastroparesis.34 Vagus nerve dysfunction (Fig. 1B) is also thought to mediate some of the acute effects of hyperglycemia such as reduced pyloric relaxation, as a similar effect can be induced by subdiaphragmatic vagotomy.35 Morphologically, both myelinated and unmyelinated nerve fibers of the vagus nerve have been noted to be smaller in the Bio Breeding (BB) rat model of spontaneous diabetes.36, 37 Similar to the parasympathetic nervous system, changes have also been described in the axons and dendrites within the prevertebral sympathetic ganglia.38, 39 Pain, which is a predominant symptom in a subset of patients with diabetic gastroparesis, is often attributed to neuropathy but better studies are needed to test if this is indeed the case.
The enteric nervous system consists of nearly one hundred million neurons distributed in the myenteric and submucosal plexi and can operate autonomously. Normal gut function requires a balance between the release of excitatory neurotransmitters such as acetylcholine and substance P 40 and inhibitory transmitters such as nitric oxide (NO)41 and VIP. NO and the enzyme responsible for its generation, neuronal nitric oxide synthase (nNOS), have been consistently noted to be decreased in patients and animal models of diabetic gastroparesis and have therefore been proposed to be central to the development of delayed gastric emptying. (Fig. 1B) Animal studies, both in rat model of spontaneous diabetes and in STZ-induced diabetes, have shown impaired gastric relaxation and decrease in nNOS expression and activity in the myenteric plexus.42–45 The loss of nNOS expression cannot simply be explained by neuronal loss as it appears to predominantly occur without accompanying loss of gastric enteric neurons,46 although some animal studies and case reports in humans do also show neuronal cell death which would contribute to the decrease in nNOS expression.47–49 The relative preservation of enteric nerves offers a therapeutic opportunity to target the remaining nNOS expressing neurons, and/or increase nNOS expression, or target dimerization of nNOS as a treatment for diabetic gastroparesis. The regulation of nNOS is complex and can be influenced by glucose, insulin or insulin like growth factor.50, 51 Loss of nNOS expression seen in diabetes may represent inhibition of nNOS by advanced glycation products which can bind to myenteric neurons and inhibit nNOS.52 A more recent study shows gastric relaxation correlates better with the dimerized form of nNOS rather than absolute nNOS levels as seen in female diabetic rats suggesting post-translational modification is important.17
While a decrease in nNOS expression is now well established in diabetic gastroparesis, it is unclear if loss of nNOS expression on its own leads to development of delayed gastric emptying. Previous efforts to increase NO using nitroglycerin or using sildenafil to exploit the downstream effector pathway of NO have not shown any clinical benefit in humans.53, 54 In the non-obese diabetic (NOD) mouse model of type 1 diabetes, loss of nNOS expression is one of the earliest events associated with development of hyperglycemia.55 However, the loss of nNOS by itself was not predictive of developing delayed gastric emptying55 and was present irrespective of whether gastric emptying was normal or delayed.55 These data suggest that nNOS may act as a co-factor in the pathogenesis of diabetic gastroparesis or that the form of nNOS may be more relevant than the total amount of the protein. Better methods to deliver NO to the stomach, studies on dimerized versus non-dimerized nNOS are needed to clarify the role of NO in the development of delayed gastric emptying in diabetes as well as its therapeutic potential.
ICC serve multiple functions in the gastrointestinal tract. ICC generate slow waves which are then transmitted to the smooth muscles, are involved in aspects of neurotransmission, set the smooth muscle membrane potential gradient and are involved in mechanotransduction.56 Given these multiple functions, it is not surprising that alterations in ICC networks have been looked for in diabetic gastroparesis. Indeed, loss of ICC is one of the most consistent histological finding in diabetic gastroparesis.(Fig. 1B) Loss of ICC in the stomach has been well established both in animal models of diabetes as well as in humans with diabetic gastroparesis.47, 48, 57–61 The NOD mouse is the best studied model of type 1 diabetes and several studies have reported loss of ICC networks both in the corpus and antrum, as examined by Kit expression.55 Kit is a receptor tyrosine kinase expressed in ICC and mast cells in the gastrointestinal tract. More recently Ano-1 has been shown to be a marker for ICC throughout the GI tract.62 Ano-1 is a calcium-activated chloride channel expressed in ICC. In the NOD mouse model, loss of Kit expression tightly correlated with development of delayed gastric emptying.55 In that study, all mice that developed delayed gastric emptying had loss of Kit expression while all mice that were resistant to development of delayed gastric emptying had normal Kit expression. Myenteric and intramuscular ICC are decreased in the stomach of db/db mice (leptin receptor mutant), a model of type 2 diabetes.63 In streptozotocin-induced diabetic rats, significant loss of ICC was observed in the gastric antrum by 12 weeks.64 Therefore, there is a strong body of literature linking ICC loss to gastroparesis in animal models of diabetes.
Human studies are limited given the difficulty obtaining full thickness biopsies and the difficulty in obtaining control tissue from anatomically corresponding regions. This has resulted in the use of paraffin–fixed human tissue that is not optimal for examination of ICC networks. With these limitations in mind, retrospective human studies have reported significant loss of ICC in diabetic patients with gastroparesis. McCallum et. al. reported profound loss of ICC in the antrum in nine out of twenty three patients with refractory diabetic gastroparesis.65 Similarly, Iwasaki et. al. reported loss of intramuscular ICC in eight patients with severe diabetes, along with loss of nNOS positive neurons.61 To address the current limitations, the National Institute of Health in the United States has established a Gastroparesis Clinical Research Consortium (GpCRC). Patients enrolled in this study represent the largest cohort of gastroparesis patients who are being followed longitudinally. In a subset of these patients, full thickness biopsies are collected prospectively and stored appropriately. Preliminary data for this consortium suggest that about 50% of patients with diabetic gastroparesis have a significant (>50%) decrease in ICC.46
Loss of ICC has been shown to result in impaired gastric function. Loss of ICC observed in diabetic gastroparesis is associated with disruption of the generation and propagation of electrical slow waves resulting in gastric dysrhythmias. Both bradygastria and tachygastria have been seen in diabetic patients, with symptoms related to meals.66 Gastric dysrhythmias predict abnormal gastric emptying.67 Both tachygastria and bradygastria may result from ICC loss. A patchy disruption of ICC networks may result in reentrant tachy-arrhythmias as well as loss of generation of the slow waves resulting in brady-arrhythmias. A recent study reported severe ICC loss in 12 out of 34 patients with refractory diabetic gastroparesis and correlated loss of ICC with an abnormal electrogastrogram showing tachygastria.59 Similar findings have also been reported in idiopathic gastroparesis with loss of ICC suggesting that abnormal function is a result of loss of ICC rather than an effect of diabetes. In animal models of diabetes, abnormal slow wave activity has been reported in STZ-induced diabetic rats, Otsuka Long-Evans Tokushima Fatty (OLETF) rats (model of type 2 diabetes) 68 and NOD mice (model of type 1 diabetes).
The mechanism for ICC loss in diabetic gastroparesis has been the focus of recent studies. Loss of ICC results from imbalance between the processes that injure ICC networks and processes that generate and maintain ICC.56 One factor is smooth muscle atrophy as a result of relative insulopenia and IGF-1 deficiency in diabetes. This leads to depletion of smooth muscle cell-produced stem cell factor, an important ICC survival factor.58 Smooth muscle degeneration and fibrosis (Fig. 1B) has been described in diabetes.69 Also, in a rodent model of diabetes, loss of protein kinase C activation and smooth muscle dysfunction has been reported.70 Diabetes is a high oxidative stress state. Recent data obtained using the NOD model of type 1 diabetes suggests that critical to the development of diabetic gastroparesis is the loss of mechanisms that normally counteract increased oxidative stress, such as upregulation of macrophage heme oxygenase-1. This leads to loss of ICC and development of delayed gastric emptying.55 Upregulation of heme oxygenase-1 by hemin increases expression of Kit and nNOS and completely reverses the delay in gastric emptying. This finding has clinical implications as hemin can also increase heme oxygenase activity in humans.71 A preliminary study suggests that carbon monoxide mediates the protective effects of heme oxygenase-1 in vivo.72 The studies on IGF-1 and on the heme oxygenase/carbon monoxide pathways provide new avenues for development of therapies for diabetic gastroparesis based on the underlying pathogenesis.
Immune cells have recently been identified as potentially playing a role in development of diabetic gastroparesis. Upregulation of the anti-oxidant heme oxygenase-1 protects ICC from damage.55 Upregulation of heme oxygenase-1 occurs in CD206 positive cytoprotective M2 macrophages (Fig. 1B), while loss of expression of heme oxygenase-1 in these cells leads to development of delayed gastric emptying.73 In a preliminary study from the gastroparesis consortium, abnormal CD45 (a protein tyrosine phosphatase found on leucocytes and other cells of hematological origin) staining was seen in 9 out of 20 patients with diabetic gastroparesis46 suggesting that immune dysregulation may be an under appreciated feature of diabetic gastroparesis. Future studies will need to address whether the changes in macrophages seen in mice also occur in humans and whether immune cell changes are associated with defined changes in cytokines that may alter gastric function.
Diagnosis
Despite the poor correlation between currently available methods to assess global gastric emptying and symptoms, documentation of delayed gastric emptying to solids is required before a diagnosis of diabetic gastroparesis can be made. Abnormal gastric emptying still remains the only objective marker of an underlying defect in the neuromuscular apparatus of the stomach.
Scintigraphy is the most common and widely available modality for measuring gastric emptying. It is however relatively expensive, associated with some radiation exposure, and despite position papers published on the topic, still not standardized across medical centers. The standard technique involves scintigraphic determination of emptying of a solid meal. The American Neurogastroenterology and Motility society recommends use of 99mTc (technetium) sulphur colloid-labeled egg sandwich as a test meal.74 It is required that scintigraphic measurement continues to 4 hours as this has been shown to improve the accuracy of the test.74 However, several centers continue to extrapolate gastric emptying data from 90–120 minute readings. The results of tests shorter than 4 hours should not be used to make a diagnosis of gastroparesis. In spite of these limitations scintigraphy remains the test of choice and is considered the gold standard for comparison of newer diagnostic modalities. Imaging should be obtained in relaxed environments and can be obtained sitting or standing as long as the same posture is maintained throughout the test. Hyperglycemia will delay gastric emptying and therefore needs to be tested for and corrected before carrying out the test. Recent advances allow some separation of distal and proximal gastric function.75 SPECT imaging following labeling of the gastric mucosa with 99mTc is helpful in determining gastric volume and can be combined with scintigraphy to measure gastric emptying and accommodation concurrently.76, 77
Gastric emptying tests that utilize non-radioactive forms of carbon incorporated in safely ingestible food or liquid products, such as octanoic acid, and acetate, correlate well with scintigraphy78 and offer the advantage of being able to be carried out in office settings. Gastric emptying breath test using 13C, a stable (non radioactive isotope) has been well studied both in human and animal models.79, 80 The test relies on the fact that the rate limiting step in metabolism of 13C substrate is emptying of the substrate from the stomach to the intestine. The substrate is rapidly absorbed once it is in the intestine as it does not need digestion; it is catabolized in the liver and excreted as 13C in the breath. The ratio of 13C to 12C provides a reliable measure of gastric emptying rate. This test has been validated in diabetics.81 Given the non–radioactive nature of the test, it can be used to measure changes in gastric emptying over time and has a sensitivity and specificity of 75 and 86% respectively.82 A variation of the 13C octanoic acid technique is the 13C Spirulina platensis breath test. A recent study validated a gastric emptying breath test using a shelf stable 238 kcal meal consisting of freeze-dried egg mix, saltine crackers and 100mg of 13C Spirulina platensis. The test was 89% sensitive and 80% specific in identifying delayed gastric emptying using breath samples at 150 and 180 min when compared with gastric scintigraphy.83
Gastroduodenal manometry is invasive, expensive, uncomfortable, and of very limited availability. However, when available, it offers the ability to assess frequency and strength of antral and proximal intestinal contractility, antro-duodenal coordination, and of the presence or absence of phase three of the migratory motor complex. This information can help differentiate between predominantly neuropathic versus non-neuropathic processes, as well as predict tolerability of gastric or small intestinal tube feedings.84
Transabdominal ultrasonography represents a simple non-invasive technique to evaluate gastric function. However studies are still limited and the technique requires considerable technical expertise. When carried out correctly, transabdominal ultrasonography provides information on global gastric emptying, with high correlation with scintigraphy, and also of accommodation and movement of intragastric contents. 2-Dimensional ultrasound can indirectly measure gastric emptying by quantifying changes in antral area over time and studies have shown increased antral area both in the fasting state and after meals in diabetes.85 3-Dimensional ultrasound provides better information of gastric pathophysiology by allowing assessment of intragastric meal distribution and gastric volume86 but is time consuming and requires an even more experienced operator. A particular problem with transabdominal ultrasonography is that it is harder in obese patients, which can be an issue is patients with obesity-related type 2 diabetes.
Magnetic resonance imaging (MRI) of the stomach also correlates well with scintigraphy.87 It has the additional advantage over ultrasound in that it can differentiate solid and liquid components of intragastric content, secretion and air. The utility of the test is currently limited by the speed that images can be acquired using most of the current machines, the procedure cost, and the time required for interpretation. Newer, faster, higher Tesla machines and better software should make MRI, a more attractive option in the future.
Recently, a non-digestible capsule that records pH, pressure and temperature as it travels through the gastrointestinal tract has been introduced as another option to measure gastric emptying. The change in pH between the distal stomach and proximal small intestine allows documentation of egress of the capsule from the stomach and therefore documentation of the time from ingestion to arrival in the small intestine.88 The human pylorus prevents movement of particles bigger than 2–3 mm from the stomach to the small intestine; therefore emptying of the capsule likely coincides with onset of the phase III migrating motor complex. It can discriminate between normal and delayed gastric emptying with a sensitivity of 87% and specificity of 92% compared to radiopaque markers.89
A challenge to the development of new therapies is the lack of acceptance of tools in clinical trials to assess improvement on treatment by the agencies that approve new drugs. Non-radioactive-based gastric emptying techniques are one option as is the use of patient reported outcome measures such as Diabetes Bowel Symptom Questionnaire, a useful measure of gastrointestinal symptoms and glycemic control in both type1 and type2 diabetic patients90 and the Gastroparesis Cardinal Symptom index (GCSI), a patient reported outcome measure of symptoms of gastroparesis consisting of nine commonly reported symptoms.91 A further modification of the initial questionnaire, the GCSI-DD has been developed, where patients record symptoms everyday allowing clinicians to capture the daily variability of patient symptoms.92 Validation of the GCSI to meet approval agencies requirements is currently underway.
Treatment
The past 5 years have seen significant advances in our understanding of the cellular changes that give rise to diabetic gastroparesis and the introduction of several new diagnostic modalities. In contrast, there are fewer options available to treat diabetic gastroparesis than were available 5 years ago. The available treatment options include nutritional support, improvement of gastric emptying using prokinetics, symptom control and, in refractory cases, use of a gastric electric stimulator, although the use of the latter is still controversial. The paucity of approved drugs to treat diabetic gastroparesis is somewhat mitigated by an increasing number of drugs under development with different mechanisms of action.
Nutritional support is often overlooked in patients with diabetic gastroparesis and there is a lack of randomized controlled trials assessing the effect of nutritional intervention on outcome. Patients are often advised to eat small frequent meals, chew their food well, avoid fiber and consume a diet low in fat as studies have shown fat can slow gastric emptying in healthy volunteers.93 This advice makes physiological sense and should be given. However, there are little data to show how these nutritional interventions compare with other treatment modalities for gastroparesis or if they affect natural history and outcome of patients with diabetic gastroparesis.
In the absence of drugs that target the underlying mechanisms, the aims of treatment of diabetic gastroparesis should be to tighten glycemic control, treat symptoms, and optimize nutritional intake. The effect of erratic gastric emptying on glycemic control is becoming increasingly clear. Therefore, there may be advantages to the use of prokinetics even in the absence of significant symptom relief. Care however must be taken to exclude rapid gastric emptying which is being increasingly recognized as occurring in a subset of patients with diabetes.5
It is important to asses the nutritional status of patients with diabetic gastroparesis as they can have unintentional weight loss, dehydration, and electrolyte imbalance secondary to vomiting. Malnutrition can often be missed due to the higher starting weight of patients with gastroparesis and type 2 diabetes.
In milder cases of malnutrition, oral nutrient drinks can be offered but in malnourished patients with greater than 5% weight loss over three months, one should consider enteral feeding to bypass the dysfunctional stomach. A nasojejunal feeding tube trial should be carried out for several days prior to placement of endoscopic or surgical feeding tube as this may unmask any underlying small bowel dysmotility that would result in failure of tube feedings. Gastroduodenal manometry, when available, can be used to assess the small bowel as potentially can the Smart Pill®. It is helpful to keep patients fasting during a trial as it is often difficult to differentiate intolerance to oral feeds and enteral feeds when they continue to eat. Venting gastrotomy may be helpful in some patients and can prevent repeated hospitalization but the data to support its benefit is limited. Total parenteral nutrition should be reserved only for patients who fail enteral feeding trial. With gastroenterology, endocrinology, and nutritional services working together, this should be a very rare occurrence.
The mainstay of treatment in patients with diabetic gastroparesis has been prokinetic medications. Both metoclopramide and domperidone are dopamine-2 receptor antagonists and equally effective in reducing symptoms of nausea and vomiting in patients with diabetic gastroparesis.94 Metoclopramide is also a weak 5-HT3 receptor antagonist and 5-HT4 receptor agonist. Domperidone does not cross the blood brain barrier and is associated with fewer CNS effects. Domperidone is widely available except in the United States where it requires Institutional Review Board approval and an FDA exemption. Metoclopramide remains the primary prokinetic in the United States and is available in liquid and oral dissolving tablets which may be tolerated better. It is also been shown to be effective by the subcutaneous route, bypassing issues associated with delayed gastric emptying.95 However, a well recognized complication of metoclopramide is tardive dyskinesia96 and since February 2009 the drug carries a box warning in the United States alerting patients about the risks of long term and high dose use. This has limited its use. A recent study in rodents shows dopamine-3 receptor agonists inhibit stimulated pyloric relaxation and gastric emptying97 suggesting dopamine-3 receptor antagonists given along with dopamine-2 receptor antagonists may be more effective in treating symptoms, though further studies are needed to test this hypothesis.
Erythromycin is a potent prokinetic agent which acts by activating the motilin receptor, likely on the cholinergic neurons. It is a useful agent for short term treatment of patients in the hospital; however its long term benefit is limited due to development of tachyphylaxis. Several other motilin agonists have been developed to avoid tachyphylaxis. Mitemcinal (GM-611), a macrolide motilin receptor agonist with acid resistance, has been shown to improve gastric emptying in patients with diabetic gastroparesis.98 GSK962040 is a recently identified small molecule motilin receptor agonist which selectively activates the motilin receptor in humans and is being evaluated to determine safety and tolerability in humans.99 An issue with most motilin agonists is that they also increase gastric tone and therefore can make symptoms worse even when gastric emptying improves. They, of course, should not be used in the subset of patients with rapid gastric emptying. Several other agents have been evaluated for their prokinetic effect including SK-951, a benzofuran derivative which improves gastric emptying in STZ- induced diabetic dogs,100 and epalrestat, an aldose reductase inhibitor which increases the amplitude of 3 cycles per minute waves on EGG in patients with diabetic gastroparesis.101 The 5HT4 agonists, prucalopride and TD-5108 accelerate gastric emptying but have not been tested in gastroparesis.102, 103 Phase III clinical trials will be required for all these drugs before their potential role in the treatment of diabetic gastroparesis is established.
Pylorospasm has been reported in diabetes104 and it has been hypothesized to act as a resistance to emptying of gastric content. This has led to the use of intrapyloric injection of botulinum toxin injection to open the pylorus. While initial open label pilot studies were encouraging, recent randomized controlled trials have failed to show improvement in symptoms.105 It therefore does not appear to have a place in the management of diabetic gastroparesis.
Ghrelin is an endogenous ligand for growth hormone secretagogue receptor expressed on the vagal afferent neurons and enteric neurons in the stomach.106 Ghrelin has been shown to improve gastric emptying in patients with diabetic gastroparesis in a placebo controlled study; however the mechanism by which it exerts the prokinetic effect is unclear and it has a short half life.107 The ghrelin receptor agonist (TZP-101) has been evaluated in humans and appears to be well tolerated in patients with diabetic gastroparesis, improving gastric emptying in these patients.108
New drugs have been recently introduced to control post prandial hyperglycemia such as amylin analogs and glucagon like peptide-1 (GLP-1) receptor analogs and agonists. These drugs can delay gastric emptying. The amylin (normally co-secreted with insulin by β cells) analog pramlintide delays gastric emptying in both patients with type 1 and type 2 diabetes.109 The effects of pramlintide on gastric emptying appear to be mediated by the vagal nerve supply to the stomach. Therefore in patients with vagal dysfunction, the delay in gastric emptying induced by pramlintide may be reduced. Exenatide is a GLP-1 mimetic used in type 2 diabetes and like the short acting native GLP-1 hormone can delay gastric emptying, though this effect may be less pronounced in patients in whom gastric emptying is already delayed.110 We are still learning about the effect of these newer classes of drugs on the gastrointestinal tract and on symptoms in patients with diabetic gastroparesis. Our current understanding is that the benefit of the drugs on glycemic control outweigh their effects on gastric emptying but these drugs need to be kept in mind when assessing an abnormal gastric emptying study.
Nausea and vomiting are often the most debilitating symptoms for patients with diabetic gastroparesis. Phenothiazines such as prochlorperazine are one of the most commonly utilized antiemetics, exerting a central effect by acting at the dopaminergic and cholinergic receptors but carry risk of extrapyramidal side effects such as tardive dyskinesia. Other antiemetics such as the 5HT3 antagonists odansetron and granisetron primarily developed for chemotherapy induced nausea and vomiting, cannabinoids, opioid agonists, benzodiazepines, and H1 receptor agonists such as diphenhydramine can be used for symptomatic control of nausea and vomiting, though they have not been evaluated in diabetic gastroparesis. Low dose tricyclic antidepressants have shown to be effective in improving symptoms of nausea and vomiting in some patients with delayed gastric emptying.111 The neurokinin-receptor antagonist aprepitant has recently been shown to effectively control vomiting in a patient with refractory diabetic gastroparesis.112 Similarly, the antidepressant mirtazapine, which acts on the 5HT3 receptor, has also shown to be effective in a patient with refractory gastroparesis.113 However, controlled studies are needed to further evaluate the benefit of these medications in improving symptoms in patients with diabetic gastroparesis. Among alternative therapies, ginger114, and acupuncture have been evaluated. Acupuncture has shown benefit in patients with diabetic gastroparesis.115
Gastric electrical stimulation is being increasingly used for patients with diabetic gastroparesis with refractory nausea and vomiting. Initial work in the field focused on pacing the stomach to increase gastric emptying. In contrast, currently available gastric electrical stimulation uses low energy, high frequency stimulation with no substantial effect on gastric emptying.116 The Enterra system, a low energy high frequency gastric electric stimulation device was approved by the FDA under the humanitarian use device (HUD) designation in 2000 for treatment of patients with refractory nausea and vomiting due to gastroparesis. A review of the literature published to date suggest that symptoms of nausea and vomiting improve with implantation of the device, as do quality of life and nutritional status; however, most of the studies are open labeled single center studies. The mechanism of action of gastric electrical stimulation is still not known, the data suggest afferent neural mechanisms and perhaps modulation of gastric biomechanical activity play a role.117 Gastric electrical stimulation is invasive and there are surgical complications associated with its use. It therefore should be considered only in refractory diabetic gastroparesis after exhaustion of other therapeutic options and only when the main symptoms are nausea and vomiting and not pain. Temporary gastric electrical stimulation may help predict who will respond to gastric electrical stimulation.118 There are currently several alternatives being studied that include long pulse high energy stimulation, as well as single and multiple channel gastric electric stimulation with a goal of achieving sustainable pacing. Preliminary data presented in abstract form shows benefit in patients with severe diabetic gastroparesis using two channel gastric pacing at 1.1 times the intrinsic frequency which normalized gastric dysrhythmia and helped entrain gastric slow waves.119
Pain management is often challenging in patients with gastroparesis as there is a lack of clear understanding of the physiological defect causing pain in these patients. While patients may respond to prokinetics and other conventional treatments used to improve gastric function, some patients require additional medications to help control pain. Medications commonly used for chronic abdominal pain such as tricyclic and tetracyclic antidepressants, gabapentin, and pregabalin can be used to treat pain though there have been no specific studies evaluating their role in gastroparesis. Pain management is best undertaken using a multidisciplinary approach targeting underlying motility disorder as well as peripheral and central circuits. Opiates should be used very sparingly and only in refractory cases. If needed, a weaker opiate such as tramadol or a kappa agonist, Asimadoline,120 should be considered.
Future directions
The past 5 years have seen significant advances in our understanding of the pathophysiology of diabetic gastroparesis and the development of several new diagnostic modalities. While treatment modalities are currently severely limited, there do appear to be several promising new drugs in the pharmaceutical pipeline. Overall, the outlook is optimistic. Future therapies will be guided by our increased understanding of the pathophysiology of the disease and we propose can be hastened by the following:
Development of non-surgical approaches to full thickness biopsies of the stomach. While animal models have proven to be very useful, we ultimately need human tissue to determine if what we have learned in animal models also applies to humans. Also, the non-specific nature of the symptoms of diabetic gastroparesis, often indistinguishable from patients with diabetes and normal gastric emptying, make a tissue diagnosis attractive as long as it can be carried out without the need for surgery. Recent reports have described non invasive endoscopic technique to obtain full thickness gastric biopsies.121, 122 Human trials will be needed to determine the viability of these approaches and whether the size of the biopsy obtained is sufficient to examine the key gastric wall components.
New therapy based on our increased understanding of the pathophysiology of the disease. Drugs that target the IGF-1 pathway, target reduction in oxidative stress, target ICC, target dimerization of nNOS and target immune cells; all may be of therapeutic utility. Of particular interest is the potential use of drugs such as hemin that are already approved for use in humans and target expression of heme oxygenase-1 or drugs that replace its products such as carbon monoxide. In animal models this approach has been shown to reverse changes in ICC, nNOS expression, and macrophages.55
New prokinetics. While the relationship between current measures of gastric emptying and symptoms is weak, control of gastric emptying is still important to regulate delivery of nutrients to the small intestine. Testing of a variety of ways to better deliver energy to allow true gastric pacing may also serve to achieve the same goals.
Studies aimed at better understanding the correlation between electrophysiology, global and segmental gastric function and symptoms.
Prospective follow up studies to determine the long term outcome of diabetic gastroparesis as well as the long term outcome of therapies.
Development of predictors of which patients will go on to develop diabetic gastroparesis. While duration of diabetes is well known to be associated with development of diabetic gastroparesis, many patients with diabetes never develop gastrointestinal complications of diabetes while others develop diabetic gastroparesis within a few years of onset of diabetes.
Development and validation of better animal models, especially of type 2 diabetes.
Studies on the potential use of stem cell-based therapies. Given we now have a better understanding of the cellular defects, recent advances in regenerative medicine also hold promise for future treatment options in patients with diabetic gastroparesis by restoring tissue integrity. Studies in this area are needed to determine the feasibility of this approach.
Pathophysiological mechanisms responsible for diabetic gastroparesis.
Autonomic neuropathy
Loss of neuronal nitric oxide synthase leading to loss of nitric oxide
Increased oxidative stress with loss of upregulation of protective enzymes such as heme oxygenase-1
Loss of interstitial cells of Cajal with resultant gastric arrhythmia and delayed gastric emptying
Smooth muscle atrophy and loss of IGF-1 from smooth muscle
Loss of macrophages expressing heme oxygenase-1
Diagnosis.
4 hours gastric scintigraphy is the gold standard
13C based breath tests can be used in the office setting
Ultrasonography is non invasive and provides information on emptying as well as segmental function but depends on operator expertise
Capsule allows measurement of pH, pressure, temperature and emptying at the same time
MRI can help determine intragastric content and emptying without radiation exposure but still requires significant experience to interpret
SPECT imaging allows measurement of gastric emptying and accommodation concurrently
Acknowledgments
We thank Kristy Zodrow for secretarial assistance and Peter Strege for technical assistance. Grant Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK68055 and DK57061 and by the National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Clinical Research Consortium (GpCRC).
Abbreviations
- ICC
Interstitial Cells of Cajal
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- CO
carbon monoxide
- GI
gastrointestinal
- VIP
vasoactive, intestinal peptide
- STZ
streptozocin
- IGF-1
insulin like growth factor 1
- SPECT
Single photon emission computed tomography
- CNS
central nervous system
- MRI
Magnetic resonance imaging
- FDA
Food and Drug Administration
- GLP-1
glucagon like peptide-1
Footnotes
Publisher's Disclaimer: License for Publication: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in your license (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Competing interests: none to declare
References
- 1.Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 3.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–96. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 4.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313–22. doi: 10.1111/j.1572-0241.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–20. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko GT, Chan WB, Chan JC, et al. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670–4. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Enck P, Rathmann W, Spiekermann M, et al. Prevalence of gastrointestinal symptoms in diabetic patients and non-diabetic subjects. Z Gastroenterol. 1994;32:637–41. [PubMed] [Google Scholar]
- 8.Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–22. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 9.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 10.Annese V, Bassotti G, Caruso N, et al. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol. 1999;29:171–7. doi: 10.1097/00004836-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 12.Hoogerwerf WA, Pasricha PJ, Kalloo AN, et al. Pain: the overlooked symptom in gastroparesis. Am J Gastroenterol. 1999;94:1029–33. doi: 10.1111/j.1572-0241.1999.01008.x. [DOI] [PubMed] [Google Scholar]
- 13.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–52. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 15.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204–7. [PubMed] [Google Scholar]
- 16.Gonenne J, Esfandyari T, Camilleri M, et al. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil. 2006;18:911–8. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 17.Gangula PR, Maner WL, Micci MA, et al. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–33. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong MF, Horowitz M, Jones KL, et al. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz M, Maddox AF, Wishart JM, et al. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18:229–34. doi: 10.1007/BF00186645. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–58. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Schvarcz E, Palmer M, Aman J, et al. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–6. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 22.Fraser R, Horowitz M, Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991;32:475–8. doi: 10.1136/gut.32.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebbink RJ, Samsom M, Bruijs PP, et al. Hyperglycemia induces abnormalities of gastric myoelectrical activity in patients with type I diabetes mellitus. Gastroenterology. 1994;107:1390–7. doi: 10.1016/0016-5085(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 24.Rayner CK, Su YC, Doran SM, et al. The stimulation of antral motility by erythromycin is attenuated by hyperglycemia. Am J Gastroenterol. 2000;95:2233–41. doi: 10.1111/j.1572-0241.2000.02250.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones KL, Berry M, Kong MF, et al. Hyperglycemia attenuates the gastrokinetic effect of erythromycin and affects the perception of postprandial hunger in normal subjects. Diabetes Care. 1999;22:339–44. doi: 10.2337/diacare.22.2.339. [DOI] [PubMed] [Google Scholar]
- 26.Schvarcz E, Palmer M, Aman J, et al. Hypoglycaemia increases the gastric emptying rate in patients with type 1 diabetes mellitus. Diabet Med. 1993;10:660–3. doi: 10.1111/j.1464-5491.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz JG, Green GM, Guan D, et al. Rapid gastric emptying of a solid pancake meal in type II diabetic patients. Diabetes Care. 1996;19:468–71. doi: 10.2337/diacare.19.5.468. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–9. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 29.Samsom M, Salet GA, Roelofs JM, et al. Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci. 1995;40:2037–42. doi: 10.1007/BF02208676. [DOI] [PubMed] [Google Scholar]
- 30.Darwiche G, Almer LO, Bjorgell O, et al. Delayed gastric emptying rate in Type 1 diabetics with cardiac autonomic neuropathy. J Diabetes Complications. 2001;15:128–34. doi: 10.1016/s1056-8727(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz M, Edelbroek MA, Wishart JM, et al. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857–62. doi: 10.1007/BF00400362. [DOI] [PubMed] [Google Scholar]
- 32.Jones KL, Horowitz M, Carney BI, et al. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643–8. [PubMed] [Google Scholar]
- 33.Lysy J, Israeli E, Strauss-Liviatan N, et al. Relationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patients. Neurogastroenterol Motil. 2006;18:433–40. doi: 10.1111/j.1365-2982.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 34.Gaddipati KV, Simonian HP, Kresge KM, et al. Abnormal ghrelin and pancreatic polypeptide responses in gastroparesis. Dig Dis Sci. 2006;51:1339–46. doi: 10.1007/s10620-005-9022-z. [DOI] [PubMed] [Google Scholar]
- 35.Ishiguchi T, Nakajima M, Sone H, et al. Gastric distension-induced pyloric relaxation: central nervous system regulation and effects of acute hyperglycaemia in the rat. J Physiol. 2001;533:801–13. doi: 10.1111/j.1469-7793.2001.t01-1-00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagihashi S, Sima AA. Diabetic autonomic neuropathy in BB rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733–43. doi: 10.2337/diab.35.7.733. [DOI] [PubMed] [Google Scholar]
- 37.Kniel PC, Junker U, Perrin IV, et al. Varied effects of experimental diabetes on the autonomic nervous system of the rat. Lab Invest. 1986;54:523–30. [PubMed] [Google Scholar]
- 38.Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol. 2002;50:257–92. doi: 10.1016/s0074-7742(02)50080-5. [DOI] [PubMed] [Google Scholar]
- 39.Carroll SL, Byer SJ, Dorsey DA, et al. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:1144–54. doi: 10.1093/jnen/63.11.1144. [DOI] [PubMed] [Google Scholar]
- 40.Costa M, Glise H, Sjodahl R. The enteric nervous system in health and disease. Gut. 2000;47:iv1. doi: 10.1136/gut.47.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah V, Lyford G, Gores G, et al. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903–13. doi: 10.1053/j.gastro.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson KM, Reid JJ. Effect of diabetes on relaxations to non-adrenergic, non-cholinergic nerve stimulation in longitudinal muscle of the rat gastric fundus. Br J Pharmacol. 1995;116:1551–6. doi: 10.1111/j.1476-5381.1995.tb16372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrzos HF, Cruz A, Polavarapu R, et al. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106–10. doi: 10.1023/a:1018830820537. [DOI] [PubMed] [Google Scholar]
- 45.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–60. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lurken MS, Parkman HP, Abell TL, et al. Histological changes in idiopathic and diabetic gastroparesis. Neurogastroenterol Motil. 2009;21:74. [Google Scholar]
- 47.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095–e92. doi: 10.1111/j.1365-2982.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 48.Zarate N, Mearin F, Wang XY, et al. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–70. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasricha PJ, Pehlivanov ND, Gomez G, et al. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall AV, Antoniou H, Wang Y, et al. Structural organization of the human neuronal nitric oxide synthase gene (NOS1) J Biol Chem. 1994;269:33082–90. [PubMed] [Google Scholar]
- 51.Wang Y, Marsden PA. Nitric oxide synthases: gene structure and regulation. Adv Pharmacol. 1995;34:71–90. doi: 10.1016/s1054-3589(08)61081-9. [DOI] [PubMed] [Google Scholar]
- 52.Korenaga K, Micci MA, Taglialatela G, et al. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392–400. doi: 10.1111/j.1365-2982.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 53.Dishy V, Cohen Pour M, Feldman L, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther. 2004;76:281–6. doi: 10.1016/j.clpt.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Sun WM, Doran S, Jones KL, et al. Effects of nitroglycerin on liquid gastric emptying and antropyloroduodenal motility. Am J Physiol. 1998;275:G1173–8. doi: 10.1152/ajpgi.1998.275.5.G1173. [DOI] [PubMed] [Google Scholar]
- 55.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–64. 64, e1–2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20 (Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 57.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 58.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 60.He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 61.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–81. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto T, Watiabe K, Nakahara M, et al. Involvement of interstitial cells of cajal in gastrointestinal dysmotility of diabetic db/db mice. Gastroenterology. 2006;130:A90. [Google Scholar]
- 64.Wang XY, Huizinga JD, Diamond J, et al. Loss of intramuscular interstitial cells of cajal and enteric nerves in streptozotocin-induced diabetic rat stomach. Neurogastroenterol Motil. 2006;18:758. [Google Scholar]
- 65.McCallum RW, Lin Z, Damjanov I, et al. Interstitial cells of cajal in the stomach of patients with gastroparesis. Neurogastroenterol Motil. 2006;18:758. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 66.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–75. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 67.Chen JD, Lin Z, Pan J, et al. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–45. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 68.Takano H, Imaeda K, Koshita M, et al. Alteration of the properties of gastric smooth muscle in the genetically hyperglycemic OLETF rat. J Auton Nerv Syst. 1998;70:180–8. doi: 10.1016/s0165-1838(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 69.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–95. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi T, Kojima Y, Tsunoda Y, et al. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol. 1996;270:G411–7. doi: 10.1152/ajpgi.1996.270.3.G411. [DOI] [PubMed] [Google Scholar]
- 71.Bharucha AE, Kulkarni A, Choi KM, et al. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2009 doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashyap PC, Choi KM, Lurken MS, et al. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am J Physiol: GI. 2010 doi: 10.1152/ajpgi.00069.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi KM, Kashyap PC, Dutta N, et al. CD206 positive M2 macrophages expressing HO1 protect against the development of delayed GE in a mouse model of diabetic gastroparesis. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.02.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–63. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 75.Gonlachanvit S, Maurer AH, Fisher RS, et al. Regional gastric emptying abnormalities in functional dyspepsia and gastro-oesophageal reflux disease. Neurogastroenterol Motil. 2006;18:894–904. doi: 10.1111/j.1365-2982.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 76.Simonian HP, Maurer AH, Knight LC, et al. Simultaneous assessment of gastric accommodation and emptying: studies with liquid and solid meals. J Nucl Med. 2004;45:1155–60. [PubMed] [Google Scholar]
- 77.Burton DD, Kim HJ, Camilleri M, et al. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol. 2005;289:G261–6. doi: 10.1152/ajpgi.00052.2005. [DOI] [PubMed] [Google Scholar]
- 78.Bromer MQ, Kantor SB, Wagner DA, et al. Simultaneous measurement of gastric emptying with a simple muffin meal using [13C]octanoate breath test and scintigraphy in normal subjects and patients with dyspeptic symptoms. Dig Dis Sci. 2002;47:1657–63. doi: 10.1023/a:1015856211261. [DOI] [PubMed] [Google Scholar]
- 79.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–7. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 80.Choi KM, Zhu J, Stoltz GJ, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–45. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 81.Zahn A, Langhans CD, Hoffner S, et al. Measurement of gastric emptying by 13C-octanoic acid breath test versus scintigraphy in diabetics. Z Gastroenterol. 2003;41:383–90. doi: 10.1055/s-2003-39331. [DOI] [PubMed] [Google Scholar]
- 82.Ziegler D, Schadewaldt P, Pour Mirza A, et al. [13C]octanoic acid breath test for non-invasive assessment of gastric emptying in diabetic patients: validation and relationship to gastric symptoms and cardiovascular autonomic function. Diabetologia. 1996;39:823–30. doi: 10.1007/s001250050516. [DOI] [PubMed] [Google Scholar]
- 83.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635–43. e1. doi: 10.1016/j.cgh.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thumshirn M, Bruninga K, Camilleri M. Simplifying the evaluation of postprandial antral motor function in patients with suspected gastroparesis. Am J Gastroenterol. 1997;92:1496–500. [PubMed] [Google Scholar]
- 85.Undeland KA, Hausken T, Svebak S, et al. Wide gastric antrum and low vagal tone in patients with diabetes mellitus type 1 compared to patients with functional dyspepsia and healthy individuals. Dig Dis Sci. 1996;41:9–16. doi: 10.1007/BF02208577. [DOI] [PubMed] [Google Scholar]
- 86.Tefera S, Gilja OH, Olafsdottir E, et al. Intragastric maldistribution of a liquid meal in patients with reflux oesophagitis assessed by three dimensional ultrasonography. Gut. 2002;50:153–8. doi: 10.1136/gut.50.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feinle C, Kunz P, Boesiger P, et al. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut. 1999;44:106–11. doi: 10.1136/gut.44.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–9. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 89.Rao SSC, Kuo B, McCallum RW, et al. Investigation of colonic and whole gut transit with wireless motility capsule and radioopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–44. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 90.Quan C, Talley NJ, Cross S, et al. Development and validation of the Diabetes Bowel Symptom Questionnaire. Aliment Pharmacol Ther. 2003;17:1179–87. doi: 10.1046/j.1365-2036.2003.01553.x. [DOI] [PubMed] [Google Scholar]
- 91.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 92.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–80. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 93.Parrish CR. Nutrition concerns for the patient with gastroparesis. Curr Gastroenterol Rep. 2007;9:295–302. doi: 10.1007/s11894-007-0033-0. [DOI] [PubMed] [Google Scholar]
- 94.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–4. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 95.McCallum RW, Valenzuela G, Polepalle S, et al. Subcutaneous metoclopramide in the treatment of symptomatic gastroparesis: clinical efficacy and pharmacokinetics. J Pharmacol Exp Ther. 1991;258:136–42. [PubMed] [Google Scholar]
- 96.Pasricha PJ, Pehlivanov N, Sugumar A, et al. Drug Insight: from disturbed motility to disordered movement--a review of the clinical benefits and medicolegal risks of metoclopramide. Nat Clin Pract Gastroenterol Hepatol. 2006;3:138–48. doi: 10.1038/ncpgasthep0442. [DOI] [PubMed] [Google Scholar]
- 97.Kashyap P, Micci MA, Pasricha S, et al. The D2/D3 agonist PD128907 (R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4- oxazin-9-ol) inhibits stimulated pyloric relaxation and spontaneous gastric emptying. Dig Dis Sci. 2009;54:57–62. doi: 10.1007/s10620-008-0335-6. [DOI] [PubMed] [Google Scholar]
- 98.McCallum RW, Cynshi O. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121–30. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 99.Sanger GJ, Westaway SM, Barnes AA, et al. GSK962040: a small molecule, selective motilin receptor agonist, effective as a stimulant of human and rabbit gastrointestinal motility. Neurogastroenterol Motil. 2009;21:657–64. e30–1. doi: 10.1111/j.1365-2982.2008.01270.x. [DOI] [PubMed] [Google Scholar]
- 100.Takeda M, Mizutani Y, Yamano M, et al. Gastric emptying in diabetic gastroparetic dogs: ffects of SK-951, a novel prokinetic agent. Pharmacology. 2001;62:23–8. doi: 10.1159/000056068. [DOI] [PubMed] [Google Scholar]
- 101.Okamoto H, Nomura M, Nakaya Y, et al. Effects of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy and gastroparesis. Intern Med. 2003;42:655–64. doi: 10.2169/internalmedicine.42.655. [DOI] [PubMed] [Google Scholar]
- 102.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–60. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 103.Manini ML, Camilleri M, Goldberg M, et al. Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2010;22:42–E8. doi: 10.1111/j.1365-2982.2009.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–25. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 105.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–8. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 106.Dass NB, Munonyara M, Bassil AK, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–53. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 107.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–8. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–87. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 109.Vella A, Lee JS, Camilleri M, et al. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil. 2002;14:123–31. doi: 10.1046/j.1365-2982.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 110.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151:123–9. doi: 10.1016/j.regpep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Sawhney MS, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–24. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 112.Chong K, Dhatariya K. A case of severe, refractory diabetic gastroparesis managed by prolonged use of aprepitant. Nat Rev Endocrinol. 2009;5:285–8. doi: 10.1038/nrendo.2009.50. [DOI] [PubMed] [Google Scholar]
- 113.Kim SW, Shin IS, Kim JM, et al. Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment. Psychosomatics. 2006;47:440–2. doi: 10.1176/appi.psy.47.5.440. [DOI] [PubMed] [Google Scholar]
- 114.Gupta YK, Sharma M. Reversal of pyrogallol-induced delay in gastric emptying in rats by ginger (Zingiber officinale) Methods Find Exp Clin Pharmacol. 2001;23:501–3. doi: 10.1358/mf.2001.23.9.662137. [DOI] [PubMed] [Google Scholar]
- 115.Wang L. Clinical observation on acupuncture treatment in 35 cases of diabetic gastroparesis. J Tradit Chin Med. 2004;24:163–5. [PubMed] [Google Scholar]
- 116.Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–12. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 117.McCallum RW, Dusing RW, Sarosiek I, et al. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil. 2010;22:161–7. doi: 10.1111/j.1365-2982.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 118.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–61. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 119.Sarosiek I, Forster J, Roeser K, et al. Effect of multi-point gastric electrical pacing (MGP) on symptoms, gastric emptying and electrical activity in diabetic gastroparesis. Gastroenterology. 2009;134:A123. [Google Scholar]
- 120.Camilleri M. Novel pharmacology: asimadoline, a kappa-opioid agonist, and visceral sensation. Neurogastroenterol Motil. 2008;20:971–9. doi: 10.1111/j.1365-2982.2008.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fraser H, Neshev E, Storr M, et al. A novel method of full thickness gastric biopsy by a percutaneous endoscopic transenteric (PETE) approach. Neurogastroenterol Motil. 2009;21:6. [Google Scholar]
- 122.Rajan E, Gostout CJ, Lurken MS, et al. Endoscopic “no hole” full-thickness biopsy of the stomach to detect myenteric ganglia. Gastrointest Endosc. 2008;68:301–7. doi: 10.1016/j.gie.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]