Abstract
Cocaine produces a persistent reduction in cystine–glutamate exchange via system xc− in the nucleus accumbens that may contribute to pathological glutamate signaling linked to addiction. System xc− influences glutamate neurotransmission by maintaining basal, extracellular glutamate in the nucleus accumbens, which, in turn, shapes synaptic activity by stimulating group II metabotropic glutamate autoreceptors. In the present study, we tested the hypothesis that a long-term reduction in system xc− activity is part of the plasticity produced by repeated cocaine that results in the establishment of compulsive drug seeking. To test this, the cysteine prodrug N-acetylcysteine was administered before daily cocaine to determine the impact of increased cystine–glutamate exchange on the development of plasticity-dependent cocaine seeking. Although N-acetylcysteine administered before cocaine did not alter the acute effects of cocaine on self-administration or locomotor activity, it prevented behaviors produced by repeated cocaine including escalation of drug intake, behavioral sensitization, and cocaine-primed reinstatement. Because sensitization or reinstatement was not evident even 2–3 weeks after the last injection of N-acetylcysteine, we examined whether N-acetylcysteine administered before daily cocaine also prevented the persistent reduction in system xc− activity produced by repeated cocaine. Interestingly, N-acetylcysteine pretreatment prevented cocaine-induced changes in [35S]cystine transport via system xc−, basal glutamate, and cocaine-evoked glutamate in the nucleus accumbens when assessed at least 3 weeks after the last N-acetylcysteine pretreatment. These findings indicate that N-acetylcysteine selectively alters plasticity-dependent behaviors and that normal system xc− activity prevents pathological changes in extracellular glutamate that may be necessary for compulsive drug seeking.
Keywords: extrasynaptic glutamate, addiction, system xc−, nucleus accumbens, nonvesicular, cystine–glutamate antiporter
Introduction
Repeated cocaine alters glutamate neurotransmission even after protracted withdrawal (Wolf et al., 2004; Kalivas et al., 2005), and this likely contributes to addiction because abnormal activation of corticostriatal pathways correlates with craving in humans (Breiter et al., 1997; Volkow et al., 1999, 2005) and is necessary for cocaine seeking in rodents (Park et al., 2002; McFarland et al., 2003; Schmidt et al., 2005). Revealing cellular mechanisms underlying altered corticostriatal activation should advance our understanding of the neurobiological basis of addiction and identify novel therapeutic targets (Volkow and Fowler, 2000; Dackis, 2004).
Models of pathological glutamate signaling proposed to underlie addiction need to account for the existence of multiple pools of extracellular glutamate. Aside from synaptic glutamate maintained by vesicular release, extrasynaptic glutamate is sustained primarily by nonvesicular release (Herrera-Marschitz et al., 1996; Timmerman and Westerink, 1997). In support, basal extrasynaptic glutamate sampled using microdialysis are mostly independent of vesicular glutamate (Westerink, 1995). Glutamate transporters may partition the two pools by limiting glutamate overflow from the synapse into extrasynaptic compartments (Danbolt, 2001), and restricting entry of nonvesicular glutamate into synapses (Jabaudon et al., 1999). Although confined to the extrasynaptic compartment, nonvesicular glutamate regulates neurotransmission by stimulating group II metabotropic glutamate receptors (mGluRs) (Xi et al., 2002a; Baker et al., 2003), which are extrasynaptic receptors capable of inhibiting vesicular release (Baskys and Malenka, 1991; Cochilla and Alford, 1998; Schoepp, 2001). Thus, extrasynaptic receptors permit cross talk between the two pools and indicate that altered nonvesicular glutamate release may contribute to pathological glutamate signaling linked to addiction.
Cystine–glutamate exchange via system xc− may be critical in the capacity of extrasynaptic glutamate to regulate corticostriatal signaling in the normal and pathological states. First, nonvesicular release from cystine–glutamate exchange maintains basal extracellular glutamate in the nucleus accumbens (Baker et al., 2002; Xi et al., 2002a), and thereby regulates the extent of endogenous group II mGluR stimulation (Baker et al., 2002; Melendez et al., 2005; Moran et al., 2005). Repeated cocaine blunts system xc− activity, which leads to reduced basal and increased cocaine-evoked glutamate in the nucleus accumbens that persists for at least 3 weeks after the last cocaine treatment (Baker et al., 2003). These changes are relevant for drug seeking because N-acetylcysteine, a cysteine prodrug used to drive system xc− (Williamson and Meister, 1981; Meister, 1985; Pileblad and Magnusson, 1992), blocks cocaine-evoked glutamate in the nucleus accumbens and subsequent cocaine-induced reinstatement (Baker et al., 2003).
In these studies, we tested the hypothesis that reduced system xc− activity is part of the pathological plasticity produced by repeated cocaine that results in compulsive drug seeking. To explore this, we assessed the capacity of N-acetylcysteine administered before daily cocaine to prevent behavioral plasticity and reduced function of system xc−. These data have the potential to extend our earlier observation that acute N-acetylcysteine administered before testing blocks reinstatement by demonstrating that plasticity-dependent behavior fails to materialize in the absence of reduced system xc− activity, even when testing occurs weeks after the last N-acetylcysteine treatment.
Materials and Methods
Animals
These experiments used male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–325 g on arrival. Rats were individually housed in a temperature-controlled colony room with a 12 h reversed light/dark cycle. Housing conditions and experimental protocols were approved by the Marquette University Institutional Animal Care and Use Committee and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996).
Surgeries
Rats included in self-administration studies were implanted with indwelling catheters under ketamine HCl (100 mg/kg, i.p.; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (2 mg/kg, i.p.; Lloyd Laboratories, Shenandoah, IA) anesthesia. A silicon tubing catheter (Dow Corning, Midland, MI; 0.64 mm inner diameter; 1.19 mm outer diameter) was implanted such that it entered the jugular vein through the right posterior facial vein and terminated at the right atrium. The catheter was sutured to the vein at the entry point. The distal aspect of the catheter, which consisted of a 22-gauge guide cannula (Plastics One, Roanoke, VA) attached with dental acrylic to a piece of polypropylene monofilament surgical mesh (Atrium Medical, Hudson, NH), exited 2 cm posterior to the scapulae. Throughout the experiment, catheters were filled daily with a heparin solution (83 i.u./ml; Elkins-Sinn, Cherry Hill, NJ) and capped when disconnected from the leash/delivery line assembly.
Rats included in microdialysis studies were also implanted with indwelling bilateral guide cannulas (20 gauge, 14 mm; Plastics One) using the following coordinates derived from Paxinos and Watson (1986): +0.9 mm anterior, ±2.5 mm mediolateral to bregma, and −4.4 mm from the surface of the skull at a 6° angle from vertical. The placement of the active region of the microdialysis probe, which began 2 mm beyond the ventral tip of the guide cannulas, was primarily in the nucleus accumbens core, although regions immediately dorsal and ventral to this were also likely sampled. After surgery, rats were given at least 5 d to recover before testing. During this time, rats were provided acetaminophen (480 mg/L) in their drinking water and injected daily with a sterile cefazolin antibiotic solution (15 mg, i.v.; West-Ward Pharmaceutical, Eatontown, NJ).
Self-administration training
Self-administration occurred in operant chambers (ENV-008CT; MED-Associates, St. Albans, VT) housed in sound-attenuating cubicles (ENV-016M; MED-Associates) and equipped with two retractable levers, two stimulus lights, and a water bottle. At least 5 d after surgery, rats were food restricted for 18 h with water available ad libitum. Rats were then placed into the operant chambers overnight and responses on the lever designated as active resulted in the delivery of food pellets under a fixed ratio 1 schedule of reinforcement. Daily food training continued until subjects received at least 150 food rewards in a session, which typically occurred after the first session. Rats then underwent drug self-administration training during daily 2 h sessions in which operant responses on the active lever were reinforced with an infusion of saline or cocaine (0.5 mg · kg−1 · 200 μl−1, i.v.; National Institute on Drug Abuse, Bethesda, MD) under a fixed ratio 1 schedule of reinforcement. Each reinforced lever response resulted in the illumination of the stimulus light located above the active lever and was followed by a 25 s time-out period. Responding on a second, inactive lever located on the back wall was recorded but had no programmed consequences. Acquisition of cocaine self-administration was operationally defined as <10% variation in daily responding over at least three consecutive sessions. Once rats met the acquisition criteria, they were advanced to maintenance sessions in which saline or cocaine was self-administered under short- (0.5 mg · kg−1 · 200 μl−1, i.v.; 2 h/d for 10 d) or extended-access conditions (1.0 mg · kg−1 · 200 μl−1, i.v.; 6 h/d for 11 d). Rats received daily N-acetylcysteine (0 or 60 mg/kg, i.p.) 60 min before each acquisition or maintenance self-administration session.
Experiment 1: impact of N-acetylcysteine pretreatment on escalation of drug intake
Self-administration under extended access conditions has been shown to produce an increase in cocaine intake that may model loss of control in human abusers (Ahmed and Koob, 1998; Mantsch et al., 2004; Ahmed and Cador, 2006; Kippin et al., 2006). In the current study, rats were pretreated with saline or N-acetylcysteine (60 mg/kg, i.p.) 60 min before self-administering saline or cocaine (1.0 mg · kg−1 · 200 μl−1, i.v.; 6 h/d; 11 daily sessions). The pretreatment/self-administration drug assignments resulted in the following groups: saline–saline (N = 19), N-acetylcysteine–saline (N = 10), saline–cocaine (N = 18), and N-acetylcysteine–cocaine (N = 12). Escalation was evident as a statistically significant increase in the number of infusions obtained relative to the first 6 h session.
Experiment 2: impact of N-acetylcysteine pretreatment on persistent cocaine-induced behavioral sensitization
Behavioral sensitization represents one of the most used paradigms in attempts to identify the relevance of drug-induced plasticity and may model changes in sensitivity to cocaine arising after repeated drug exposure (Wolf, 1998; Vanderschuren and Kalivas, 2000). On days 1–7 of the current experiment, rats received daily injections of saline or N-acetylcysteine (60 mg/kg, i.p.) followed 120 min later by saline or cocaine (15 mg/kg, i.p., on days 1 and 7; 30 mg/kg, i.p., on days 2–6). These drug assignments resulted in the following groups: saline–saline (N = 11), N-acetylcysteine–saline (N = 11), saline–cocaine (N = 13), and N-acetylcysteine–cocaine (N = 15). Rats then remained in the home cage for a 21 d drug-free period (day 28 of the experiment), and were then challenged with saline or cocaine (15 mg/kg, i.p.) in the absence of N-acetylcysteine pretreatment. As a result, behavioral sensitization is assessed on day 28, which is 3 weeks after the last N-acetylcysteine injection.
Locomotor activity was monitored using 16 Plexiglas activity chambers (22 × 43 × 33 cm3; Omnitech Electronics, Columbus, OH). Total distance (in centimeters) traveled was monitored using 16 photobeams (eight on each horizontal axis) interfaced with a Digiscan monitor (Omnitech Electronics). Activity levels were recorded 60 min before and 120 min after a saline or cocaine injection. Locomotor behavior was measured on days 1, 7, and 28.
Experiment 3: impact of daily N-acetylcysteine pretreatment on persistent cocaine-evoked changes in extracellular glutamate and reinstatement
Cocaine-primed reinstatement likely provides measures of drug seeking independent of drug reinforcement and may require reduced system xc− activity that has been observed after withdrawal from repeated cocaine (Baker et al., 2003). In the current study, microdialysis and reinstatement data were collected in parallel from the same group of rats to better correlate neurochemical and behavioral findings. Rats were pretreated with saline or N-acetylcysteine (60 mg/kg, i.p.) 60 min before self-administering saline or cocaine (0.5 mg · kg−1 · 200 μl−1, i.v.; 2 h/d). The pretreatment/self-administration drug assignments resulted in the following groups: saline–saline (N = 14, N-acetylcysteine–saline (N = 6), saline–cocaine (N = 14), and N-acetylcysteine–cocaine (N = 7). After self-administration, rats underwent extinction training and a subsequent microdialysis/reinstatement test day as described below.
Extinction training.
After completing 10 maintenance self-administration sessions, rats remained in their home cages for 7 d before extinction training. A 7 d delay was used to ensure an adequate drug-free period before reinstatement, even in rats that quickly extinguished responding. Extinction training involved placing rats into the operant chambers for 2 h/d as described above in the self-administration section except each active lever press now resulted in an infusion of saline. This continued until the mean number of lever presses was ≤15 responses across at least three sessions, at which point rats were tested for drug-primed reinstatement. Because the average number of extinction sessions needed to meet criteria (±SEM) was 11.7 ± 1.10, reinstatement testing occurred ∼18 d after the last self-administration session.
In vivo microdialysis and reinstatement test day.
On the night before the reinstatement test, microdialysis probes, constructed as previously described (Baker et al., 2003), were inserted into indwelling guide cannula. Rats were then housed overnight in the self-administration chambers. The next day, dialysis buffer (5 mm glucose, 140 mm NaCl, 1.4 mm CaCl2, 1.2 mm MgCl2, and 0.15% PBS, pH 7.4) was pumped through the probes at a rate of 2 μl/min for at least 3 h to permit an adequate period of time for glutamate levels to stabilize. After this, basal glutamate levels were sampled over 60 min. Rats were then injected with cocaine (10 mg/kg, i.p.) and the levers were extended. Extracellular glutamate and operant responding were assessed over the next 120 min (N = 6–14/group).
Quantification of glutamate.
The concentration of glutamate in dialysis samples was quantified by comparing peak heights from samples and external standards using HPLC coupled to fluorescence detection. Precolumn derivatization of glutamate with opthaldehyde was performed using a Shimadzu LC10AD VP autosampler. The mobile phase consisted of 13% acetonitrile, 100 mm Na2HPO4, and 0.1 mm EDTA, pH 5.90. Glutamate was separated using a reversed-phase column (4 μm; 140 × 6.0 mm; Phenomenex, Torrance, CA), and detected using a Shimadzu 10RF-AXL fluorescence detector with excitation and emission wavelengths of 320 and 400 nm, respectively.
Histology.
Rats included in the microdialysis studies were given an overdose of pentobarbital (60 mg/kg, i.p.), and the brains were fixed by intracardiac infusion of 0.9% saline followed by 2.5% formalin solution. Brains were removed and stored in 2.5% formalin for at least 7 d before sectioning. The tissue was then blocked, and coronal sections (100 μm) were cut and stained with cresyl violet to verify probe placements.
Experiment 4: impact of N-acetylcysteine pretreatment on persistent cocaine-induced changes in system xc− activity
Repeated cocaine produces a persistent reduction in system xc− activity in the nucleus accumbens (Baker et al., 2003). In the present study, cystine–glutamate exchange was assessed in rats pretreated with saline or N-acetylcysteine (60 mg/kg, i.p.) 60 min before self-administering saline or cocaine (1.0 mg · kg−1 · 200 μl−1, i.v.; 6 h/d; 11 daily sessions). The pretreatment/self-administered drug assignments resulted in the following groups: saline–saline (N = 12), N-acetylcysteine–saline (N = 5), saline–cocaine (N = 7), and N-acetylcysteine–cocaine (N = 11). After self-administration, rats remained in the home cage for 23–34 d. As a result, the status of system xc− was determined at least 3 weeks after the last N-acetylcysteine treatment.
Rats were decapitated and the brains were rapidly extracted and cut into 2 mm coronal slices using a brain matrix. Tissue punches (1.25 mm diameter; Stoelting, Wood Dale, IL) were collected from the nucleus accumbens core and incubated at 37°C for ∼30 min on a nylon bolting cloth platform, submerged beneath 2 mm of standard buffer (Lobner and Lipton, 1993); the standard buffer contained 124 mm NaCl, 3.0 mm KCl, 1.4 mm KH2PO4, 1.3 mm MgSO4, 26 mm NaHCO3, 2.4 mm CaCl2, and 4 mm glucose, equilibrated with 95% O2, 5% CO2, pH 7.4. After a 30 min wash, the tissue was incubated for 30 min in standard buffer containing [35S]cystine (4 μCi/ml; GE Healthcare, Piscataway, NJ). d-Aspartate (1 mm) was also added to the incubation buffer to prevent uptake via XAG. After 30 min incubation, the tissue punches were washed four times in ice-cold standard buffer and dissolved in 250 μl of 1% SDS. One aliquot (100 μl) was used to measure [35S]cystine uptake by scintillation counting; a second aliquot (100 μl) was used to assess protein content by the BCA method. Additional punches from each subject were processed as described above with the exception that unlabeled CPG [(S)-4-carboxyphenylglycine] (1 mm) was added 10 min before and for the duration of the 30 min incubation with [35S]cystine (4 μCi/ml). These values reflect labeling independent of system xc− and were subtracted from the total labeling obtained from each subject to get a measure that solely reflected uptake through cystine–glutamate exchange.
Statistical analyses for experiments 1–4
The SPSS statistics package was used to perform the statistical analyses. Data were analyzed using ANOVA with drug treatment (e.g., dose of N-acetylcysteine and cocaine) as between-subject factors and time (day and/or 20 min interval) as a repeated factor. Significant main effects and interactions were further evaluated using Dunnett's t test with cocaine controls designated as the comparison group.
Results
Daily N-acetylcysteine pretreatment blunts cocaine-induced behavioral plasticity
In the present studies, we tested the hypothesis that N-acetylcysteine administered before each daily cocaine session would prevent the development of compulsive drug seeking. Figure 1 illustrates the impact of N-acetylcysteine pretreatment on daily cocaine self-administration under extended access conditions (6 h/d). A comparison of daily infusions during each self-administration session produced a three-way interaction between cocaine treatment, N-acetylcysteine treatment, and self-administration session (ANOVA: F(10,550) = 3.05; p = 0.001). Post hoc analyses indicated that cocaine self-administering rats pretreated with saline exhibited escalation, which was evident as an increase from day 1 in cocaine intake that was significant by the fifth session, and that this effect was prevented in cocaine self-administering rats pretreated with N-acetylcysteine (Fig. 1a) (Dunnett's t, p < 0.05). Furthermore, N-acetylcysteine did not significantly alter the number of cocaine infusions obtained until escalation of intake was evident in cocaine rats pretreated with saline (Fig. 1a) (Dunnett's t, p < 0.05). The magnitude of escalation is evident when directly comparing the difference in intake on the last and first sessions (Fig. 1b). Analysis of these data produced a significant interaction between N-acetylcysteine and cocaine treatments (ANOVA: F(10,550) = 3.05; p = 0.001); post hoc tests confirmed that only cocaine rats pretreated with saline exhibited escalation (Dunnett's t, p < 0.05).
Figure 1.
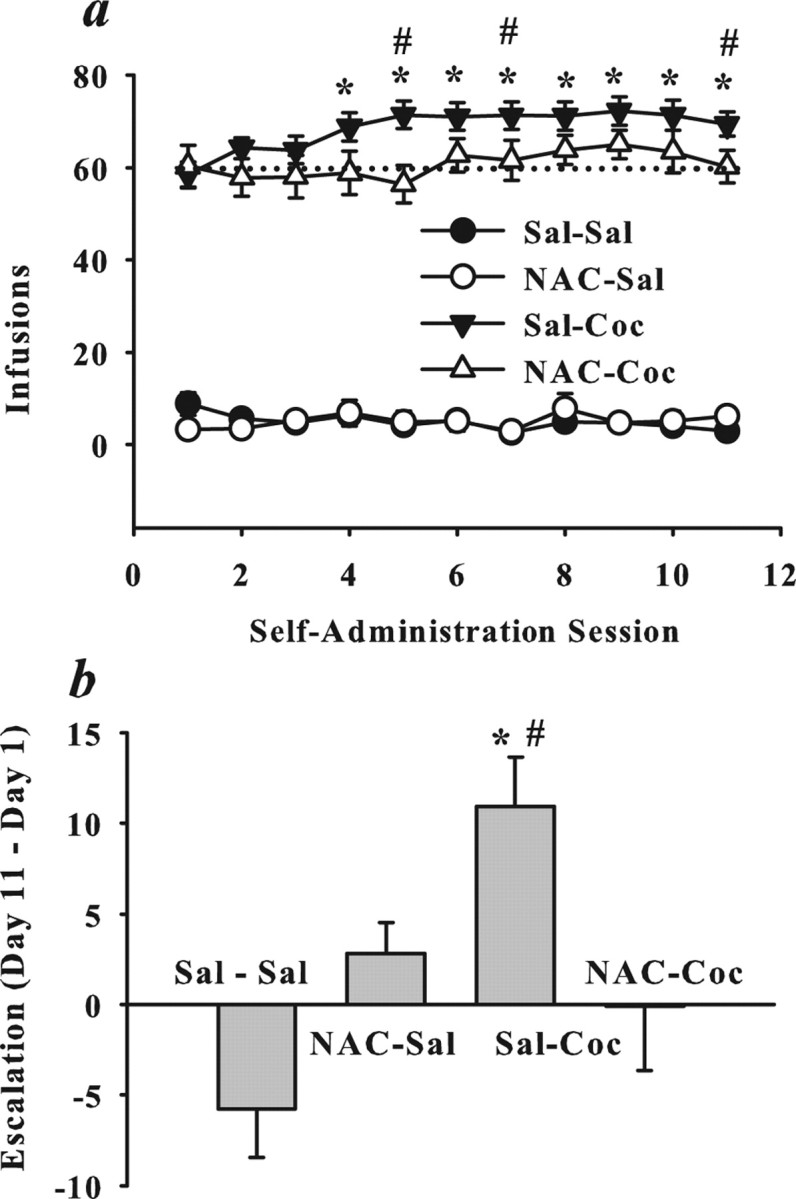
N-Acetylcysteine pretreatment prevents escalation of drug intake during daily extended-access cocaine self-administration. a, b, Data depict mean ± SEM daily cocaine intake (mg/kg, i.v.) across 11 maintenance self-administration sessions (a) and difference in intake between days 11 and 1 to illustrate escalation in intake (b). Rats received saline (Sal) or N-acetylcysteine (NAC) (60 mg/kg, i.p.) before daily saline or cocaine (Coc) (1.0 mg/kg, i.v./infusion; 6 h/d) self-administration sessions. The pretreatment/self-administration drug assignments resulted in the following groups: Sal–Sal (N = 19), NAC–Sal (N = 10), Sal–Coc (N = 18), and NAC–Coc (N = 12). Escalation is operationally defined as a significant increase in the number of infusions obtained relative to day 1. *Significant difference from day 1 intake (a) or rats pretreated with saline before saline self-administration training sessions (Sal–Sal) (b); Dunnett's t, p < 0.05. #Significant difference from NAC–Coc rats.
Figure 2 illustrates the impact of N-acetylcysteine administered before seven daily injections of cocaine on the establishment of behavioral sensitization. A comparison of total distance traveled on day 1 of the experiment resulted in a significant two-way interaction between time (18 10 min intervals) and cocaine treatment (ANOVA: F(17,782) = 14.03; p < 0.001). Post hoc analyses revealed that all rats treated with cocaine, regardless of N-acetylcysteine pretreatment, exhibited significantly higher levels of locomotor activity when compared with rats treated with saline (Dunnett's t, p < 0.05) indicating that N-acetylcysteine did not alter acute cocaine-induced locomotor activity (Fig. 2a). Separate ANOVAs comparing total distance traveled on days 7 and 28 of the experiment each produced a significant three-way interaction between time (18 10 min intervals), N-acetylcysteine treatment, and cocaine treatment (day 7: F(17,782) = 1.92, p = 0.01; day 28: F(17,782) = 2.69, p < 0.001). Post hoc analyses indicated that both cocaine groups exhibited higher levels of locomotor activity relative to saline controls on days 7 and 28 of the experiment (Dunnett's t, p < 0.05). Furthermore, cocaine rats pretreated with saline exhibited higher levels of activity relative to those pretreated with N-acetylcysteine after the last of seven daily cocaine injections (day 7) and after a cocaine challenge given 21 d after the last N-acetylcysteine pretreatment (day 28) (Fig. 2b,c) (Dunnett's t, p < 0.05). To test for behavioral sensitization, we compared total distance across all three test days. This ANOVA produced an interaction between test day, N-acetylcysteine, and cocaine (F(2,92) = 3.15; p = 0.05). Post hoc analyses indicated that rats pretreated with saline, but not N-acetylcysteine, exhibited cocaine-induced behavioral sensitization, evident as a significant increase in cocaine-induced activity on days 7 and 28 relative to day 1 (Fig. 2c) (Dunnets t, p < 0.05).
Figure 2.
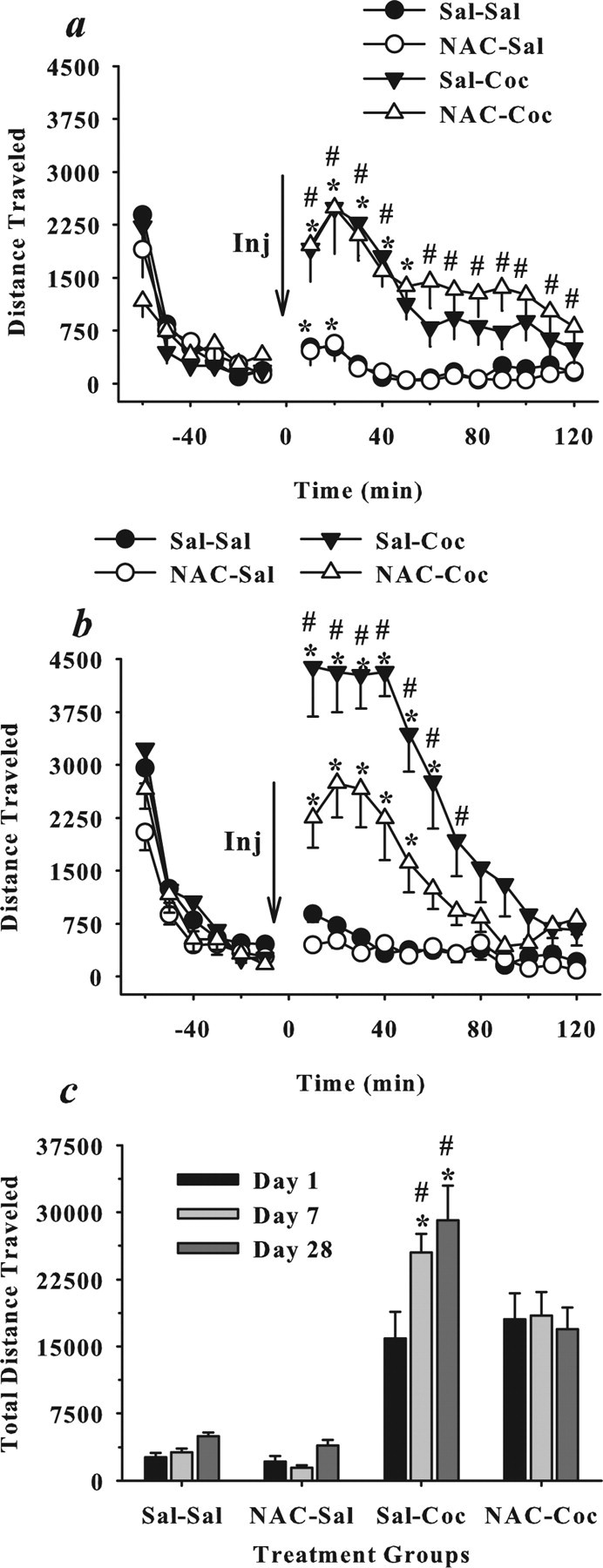
Daily N-acetylcysteine prevents cocaine-induced behavioral sensitization without altering acute locomotor activity. a–c, Total distance traveled (mean centimeters ± SEM) is depicted in 10 min intervals (a, b) or summed across the 2 h session (c). Group designations refer only to treatments on days 1–7, at which time rats received saline or N-acetylcysteine (0 or 60 mg/kg, i.p.) before each of seven daily injections of saline or cocaine (15 mg/kg, i.p., on days 1 and 7; 30 mg/kg, i.p., on days 2–6). These drug assignments resulted in four groups: Sal–Sal (N = 11), NAC–Sal (N = 11), Sal–Coc (N = 13), and NAC–Coc (N = 15). After a 21 d drug-free period, locomotor activity was measured on day 28 of the experiment after a saline or cocaine (15 mg/kg, i.p.) challenge in the absence of N-acetylcysteine pretreatment. The arrow indicates the timing of the saline or cocaine injection administered on day 1 (a, c), day 7 (c), or day 28 (b, c) of the experiment. a, *Significant difference from interval immediately preceding the saline or cocaine injection regardless of N-acetylcysteine treatment; Dunnett's t, p < 0.05. #Significant difference from rats injected with saline (0 cocaine) regardless of N-acetylcysteine treatment; ANOVA, p < 0.05. b, *Significant difference from the interval immediately preceding the saline or cocaine injection; Dunnett's t, p < 0.05. #Significant difference from every other group; Dunnett's t, p < 0.05. c, *Significant difference from day 1; Dunnett's t, p < 0.05. #Significant difference from every other group; Dunnett's t, p < 0.05.
Figure 3 illustrates the impact of repeated N-acetylcysteine pretreatment on self-administration under short-access conditions (2 h/d) and cocaine-induced reinstatement of extinguished lever pressing. A comparison of infusions obtained during daily self-administration sessions with N-acetylcysteine and cocaine treatments as between-subjects variables and self-administration session as a within-subjects variable yielded only a main effect of cocaine (ANOVA: F(1,37) = 115; p < 0.001), indicating that N-acetylcysteine pretreatment did not significantly alter self-administration under short-access conditions (Fig. 3a). N-Acetylcysteine administered during self-administration training also failed to alter the mean ± SEM number of extinction sessions needed to reach criteria relative to cocaine rats pretreated with saline (SEM: 11 ± 2 and 13.6 ± 1.7, respectively; p > 0.05). In contrast, a comparison of lever pressing during the reinstatement test day and the last three extinction sessions produced a three-way interaction between test day, N-acetylcysteine treatment, and cocaine treatment (ANOVA: F(3,111) = 3.06; p = 0.03). Post hoc analyses revealed that a cocaine prime reinstated extinguished drug seeking, evident as a significant increase in lever pressing on the reinstatement test relative to the last extinction session, but only in rats that had been treated with saline during cocaine self-administration (Dunnett's t, p < 0.05); indeed, this group of rats exhibited significantly higher levels of responding on the reinstatement test day than all other groups (Dunnett's t, p < 0.05). In contrast, cocaine rats pretreated with N-acetylcysteine failed to reinstate cocaine-seeking (Dunnett's t, p < 0.05) despite testing 2–3 weeks after the last N-acetylcysteine treatment (Fig. 3b).
Figure 3.
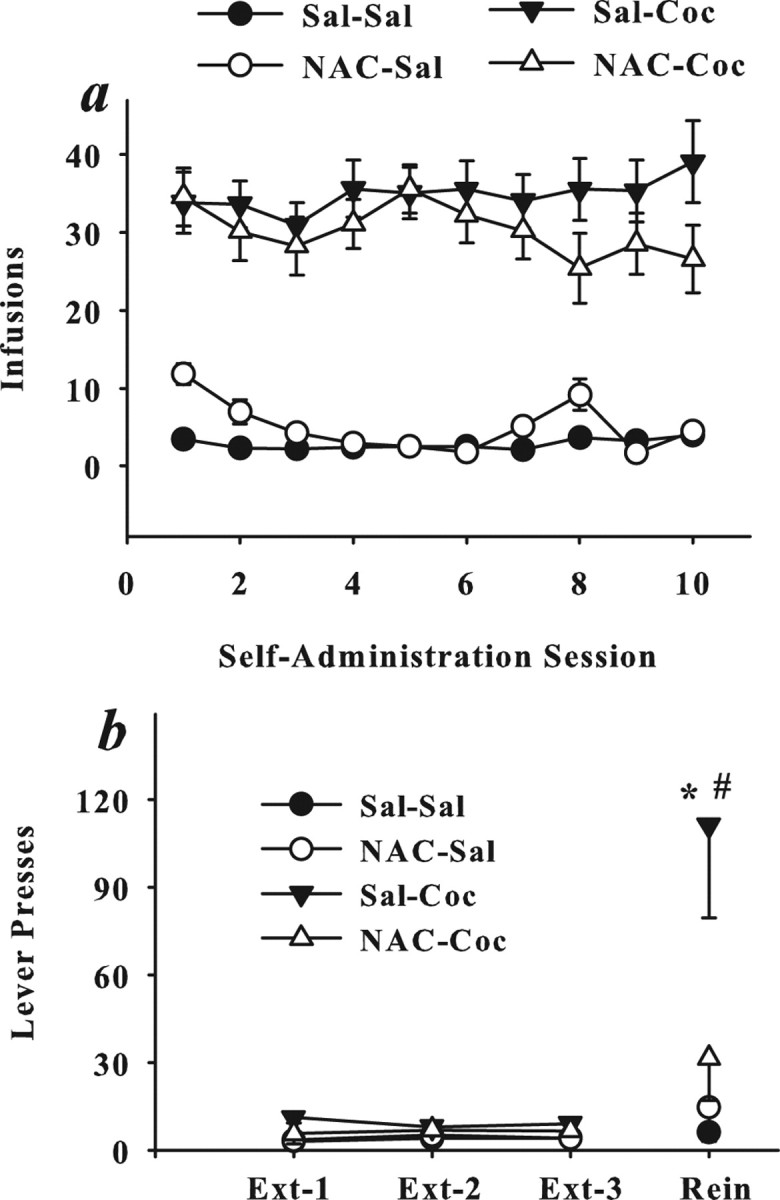
Daily N-acetylcysteine prevents cocaine-induced reinstatement without altering cocaine self-administration. a, b, Data are depicted as mean ± SEM number of infusions across daily self-administration sessions (2 h/d) (a) or responses on the active lever during the last three extinction sessions and the test for cocaine-induced reinstatement (b). Group designations refer only to treatments given during self-administration training, at which time rats received saline or N-acetylcysteine (60 mg/kg, i.p.) before self-administering saline or cocaine (0.5 mg/kg, i.v./infusion; 2 h/d). The pretreatment/self-administration drug assignments resulted in four groups: Sal–Sal (N = 14), NAC–Sal (N = 6), Sal–Coc (N = 14), and NAC–Coc (N = 7). Seven days after the last self-administration session, rats underwent daily extinction training in the absence of any drug treatments. This was followed by a test for cocaine-induced (10 mg/kg, i.p.) reinstatement in the absence of N-acetylcysteine treatment. *Significant difference from the last extinction session; Dunnett's t, p < 0.05. #Significant difference from all other groups; Dunnett's t, p < 0.05.
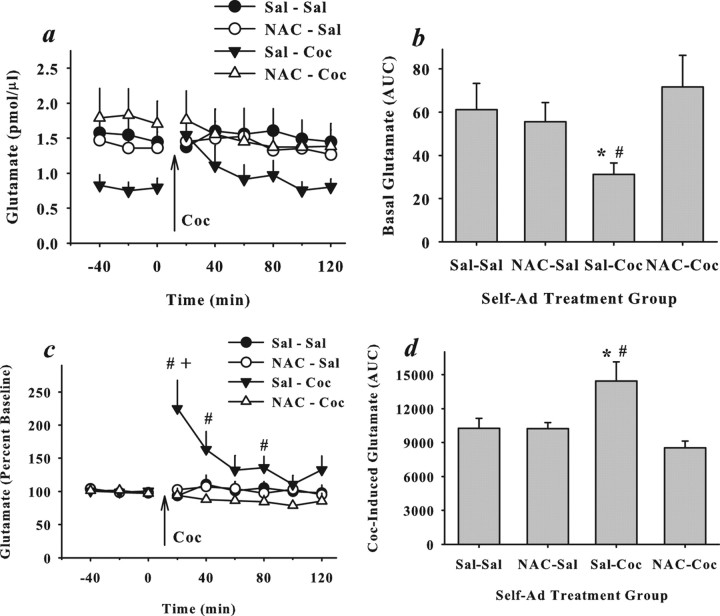
N-Acetylcysteine pretreatment prevents cocaine-induced changes in extracellular glutamate levels in the nucleus accumbens
Figure 4 illustrates extracellular basal glutamate levels sampled from the nucleus accumbens using in vivo microdialysis. An ANOVA used to compare glutamate levels (in picomoles per microliter) (Fig. 4a) with N-acetylcysteine and cocaine treatment as between-subject measures and time (nine 20 min samples) as a repeated measure did not yield a significant interaction between the variables (F(8,296) = 1.28; p = 0.25), nor a main effect of cocaine (F(1,37) = 0.54; p = 0.47), N-acetylcysteine (F(1,37) = 0.94; p = 0.34), or time (F(8,296) = 1.73; p = 0.09). A comparison of basal glutamate (e.g., before cocaine injection) (Fig. 4b) calculated as area under the curve yielded a significant interaction between N-acetylcysteine and cocaine treatments (ANOVA: F(1,37) = 4.05; p = 0.05). Subsequent post hoc analyses revealed that a reduction in basal glutamate levels relative to saline controls was evident in rats pretreated with saline before daily cocaine self-administration sessions; this change in extracellular glutamate was not evident in cocaine rats that had been treated with N-acetylcysteine during self-administration training even when sampled 2–3 weeks after the last N-acetylcysteine treatment (Dunnett's t, p < 0.05).
Figure 4.
Daily N-acetylcysteine prevents long-term changes in extracellular glutamate levels in the nucleus accumbens produced by repeated cocaine. a, b, Basal glutamate levels (mean ± SEM) are depicted as picomoles per microliter across 20 min samples (a) or area under the curve calculated using samples collected before the cocaine injection (b). c, d, Cocaine-evoked glutamate is presented as percent change from baseline (c) or area under the curve calculated using samples collected after the cocaine injection (d). Group designations refer only to treatments given during self-administration training, at which time rats received saline or N-acetylcysteine (60 mg/kg, i.p.) before self-administering saline or cocaine (0.5 mg/kg, i.v./infusion; 2 h/d). The pretreatment/self-administration drug assignments resulted in four groups: Sal–Sal (N = 14), NAC–Sal (N = 6), Sal–Coc (N = 14), and NAC–Coc (N = 7). Seven days after the last self-administration session, rats underwent daily extinction training in the absence of any drug treatments. Microdialysis was then used to sample extracellular glutamate before and after an acute cocaine challenge (10 mg/kg, i.p.) in the absence of N-acetylcysteine. *Significant difference from Sal–Sal controls; Dunnett's t, p < 0.05. +Significant difference from the last baseline sample; Dunnett's t, p < 0.05. #Significant difference from NAC–Coc rats (c); Dunnett's t, p < 0.05.
An ANOVA comparing cocaine-evoked glutamate (percent baseline) (Fig. 4c,d) with N-acetylcysteine and cocaine treatment as between-subject measures and time (nine 20 min samples) as a repeated measure yielded a significant three-way interaction (F(8,296) = 2.05; p = 0.04). Post hoc analyses indicated that cocaine increased extracellular glutamate relative to the last baseline sample in rats that had been pretreated with saline before cocaine self-administration (Dunnett's t, p < 0.05) (Fig. 4c); this effect was not obtained in rats pretreated with N-acetylcysteine during self-administration training (Dunnett's t, p < 0.05). Analyzing glutamate as area under the curve generated from samples collected after the cocaine injection also indicated that only cocaine rats pretreated with saline exhibited a significant increase in extracellular glutamate. An interaction between N-acetylcysteine and cocaine treatments group was obtained (F(1,37) = 4.05; p = 0.05), with post hoc analyses indicating a significant increase in glutamate in rats pretreated with saline before cocaine self-administration relative to saline controls or cocaine rats pretreated with N-acetylcysteine (Dunnett's t, p < 0.05). Although the area primarily sampled in this experiment is the nucleus accumbens, the microdialysis probe for some subjects also extended into the striatum dorsal to the nucleus accumbens or the olfactory tubercles (Fig. 5).
Figure 5.
A schematic illustrating the placement of the 2 mm active membrane portion of the microdialysis probe for the rats included in the microdialysis study. The active regions of the microdialysis probes were primarily located in the nucleus accumbens, although regions of the neostriatum dorsal to the nucleus accumbens as well as the olfactory tubercles were likely sampled as well.
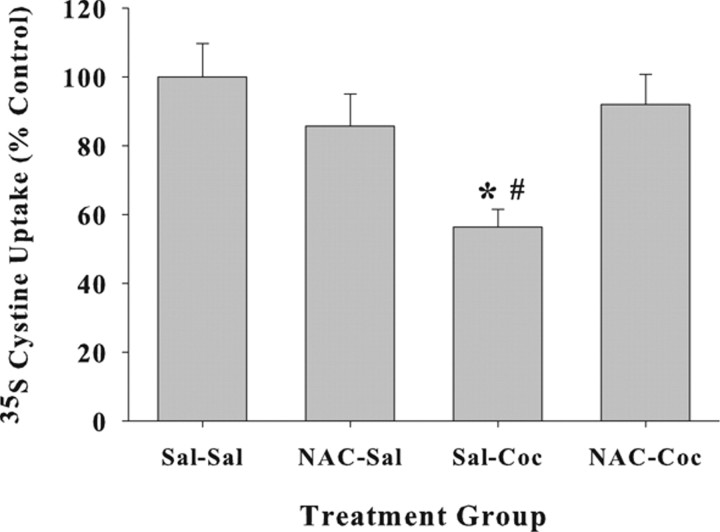
N-Acetylcysteine pretreatment prevents cocaine-induced plasticity involving system xc−
Figure 6 illustrates the impact of daily N-acetylcysteine pretreatment on cocaine-induced plasticity involving system xc−. A comparison of [35S]cystine transport produced an interaction between N-acetylcysteine and cocaine treatment conditions (F(1,31) = 7.53; p = 0.01). Post hoc analyses revealed that rats pretreated with saline before cocaine self-administration exhibited reduced [35S]cystine transport relative to saline controls (Dunnett's t, p < 0.05). Furthermore, rats pretreated with N-acetylcysteine before cocaine self-administration failed to exhibit diminished cystine–glutamate exchange, although cystine uptake was measured 23–34 d after the last treatment of either cocaine or N-acetylcysteine (Dunnett's t, p < 0.05).
Figure 6.
Daily N-acetylcysteine prevents cocaine-induced blunting of system xc− activity in the nucleus accumbens. Data depict [35S]cystine transport restricted to system xc− activity in nucleus accumbens tissue punches. Group designations refer only to treatments given during self-administration training at which time rats received saline or N-acetylcysteine (60 mg/kg, i.p.) before self-administering saline or cocaine (1.0 mg/kg, i.v./infusion; 6 h/d). The pretreatment/self-administration drug assignments resulted in the following groups: Sal–Sal (N = 12), NAC–Sal (N = 5), Sal–Coc (N = 7), and NAC–Coc (N = 11). After an extended drug-free period (23–34 d), tissue punches were obtained from the nucleus accumbens and used to measure [35S]cystine transport by system xc−. *Significant difference from Sal–Sal controls; Dunnett's t, p < 0.05. #Significant difference from NAC–Coc rats; Dunnett's t, p < 0.05.
Discussion
Abnormal glutamate signaling within corticostriatal pathways has been linked to craving in humans (Breiter et al., 1997; Volkow et al., 2005) and cocaine seeking in rodents (Park et al., 2002; McFarland et al., 2003; Schmidt et al., 2005). Plasticity involving cellular mechanisms capable of regulating glutamate signaling may underlie altered activation of glutamatergic pathways. As a result, identifying these mechanisms may advance our understanding of the neurobiological basis of addiction and facilitate the development of pharmacotherapies. In the present report, N-acetylcysteine administered before daily cocaine prevented the establishment of plasticity-dependent cocaine-seeking, even when behavior was assayed 21 d after the last injection of N-acetylcysteine. N-Acetylcysteine pretreatment also prevented reduced cystine–glutamate exchange and subsequent changes in extracellular glutamate in the nucleus accumbens after repeated cocaine. Collectively, these data indicate that reduced system xc− function represents part of the pathological plasticity needed to generate compulsive drug seeking.
Targeting system xc− to prevent behavioral plasticity
Repeated cocaine produces a long-term reduction in system xc− activity, which leads to abnormal levels of extracellular glutamate in the nucleus accumbens at baseline and after a cocaine injection (Baker et al., 2003). Acute administration of N-acetylcysteine, a cysteine prodrug used to increase cystine–glutamate exchange, blocks cocaine-evoked glutamate and blunts cocaine seeking (Baker et al., 2003). These data are compelling, in part, because they pose nonvesicular glutamate release by system xc− as a mechanism that may contribute to the neurobiological basis of addiction. We explored this hypothesis in the current studies by assessing the impact of N-acetylcysteine administered before daily cocaine on the establishment of drug seeking. Drug seeking was assessed using multiple behavioral paradigms because each may model distinct aspects of addiction. Escalation of cocaine intake during extended-access self-administration was used because this may model loss of control over drug use (Ahmed and Koob, 1998; Mantsch et al., 2004; Ahmed and Cador, 2006; Kippin et al., 2006). Interestingly, N-acetylcysteine pretreatments prevented escalation of drug intake that was evident in cocaine controls. These data are among the first to indicate that altered glutamate may be necessary for the escalation of drug intake.
Behavioral sensitization is one of the most used paradigms in attempts to identify the relevance of drug-induced plasticity and may model changes in sensitivity to cocaine arising after repeated drug exposure (Wolf, 1998; Vanderschuren and Kalivas, 2000). In the present study, N-acetylcysteine administered before daily cocaine prevented behavioral sensitization. These data are consistent with the preponderance of previous work indicating that excessive glutamatergic signaling is a precipitating event underlying sensitization (Pierce et al., 1996; Li et al., 1999; Tzschentke and Schmidt, 2000; Ghasemzadeh et al., 2003). Moreover, sensitized locomotion failed to emerge even when behavior was tested 21 d after the last N-acetylcysteine treatment, indicating that daily cocaine failed to produce the plasticity necessary for the emergence of behavioral sensitization in rats pretreated with N-acetylcysteine.
Cocaine-induced reinstatement is dependent on increased glutamatergic signaling within the prefrontal cortex or nucleus accumbens (Cornish and Kalivas, 2000; Capriles et al., 2003; McFarland et al., 2003; Baptista et al., 2004; Di Ciano and Everitt, 2004; Suto et al., 2004). In the present study, N-acetylcysteine administered before daily cocaine prevents the establishment of plasticity needed for cocaine-induced reinstatement because behavior is measured 2–3 weeks after the last N-acetylcysteine treatment. These data extend our previous observation that acute N-acetylcysteine given before testing blocks cocaine-primed reinstatement (Baker et al., 2003). Furthermore, our current finding represents one of the first chronic treatments capable of blocking cocaine-primed reinstatement, which is of particular importance because long-term treatment will likely be needed to treat addiction.
N-Acetylcysteine prevented the establishment of plasticity-dependent behaviors without altering the acute effects of cocaine (e.g., locomotor activity). One interpretation of these findings is that acute N-acetylcysteine is ineffective in blocking behaviors produced by cocaine; however, this is unlikely for two reasons. First, acute N-acetylcysteine blocks cocaine-primed reinstatement when administered on the test day (Baker et al., 2003). Second, repeated N-acetylcysteine in the present study failed to block cocaine-induced locomotion or self-administration before escalation. Instead, these data suggest that N-acetylcysteine selectively reverses plasticity-dependent behaviors. In support, N-acetylcysteine, fails to increase system xc− activity in drug-naive controls (Baker et al., 2003), indicating that it selectively alters extracellular glutamate in pathological states, which further supports the utility of this approach in treating addiction.
Targeting system xc− to prevent plasticity involving extracellular glutamate
The cysteine prodrug N-acetylcysteine may have blocked the establishment of escalation of drug intake, behavioral sensitization, and cocaine-primed reinstatement by preventing a persistent reduction in system xc− activity and subsequent changes in extracellular glutamate in the nucleus accumbens. To test this, we examined the status of cystine–glutamate exchange and extrasynaptic glutamate in the nucleus accumbens in rats treated with N-acetylcysteine before daily cocaine. The current studies revealed that repeated N-acetylcysteine prevented a long-term reduction in system xc− activity that was evident in cocaine controls. In separate rats, N-acetylcysteine also prevented the capacity of cocaine to produce a long-term reduction in extrasynaptic basal glutamate in the nucleus accumbens. This is important because this pool of glutamate provides endogenous stimulation to group II mGluRs (Xi et al., 2002a), which function as autoreceptors providing constitutive inhibition of synaptic glutamate release (Baskys and Malenka, 1991; Cochilla and Alford, 1998; Hu et al., 1999; Schoepp, 2001; Valenti et al., 2002; Xi et al., 2002b). Not surprisingly, we observed in the present study that cocaine failed to elevate extracellular glutamate in the nucleus accumbens once normal basal levels were restored. Similar to the above behavioral experiments, the lack of neurochemical changes was observed at least 2 weeks after the last N-acetylcysteine treatment.
In the current study, cocaine-induced behavioral plasticity failed to materialize in the absence of reduced cystine–glutamate exchange. One interpretation of these findings is that N-acetylcysteine maintains normal system xc− activity during repeated cocaine, which in turn prevents persistent changes in the levels of extracellular glutamate in the nucleus accumbens that are necessary for plasticity-dependent drug seeking. In support, previous studies indicate that normalizing extracellular glutamate, particularly after a cocaine prime, would be expected to block behavioral sensitization and cocaine-primed reinstatement (Pierce et al., 1998; Li et al., 1999; Tzschentke and Schmidt, 2000; McFarland and Kalivas, 2001; Baker et al., 2003). Thus, these data provide compelling evidence that nonvesicular glutamate contributes to pathological brain function, and further establish system xc− as a novel target for disorders involving abnormal glutamate. However, future studies will be needed to confirm the site of action of N-acetylcysteine because it is possible that N-acetylcysteine functions through mechanisms distinct from system xc− or by altering cystine–glutamate exchange in brain regions other than the nucleus accumbens.
It may seem paradoxical that targeting cystine–glutamate exchange, which increases nonvesicular glutamate, is effective in reducing cocaine-induced behaviors in rodents or drug craving and use in humans because these behaviors require increased glutamate signaling (Cornish and Kalivas, 2000; Baker et al., 2003; Di Ciano and Everitt, 2003; McFarland et al., 2003; Larowe et al., 2006; Mardikian et al., 2007). However, this may reflect the existence of multiple, functionally distinct pools of glutamate. Cocaine increases glutamate in the synaptic cleft after corticostriatal activation, thereby generating behaviors dependent on postsynaptic receptor stimulation (Cornish and Kalivas, 2000; McFarland et al., 2003; Di Ciano and Everitt, 2004; Suto et al., 2004). Conversely, N-acetylcysteine involves glutamate release into the extrasynaptic compartment resulting in the stimulation of group II mGluRs (Baker et al., 2002, 2003; Moran et al., 2005). Glutamate transporters may partition the two pools by limiting glutamate overflow from the synapse into the extrasynaptic compartment (Danbolt, 2001), and restricting entry of nonvesicular glutamate into the synapse (Jabaudon et al., 1999). By stimulating extrasynaptic group II mGluRs without exerting postsynaptic effects, extrasynaptic glutamate appears to inhibit synaptic pools (Baker et al., 2002; Moran et al., 2005). To the extent that NR2B receptors are located outside the synapse, this hypothesis is supported by the recent observation that glutamate release from astrocytes appears to stimulate extrasynaptic, but not synaptic NMDA receptors (D'Ascenzo et al., 2007).
The present data reveal that system xc− represents a novel target capable of preventing plasticity that underlies behaviors used to model aspects of addiction and are consistent with clinical studies indicating efficacy for N-acetylcysteine as an anticraving agent for cocaine addiction (Larowe et al., 2006; Mardikian et al., 2007). Aside from system xc−, numerous mechanisms may be capable of releasing glutamate into the extrasynaptic compartment including vesicular release from astrocytes (Bezzi et al., 2004; Zhang et al., 2004), nonvesicular release from astrocytic gap junction hemichannels (Ye et al., 2003), volume-sensitive organic anion channels (Strange et al., 1996; Basarsky et al., 1999), or hydrolysis of N-acetylaspartylglutamate (Blakely et al., 1988; Zhou et al., 2005). Thus, models of brain functioning in the normal and diseased states need to account for the existence of high, steady-state levels of glutamate in the extrasynaptic space. Moreover, mechanisms contributing to extrasynaptic glutamate represent novel opportunities to regulate glutamate and may contribute to pathological states involving altered excitatory signaling.
Footnotes
This work was supported by National Institute on Drug Abuse Grants DA17328 (D.A.B.) and DA15758 (J.R.M.). We also gratefully acknowledge the contributions of the late Ann Kelley as an insightful scientist who made innumerable advances in the field of motivated behaviors.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19:6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol (Lond) 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Robinson MB, Thompson RC, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-l-aspartyl-l-glutamate: subcellular and regional distribution, ontogeny, and the effect of lesions on N-acetylated-alpha-linked acidic dipeptidase activity. J Neurochem. 1988;50:1200–1209. doi: 10.1111/j.1471-4159.1988.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(RC89) doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA. Recent advances in the pharmacotherapy of cocaine dependence. Curr Psychiatry Rep. 2004;6:323–331. doi: 10.1007/s11920-004-0018-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, You ZB, Goiny M, Meana JJ, Silveira R, Godukhin OV, Chen Y, Espinoza S, Pettersson E, Loidl CF, Lubec G, Andersson K, Nylander I, Terenius L, Ungerstedt U. On the origin of extracellular glutamate levels monitored in the basal ganglia of the rat by in vivo microdialysis. J Neurochem. 1996;66:1726–1735. doi: 10.1046/j.1471-4159.1996.66041726.x. [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Larowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lobner D, Lipton P. Intracellular calcium levels and calcium fluxes in the CA1 region of the rat hippocampal slice during in vitro ischemia: relationship to electrophysiological cell damage. J Neurosci. 1993;13:4861–4871. doi: 10.1523/JNEUROSCI.13-11-04861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mardikian PN, Larowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Methods for the selective modification of glutathione metabolism and study of glutathione transport. Methods Enzymol. 1985;113:571–585. doi: 10.1016/s0076-6879(85)13077-6. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Pileblad E, Magnusson T. Increase in rat brain glutathione following intracerebroventricular administration of gamma-glutamylcysteine. Biochem Pharmacol. 1992;44:895–903. doi: 10.1016/0006-2952(92)90121-x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex. 2000;10:488–498. doi: 10.1093/cercor/10.5.488. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH. Brain microdialysis and its application for the study of animal behaviour. Behav Brain Res. 1995;70:103–124. doi: 10.1016/0166-4328(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Williamson JM, Meister A. Stimulation of hepatic glutathione formation by administration of l-2-oxothiazolidine-4-carboxylate, a 5-oxo-l-prolinase substrate. Proc Natl Acad Sci USA. 1981;78:936–939. doi: 10.1073/pnas.78.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002a;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002b;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]