Abstract
Ghrelin, an orexigenic hormone produced by the stomach, is secreted in anticipation of scheduled meals and in correlation with anticipatory locomotor activity. We hypothesized that ghrelin is directly implicated in stimulating locomotor activity in anticipation of scheduled meals. To test this hypothesis, we observed 24 hr patterns of locomotor activity in mice with targeted mutations of the ghrelin receptor gene (GHSR KO) and wild-type littermates, all given access to food for four hours daily for 14 days. While WT and GHSR KO mice produced increases in anticipatory locomotor activity, anticipatory locomotor activity in GHSR KO mice was attenuated (p.< 0.05). These behavioral measures correlated with attenuated levels of Fos immunoreactivity in a number of hypothalamic nuclei from GHSR KO placed on the same restricted feeding schedule for seven days and sacrificed at ZT4. Interestingly, seven daily intraperitoeneal ghrelin injections mimicked hypothalamic Fos expression patterns to those seen in mice under restricted feeding schedules. These data suggest that ghrelin acts in the hypothalamus to augment locomotor activity in anticipation of scheduled meals.
Keywords: Ghrelin, GHSR, anticipatory locomotor activity, restricted feeding schedules, hypothalamus, Fos immunoreactivity
The circadian timing system is strongly linked with the regulation of food intake and energy balance (Wiepkema et al., 1966, Turek et al., 2005). In mammals, the hypothalamic suprachiasmatic nucleus (SCN) is the “master clock” capable of maintaining circadian rhythms of physiological activity (i.e. hormone release, body temperature) and behavior (locomotor activity, drinking, feeding) (Wiepkema et al., 1966, Stephan and Zucker, 1972, Abe et al., 1979). These rhythms, however are disrupted following complete SCN lesions, but can be rescued if food is made available to the experimental animals for only a restricted number of hours during the day (Boulos et al., 1980, Stephan, 2002). Entrainment to these feeding schedules is also seen in SCN intact animals, which will show increased locomotor activity in anticipation of the daily meal (Boulos et al., 1980, Mistlberger, 1994, Saper and Fuller, 2007). It has been hypothesized that food anticipatory activity is produced by signals from a peripheral oscillator that stimulates mediobasal hypothalamic nuclei that regulate feeding and metabolism (Mendoza, 2007, Saper and Fuller, 2007).
Ghrelin, a 28-aminoacid peptide produced by the stomach, increases appetite and regulates metabolism to reduce energy expenditure and increase adiposity (Tschop et al., 2000, Nakazato et al., 2001). Ghrelin exerts these effects by the stimulation of the growth hormone secretagogue receptor (GHSR) 1a subtype that is widely expressed throughout the hypothalamus of rodents (Guan et al., 1997, Zigman et al., 2006). Interestingly, rats and other mammals under restricted feeding schedules also show altered patterns of ghrelin secretion, with ghrelin levels being elevated and peaking at about one hour prior to the time in which food is presented and at around the onset of anticipatory locomotor activity (Drazen et al., 2006). Given these data, we propose that ghrelin is a peripheral signal that targets hypothalamic nuclei to increase locomotor activity in anticipation to a scheduled meal. To test this hypothesis, we conducted experiments to confirm that ghrelin does indeed rise in anticipation of scheduled meals in mice just as it does in rats, and that inability to sense ghrelin decreases food anticipatory activity. Finally we evaluated the potential hypothalamic targets by which ghrelin could influence locomotor activity in anticipation of a scheduled meal using Fos immunocytochemistry.
METHOD
Animals
Mice with targeted mutations to the ghrelin receptor gene (GHSR KO) and their WT littermates were bred at the Carleton University Neuroscience Institute animal facilities. Mice originated from heterozygous breeding pairs obtained from Regeneron Pharmaceuticals in Tarrytown, New York. These mice were generated using a mixed C57bl/J6 and DBA strain as background. Additional mice (C57bl/J6) were obtained from Jackson Laboratories in Bar Harbor Maine. All animals had free access to food and tap water unless specified, and were kept under a 12 hour light/dark cycle with the onset of light set at 08:00hr (ZT0). Research was conducted under the guidelines of the Canadian Council on Animal Care and approved by Carleton University’s Animal Care Committee.
Ghrelin Radioimmunoassay (RIA)
Mice (C57BL/J6) fed ad lib (n=16) or under a restricted feeding schedule for 14 days (n=18) were sacrificed by rapid decapitation 60 minutes before the time at which restricted mice were fed every day (ZT3), and blood samples were collected. Samples were then centrifuged (300 rpm for 5 min. at 4°C) to separate plasma. Plasma was stored at −80°C until processing for RIA. Plasma content active (acylated) ghrelin was determined using a commercially available RIA kit (Millipore). All samples were assayed in duplicate and had intra-assay variability of 9.5% and differences were evaluated using independent group t-test. The amount of food ingested by ad lib fed mice and mice with restricted access to food was measured throughout. Differences in daily food intake, as well as food intake during the period of restricted access were examined using a mixed design with food access (Ad lib vs. restricted) as the between groups variable, and days as the within groups variable.
Behavioral Measures
Entrainment to feeding schedules
GHSR WT and KO mice were individually housed in units equipped with an activity wheel connected to a computer running software capable of quantifying the number of wheel revolutions throughout the study (Lafayette instruments, Lafayette, IN). All animals were allowed to acclimate to their new environments for at least one week before any recording occurred. Running wheel activity was then measured as the number of wheel revolutions in six-minute bins for the rest of the experiment. Three days of baseline were recorded before the restricted feeding paradigm commenced. Food intake and body weight was recorded daily. Following baseline, food was removed and returned the following day from ZT4 to ZT8 (12:00h–16:00h). This restricted feeding was carried out for 14 days and the total chow eaten by each mouse was monitored daily. The animals were then placed back on an ad libitum diet for 4 days while continuing to record their daily wheel running activity. Several measures were calculated including food anticipatory activity, total nocturnal activity, and nocturnality (defined as nocturnal activity as a percentage of total daily activity). Both the duration of food anticipatory activity and the duration of nocturnal activity were also calculated to determine any general locomotor differences that may have been due to the transgenic manipulation. Differences were analyzed using a mixed design with food access (Ad lib vs. restricted) as the between groups variable, and days as the within groups variable.
A second study was conducted using the same experimental design described above, but this time animals were monitored for spontaneous locomotor activity using a micromax activity monitor with infrared beams (Accuscan Instruments). In this second study, however, the activity of WT (n=8) and KO mice (n=9) was monitored for three days following the termination of the restricted feeding schedule to determine if the anticipatory activity persisted. After these two days, mice were food deprived for 36 hours to determine if anticipatory activity would re-emerge following the time that the animals were being refed during the scheduled feeding period. Data were organized and analyzed both in terms of total activity in anticipation of the scheduled meal as well as a ratio of anticipatory activity over the total daily activity counts.
Fos immunocytochemistry
Hypothalamic activation following food entrainment in WT and GHSR KO mice
Wild type (n=5) and GSHR KO (n=6) mice were subjected to restricted feeding schedules as described above. On the 7th day of the restricted feeding schedule they were injected with an overdose of sodium pentobarbital at ZT3 and perfused with 100 ml of saline (0.9%) followed by 250 ml of 4% paraformaldehyde. Brains were postfixed overnight and cryoprotected in 30% sucrose solution for 48hrs before being sectioned in a cryostat. One out of four 40μm sections containing the hypothalamus were processed for immunocytochemistry as described previously (Abizaid et al., 2005). Hypothalamic nuclei were identified using the Franklin and Paxinos (Franklin and Paxinos, 1997) mouse brain atlas (See Table 1 for list of regions examined). Images from different hypothalamic nuclei were captured with a digital camera connected to an Olympus BX51 microscope, and these were analyzed using the Scion Image (v 4.0) software. A total of 7–8 sections containing each hypothalamic nucleus were used for cell quantification. An exception was made for the arcuate nucleus of a WT mouse which was lost during dissection. Cell counts were performed by a rater unaware of the treatment group to which each animal belonged. These counts were organized so as to obtain a mean number of Fos immunoreactive cells for each hypothalamic nuclei of every animal, and these numbers were use to obtain group means and standard error of estimate (SE). Differences in these cell counts were analyzed using independen group t-tests.
Table 1.
Brain regions analyzed for Fos-immunoreactivity following exposure to restricted feeding schedules in GHSR KO and WT mice or repeated injections of ghrelin or saline.
| Brain Region | Location with respect to Bregma according to Franklin and Paxinos (1997) |
|---|---|
| Oval nucleus of the bed nucleus of the stria terminalis (BNSTov) | 0.40–0mm anterior |
| Suprachiasmatic nucleus (SCN) | 0.3–0.7mm posterior |
| Paraventricular nucleus of the hypothalamus (PVN) | 0.7–1.2mm posterior |
| Dorsomedial nucleus of the hypothalamus (DMH) | 1.4–2.0mm posterior |
| Ventromedial nucleus of the hypothalamus (VMH) | 1.4–2.0mm posterior |
| Arcuate nucleus (ARC) | 1.4–2.0mm posterior |
| Lateral Hypothalamus (LH) | 1.0–2.0mm posterior |
Comparison of hypothalamic activation in mice under a restricted feeding schedule and mice treated with a daily injection of ghrelin
Mice (C57BL/J6) were assigned to one of four groups: a) Ad lib fed control (n=8); b) Restricted feeding schedule (n=5); c) Saline control (n=7); and d) Ghrelin (n=9). Food restricted mice had a four hour restricted access to food for a period of 7 days. Ghrelin treated mice were given intraperitoneal injections of 10nM of ghrelin every day for 7 days at ZT3. Control injected mice received saline injections of equal volume at the same time every day for 7 days. Ad lib fed controls were left undisturbed throughout the study. On the seventh day all animals were injected with an overdose of pentobarbital, perfused with saline and fixative and processed for Fos immunocytochemistry as described above. Food was removed from the cages of injected animals right after the injection, and from Ad lib fed controls one hour before being sacrificed. Differences in cell counts were evaluated using between group ANOVAs.
Results
Mice show elevated ghrelin concentrations in anticipation of a scheduled meal
As expected, food restricted C57BL/J6 mice adjusted their feeding patterns so as to consume almost all of their normal 24 hour food intake during the restricted access period (Interaction effect F(1,14)= 4.8, p.< 0.05; see supplemental figure 1, panel A). These animals were capable of doing this within a period of 7 days after the start of the restricted feeding schedule, and consumed more than control animals during the four hour access period (F(1,14)= 198.88, p.< 0.05; see panel B). Analyses of plasma acylated ghrelin concentrations showed that mice under a restricted feeding schedule had higher plasma levels of ghrelin in anticipation of the scheduled meal compared to those of control mice at the same time of the day (t(32)= −2.69, p.< 0.05).
Impairments in food anticipation in GHSR deficient mice
In a preliminary study, young (2–3 month old mice weighing 20–30g) GHSR KO and WT mice (n=6/group) were placed in a restricted feeding schedule with four hour access to food in the middle of the light phase of the light/dark cycle. WT mice adjusted readily to this feeding schedule and after seven days were consuming most of their baseline food intake during the 4-hour period in which food was available. GHSR KO mice, however, failed to adapt to the feeding schedule and had to be removed from the experiment due to unacceptable weight loss. The rate of attrition was 100% when mice weighed 25g or less at the onset of the study.
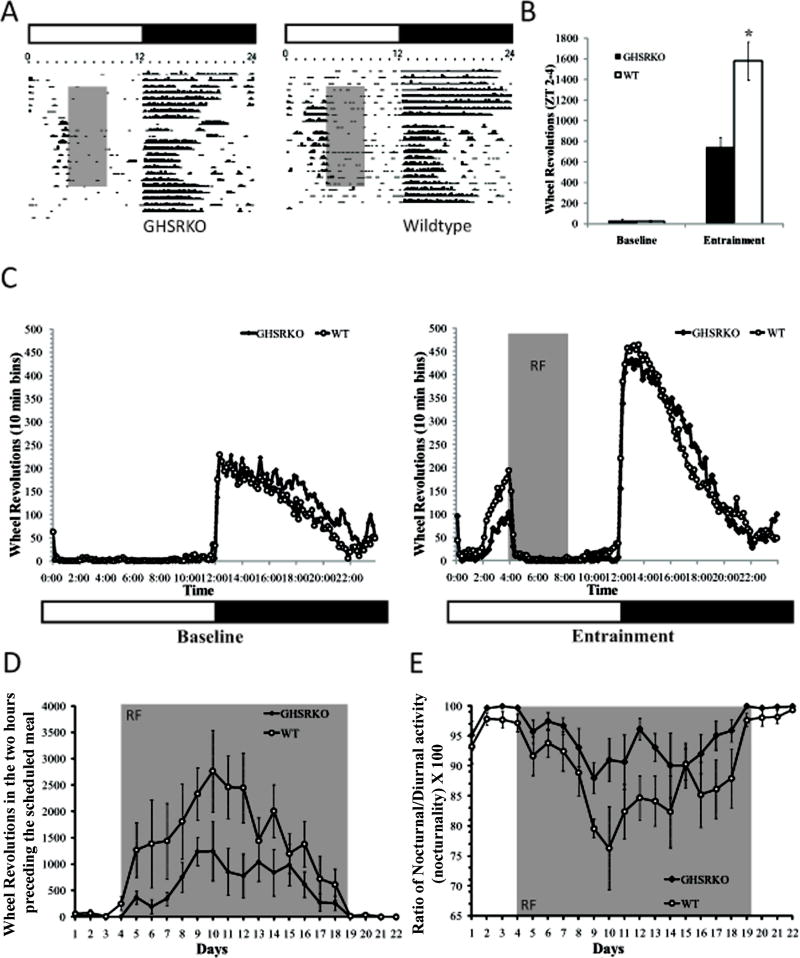
Using mice weighing 35gm or more at the onset of the study, and in order to assess the role of ghrelin in producing anticipatory locomotor activity to a scheduled meal, we placed GHSR KO mice (n=6) and their wild type littermates (n=6) under restricted feeding schedules and we monitored their locomotor activity patterns throughout the light/dark cycle for a period of two weeks. As seen in Figure 1, there were no differences in the patterns or in the amount of locomotor activity seen in GHSR KO and WT mice during the baseline period. Following the onset of the restricted feeding schedule, however, GHSR KO mice showed overall attenuated anticipatory locomotor activity compared to WT mice (F(1,13)=37.05, p.< 0.05; see figure 1 Panels a, b, and c) and these differences persisted for at least 10 days following the onset of the restricted feeding schedule(p.<0.05; figure 1 panel d). These differences were not due to differences in general locomotor activity given that GHSR KO mice showed similar levels of nocturnal activity throughout the study compared to WT mice (F(1,13)= 3.06, p.> 0.05). Lower food anticipatory responses were more likely due to diminished ability of GHSR KO mice to increase their day time activity in the way that WT mice did as reflected by nocturnality scores (F(1,13)= 16.08, p.<0.05; figure 1 panel e). Interestingly, GHSR KO mice ate as much food as WT mice before and during the restricted feeding schedule (See Supplemental figure 2).
Figure 1.
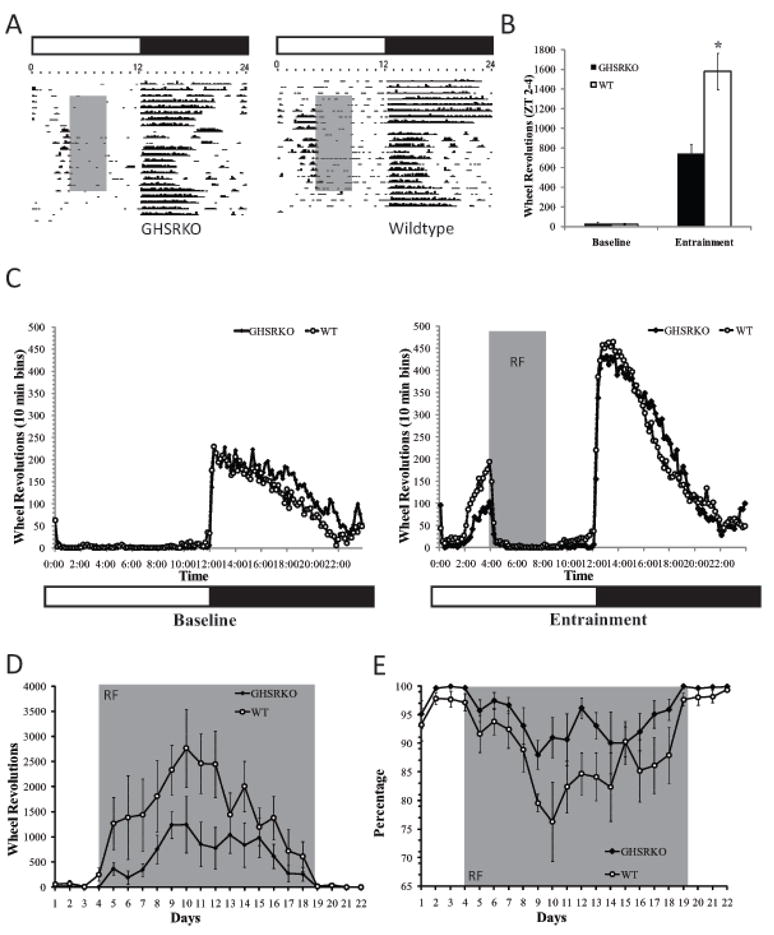
Representative actograms and group data from WT and GHSR KO mice under a 4-hr restricted feeding schedule. As seen in Panels B, C and D, anticipatory locomotor activity was significantly attenuated in GHSR KO mice (*p.<0.05), and remained attenuated for most of the restricted feeding schedule period (see Panel D). These effects are not due to locomotor deficits in these animals, but possibly due to deficits in shifting their nocturnal activity to diurnal activity as suggested by a higher ratio for nocturnal activity in GHSR KO mice (see Panel E). Gray shaded areas refer to the period of time during the day animals have access to food (Panels A,B and C), or to the number of days animals were subjected to the restricted feeding schedule (Panels D and E).
A second study looking at spontaneous locomotor activity in anticipation of the meal replicated the above described findings, showing that GHSR KO mice generated less anticipatory locomotor activity than WT when given daily restricted access to food (significant interaction effect, F(25,375)= 2.52, p.< 0.05). Interestingly, when access to food was restored to ad lib (i.e. 24 hour access), WT mice continued to show a small amount of locomotor activity in the hours prior to the time in which food was provided during the restricted access period, and this was higher than that exhibited by GHSR KO mice (p.< 0.05). Furthermore, when mice were food deprived for 36 hours evidence for anticipatory activity tended to be higher in GHSR WT mice compared to GHSR KO mice (p.=.08) (figure 2).
Figure 2.
Body weight, food intake and spontaneous locomotor activity in GHSR KO and WT mice before, during, and after a two week restricted feeding schedule, and after 36 hours of food deprivation. As seen in panels A and B, both GHSR WT and KO mice showed similar patterns of body weight and food intake throughout the study. In spite of this, GHSR KO mice moved less in anticipation to a scheduled meal (Panels C, D, E, and F). Interestingly, anticipatory activity persisted in WT mice but not in GHSR KO mice following the restricted feeding schedule under ad lib conditions. While anticipatory activity was seen in both WT and GHSR KO mice following a 36 hour food deprivation period, this tended to be lower in GHSR KO mice. * = significant differences between conditions (p. <0.05). ** = significant genotype differences (p.<0.05). Shaded area represents food restriction, RF with presentation at ZT 4, and F with total food deprivation.
Attenuated levels of hypothalamic Fos immunoreactivity in GHSR KO mice in restricted feeding schedules
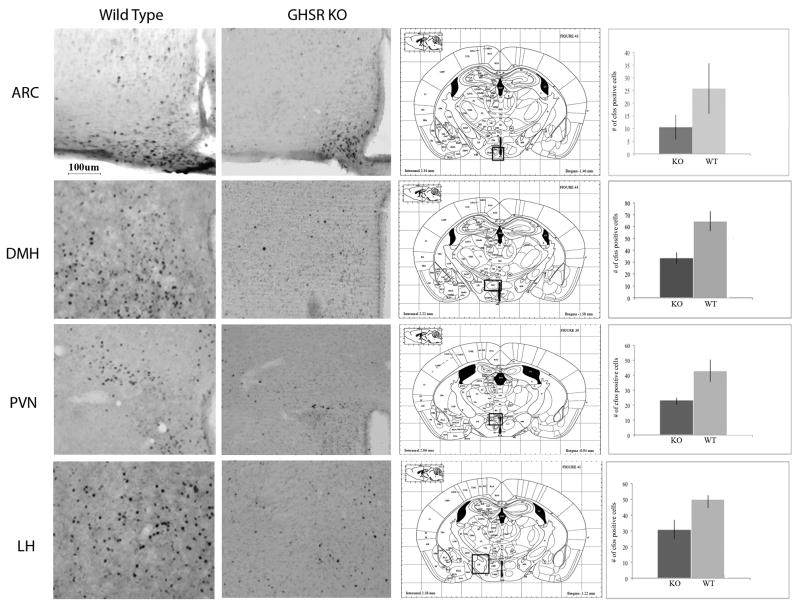
Using Fos as an index of neuronal activation, we examined patterns of activation in the hypothalamus of GHSR KO and WT mice under restricted feeding schedules. All Mice were sacrificed at the time in which they normally received their food. As seen in Figure 3, food restricted GHSR KO mice had significantly lower number of Fos-immunoreactive cells in the DMH, PVN, and LH in comparison with wild type controls (p. < 0.05). Fos expression in the ARC was lower in GHSR KO mice but differences did not achieve statistical significance. No differences in Fos expression were detected in any other region examined (see Figure 3).
Figure 3.
Fos immunoreactivity following restricted feeding schedules in GHSR KO and WT mice. As seen in this figure, both GHSR KO and WT controls showed comparable amounts of Fos immunoreactive nuclei in the ARC, but WT controls had higher levels of Fos immunoreactivity in the DMH, PVN and LH compared to food restricted GHSR KO mice (*p< 0.05).
Repeated ghrelin injections mimic hypothalamic Fos immunoreactivity patterns seen in miced in restricted feeding schedules
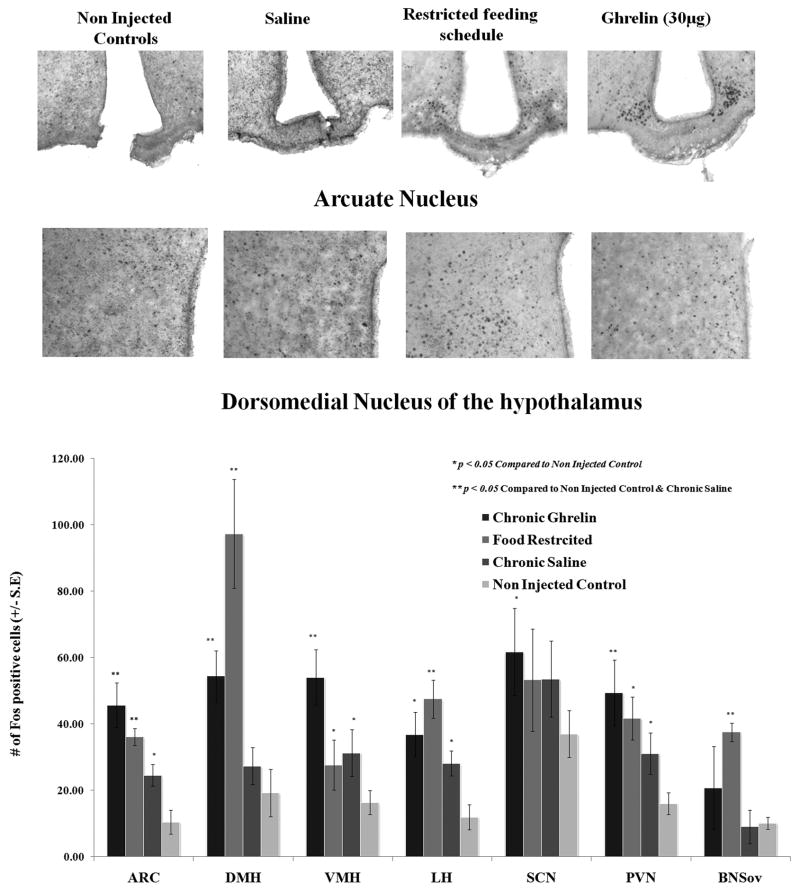
Patterns of hypothalamic Fos expression were compared between C57BL/J6 mice injected daily at ZT6 with vehicle (saline) or ghrelin (30μg/mouse) for seven days, and mice under restricted feeding schedules. Additional control non-injected mice were included in the study (Ad lib). As expected, scheduled fed mice had significantly higher number of Fos stained nuclei in the ARC, DMH, LH, PVN, and the oval nucleus of the stria terminalis (p.< 0.05). Mice receiving daily ghrelin injections also showed a larger number of Fos immunoreactive cells than mice in the ad lib control group in these nuclei, and a pattern similar to that seen in mice exposed to scheduled meals. Daily saline injections, however, appeared to increase Fos immunoreactivity to levels similar as those seen in food restricted mice (for example the PVN and LH). This was not the case in the DMH, where food restricted and ghrelin treated mice showed consistently higher levels of Fos expression than saline injected mice (p.<, 0.05; See Figure 4). While saline treated animals showed more Fos immunoreactive cells in the ARC compared to non injected ad lib controls, ghrelin treated and food restricted mice showed higher levels of Fos-labelled nuclei than saline treated mice (p.< 0.05).
Figure 4.
Chronic ghrelin injections given at ZT3 produce mimic patterns of Fos immunoreactivity seen in the hypothalamus of mice under restricted feeding schedules. Fos quantification shows that food restriction and ghrelin injections increased Fos immunoreactivity in the ARC and DMH of mice compared to levels of Fos seen in vehicle injected or non-injected control mice (p< 0.05). *= significantly different from control non injected; **= significantly different from saline injected.
Discussion
The coordination of physiological functions and behavioral responses with environmental predictors of food is a critical adaptive feature (Silver and Lesauter, 2008). Environmental cues signaling the proximity of food such as time of day, or a particular place influence a number of peripheral and central mechanisms to generate the behavioral repertoire (i.e. arousal, foraging, risk taking, hoarding, and food consumption) that is necessary to secure the needed nourishment. In this study we tested the hypothesis that ghrelin plays a key role as a signal coordinating physiological and behavioral daily rhythms, with the time at which foods are available.
A number of studies have hinted at the possibility that ghrelin is important for the generation of anticipatory locomotor responses to food. In several species of mammals, ghrelin levels peak prior to the regular onset of meals, and these peaks can shift to other times of the day in anticipation of scheduled meals (Cummings et al., 2001, Sugino et al., 2002, Drazen et al., 2006, Morgado et al., 2008, Sutton et al., 2008). Moreover, ghrelin injections increase locomotor activity in laboratory rats, and stimulate foraging-like responses in hamsters (Keen-Rhinehart and Bartness, 2005, Jerlhag, 2008). In line with this evidence, we show that ghrelin levels in mice are elevated in anticipation to a scheduled meal. Furthermore, GHSR KO mice under a similar restricted feeding schedule had difficulties adjusting to scheduled meals if they were lean, and those that did adjust exhibited significantly attenuated locomotor responses in anticipation to their scheduled meals. Differences in locomotor activity were not due to abnormalities in basal locomotor activity related to the GHSR deficiency, because GHSR KO mice displayed basal levels of activity comparable to those of WT. Nocturnality scores show, however, that while GHSR WT mice increased their day their day time activity, GHSR KO mice did not. Interestingly, both GHSR KO and WT mice show residual activity in anticipation of the previously scheduled meal after the termination of the restricted feeding schedule. Nevertheless, as seen in figure 2, WT mice show more activity in the two hours preceding the previously scheduled meal when returned to ad lib conditions, and tend to show more activity during this time when fasted for 36 hours when compared to GHSR KO mice. Thus, while GHSR KO mice show evidence of entrainment, their food anticipation is lower than that of WT mice.
Ghrelin could be acting at a number of hypothalamic regions to generate locomotor anticipatory responses. Food restricted C57BL/J6 and GHSR WT mice both showed increased levels of Fos immunoreactivity in the DMH, ARC, PVN and LH in comparison with a lib fed control C57BL/J6 or GHSR KO mice. Interestingly, repeated daily ghrelin injections given to ad lib fed mice produce neuronal activation patterns that partially parallel those seen in animals under a restricted feeding schedule. Thus, the number of Fos positive cells in the DMH, ARC, PVN and LH of ghrelin injected mice was higher than those seen in non injected control mice. Nevertheless, saline injections were sufficient to increase Fos expression in the VMH, LH, and PVN, suggesting that perhaps increased Fos expression in this regions is partially mediated by stimulation of stress pathways, and that ghrelin (or the restricted feeding schedule) augment these neuronal responses. One particular region, the DMH, was selectively stimulated by food restriction and ghrelin but not by the saline injections. Interestingly, the DMH has been proposed as a possible site for a food entrainable oscillator for a number of reasons(Chou et al., 2003, Gooley et al., 2006). For example, the DMH participates in a number of functions including food intake, thermoregulation, sympathetic activation, and sleep arousal, and increased Fos and clock gene expression in animals under restricted feeding schedules(Bellinger and Bernardis, 2002, Gooley et al., 2006, Dimicco and Zaretsky, 2007, Verwey et al., 2007). While the degree of contribution of the DMH in the entrainment of activity rhythms to scheduled meals remains under contention(Gooley et al., 2006, Landry et al., 2007), our data suggest that the DMH is especially sensitive to ghrelin secretion to generate preprandial locomotor responses. It should be noted that the DMH may not be necessary for the generation of anticipatory activity in rats and mice placed under restricted feeding schedules(Moriya et al., 2009). Moreover, clock gene expression in the DMH can be decoupled from locomotor activity in anticipation of a meal in rats under restricted feeding schedules that are provided food access at random times during the day suggesting that the role of the DMH on food anticipation in scheduled fed rodent is not as strong as previously suggested (Verwey et al., 2009). It is more likely that ghrelin targets several hypothalamic regions in parallel to enhance food anticipation, a hypothesis that seems supported by our Fos data.
Interestingly, a region that was selectively stimulated by the restricted feeding schedule and ghrelin injections, but not by saline injections was the ARC. Neurons in the ARC secrete agouti related peptide (AGRP) or α-melanocyte-stimulating hormone (α-MSH). The former stimulates whereas the latter decreases food intake and energy expenditure by acting on melanocortin receptors 3 and 4 (MC3 and MC4). These receptors are found in high density in all areas expressing Fos in response to either ghrelin or after the restricted feeding schedule, including the DMH(Cone, 2006). Recent evidence shows that MC3 KO mice have attenuated locomotor responses in anticipation of a scheduled meal similar to those seen in GHSR KO mice as well as in the expression of clock related proteins in relation to the meal(Sutton et al., 2008). It is therefore possible that anticipatory ghrelin peaks may act through the ARC nucleus to stimulate the release of AGRP, and block MC3 receptors in the DMH and PVN ultimately increasing anticipatory locomotor activity via its actions on these nuclei. Alternatively, anticipatory ghrelin peaks may stimulate hypothalamic sites through indirect pathways. For example, ghrelin may mediate hypothalamic activation via ascending noradrenergic pathways. Indeed, peripheral ghrelin stimulates cells in brain stem regions like the nucleus of the solitary tract and parabrachial nucleus, both of which convey information from the gut to the hypothalamus, and are implicated in food anticipation (Davidson et al., 2000, Angeles-Castellanos et al., 2005).
While there is a significant degree of overlap in patterns of cellular activity in the hypothalamus of food restricted mice and that of ghrelin injected mice, Fos expression appears to increase following vehicle injections. For example, the PVN and LH of saline injected mice show Fos expression that is significantly higher than non injected ad lib mice and comparable to that of ghrelin treated mice (PVN) and/or food restricted mice (LH). This is not surprising given the role of these regions in stress and arousal(Winsky-Sommerer et al., 2004, Horvath and Gao, 2005), and perhaps reflect the partial contribution of the hypothalamic-pituitary-adrenal axis and that of the orexin system in producing anticipatory feeding responses in food restricted mice (Akiyama et al., 2004, Girotti et al., 2009).
In all, our data supports the notion that ghrelin is an important signal coordinating behavioral activity rhythms with the timing of food availability. Ghrelin does this by increasing the activity of hypothalamic nuclei implicated in the regulation of energy balance, including the DMH, ARC, PVN and LH. Understanding the relative contribution of these hypothalamic nuclei as well as that of extrahypothalamic regions in conveying ghrelin signals for the generation of food anticipation will lead to a better understanding of the mechanisms regulating food intake and energy balance.
Supplementary Material
Food intake and ghrelin concentrations in C57BL/J6 mice with 4hr restricted access to food starting at ZT 4 (noon) and ending at ZT 8 (16:00hrs). Food restricted mice increased their food intake so as to consume similar amounts of food during the restricted access period to the amounts consumed by ad lib controls during a whole 24 hr. period (Panel A). Panel B shows the intake of food restricted mice in relation to the intake of ad lib fed animals during the restricted access period. Food restricted mice showed significantly higher levels of acylated ghrelin than controls 1 hr before food access began (*p.<0.05).
Food intake during restricted feeding schedules in GHSR WT and KO mice from the first behavioral study. There were no significant differences in food intake between GHSR WT and KO mice at any point during the study (p. < 0.05). Locomotor activity data from these mice are shown in Figure 1.
Acknowledgments
This project was funded by grants from the Natural Sciences and engineering Research Council of Canada (NSERC), the Canadian Funds for innovation (CFI) awarded to AA, and from National Institute of Health (NIH) grant to TLH (DK-060711).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29:119–131. doi: 10.1159/000122913. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Mezei G, Thanarajasingam G, Horvath TL. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur J Neurosci. 2005;21:1536–1546. doi: 10.1111/j.1460-9568.2005.03964.x. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Diaz-Munoz M, Escobar C. Food entrainment modifies the c-Fos expression pattern in brain stem nuclei of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R678–684. doi: 10.1152/ajpregu.00590.2004. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Cappendijk SL, Stephan FK. Feeding-entrained circadian rhythms are attenuated by lesions of the parabrachial region in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1296–1304. doi: 10.1152/ajpregu.2000.278.5.R1296. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. Mouse brain in stereotaxoc cooordinates. Academic Press; New Yrk, NY: 1997. [Google Scholar]
- Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab. 2009;296:E888–897. doi: 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Morgado E, Gordon MK, del Carmen Minana-Solis M, Meza E, Levine S, Escobar C, Caba M. Hormonal and metabolic rhythms associated with the daily scheduled nursing in rabbit pups. Am J Physiol Regul Integr Comp Physiol. 2008;295:R690–695. doi: 10.1152/ajpregu.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM. Inducible clocks: living in an unpredictable world. Cold Spring Harb Symp Quant Biol. 2007;72:543–550. doi: 10.1101/sqb.2007.72.008. [DOI] [PubMed] [Google Scholar]
- Silver R, Lesauter J. Circadian and homeostatic factors in arousal. Ann N Y Acad Sci. 2008;1129:263–274. doi: 10.1196/annals.1417.032. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino T, Hasegawa Y, Kikkawa Y, Yamaura J, Yamagishi M, Kurose Y, Kojima M, Kangawa K, Terashima Y. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochem Biophys Res Commun. 2002;295:255–260. doi: 10.1016/s0006-291x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Verwey M, Lam GY, Amir S. Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06766.x. [DOI] [PubMed] [Google Scholar]
- Wiepkema PR, de Ruiter L, Reddingius J. Circadian rhythms in the feeding behaviour of CBA mice. Nature. 1966;209:935–936. doi: 10.1038/209935a0. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Food intake and ghrelin concentrations in C57BL/J6 mice with 4hr restricted access to food starting at ZT 4 (noon) and ending at ZT 8 (16:00hrs). Food restricted mice increased their food intake so as to consume similar amounts of food during the restricted access period to the amounts consumed by ad lib controls during a whole 24 hr. period (Panel A). Panel B shows the intake of food restricted mice in relation to the intake of ad lib fed animals during the restricted access period. Food restricted mice showed significantly higher levels of acylated ghrelin than controls 1 hr before food access began (*p.<0.05).
Food intake during restricted feeding schedules in GHSR WT and KO mice from the first behavioral study. There were no significant differences in food intake between GHSR WT and KO mice at any point during the study (p. < 0.05). Locomotor activity data from these mice are shown in Figure 1.