Abstract
Twin studies indicate that both intelligence and brain structure are moderately to highly heritable. Recent bivariate studies of adult twins also suggest that intelligence and brain morphometry are influenced by shared genetic factors. The current study examines shared genetic and environmental factors between brain morphometry and intelligence in a sample of children and adolescents (twins, twin siblings, and singletons; n = 649, ages 4–19). To extend previous studies, brain morphometric data were parsed into subregions (lobar gray/white matter volumes, caudate nucleus, lateral ventricles) and intelligence into verbal and nonverbal skills (Wechsler Vocabulary and Block Design subtests). Phenotypic relationships between brain volumes and intelligence were small. Verbal skills shared unique environmental effects with gray matter volumes while nonverbal skills shared genetic effects with both global and regional gray and white matter. These results suggest that distinct mechanisms contribute to the small phenotypic relationships between brain volumes and verbal versus nonverbal intelligence.
Keywords: Twin, Magnetic resonance imaging, Brain, Intelligence, Verbal, Nonverbal
Introduction
Research investigating the genetic contributions to individual differences in intellectual ability dates to the mid-nineteenth century and Galton's (1869) famous study of the heredity of “genius” (Plomin et al. 1997a). Numerous twin, adoption and molecular genetic studies currently are focused on the genetic and environmental contributions to variation in intelligence and its neuroanatomic underpinnings, but few have utilized bivariate methods to identify shared and unique influences, particularly during childhood and adolescence. Such research has the potential to inform studies seeking to develop novel biomedical or educational interventions aimed at augmenting cognitive functioning of individuals with intellectual disabilities or other learning difficulties. We present data on a sample of typically developing pediatric twins, their siblings, and unrelated, non-twins, referred to as ‘singletons’ from this point forward. Our analyses investigate the shared genetic and environmental covariance between individual differences in brain volumes (white and gray matter overall; frontal, temporal, and parietal lobes; and the caudate and lateral ventricles) and verbal (word knowledge) and nonverbal (visuo-spatial) skills.
Univariate twin studies of intelligence and specific cognitive abilities
Twin studies of general intelligence indicate that the variance attributable to genetic effects (i.e., heritability) is moderate (approximately 50%) and increases from 20% in infancy to 40% in childhood to 60% in adulthood. In contrast, estimates of shared environmental influences decrease from approximately 60% in infancy to negligible values in adulthood (for review, see McGue et al. 1993). A recent multi-site study of over 10,000 twin pairs comprised of children, adolescents, and young adults corroborated these earlier findings of increasing genetic influences on general intelligence with increasing age (Haworth et al. 2009). In a review of studies of specific cognitive abilities (that have included both children and adults), Plomin and Craig (1997) report heritability estimates of approximately 65% for verbal ability and 55% for visuo-spatial ability. Adoption studies suggest that heritability estimates for verbal and visuo-spatial abilities, similar to heritability estimates for overall intellectual ability, increase from early to later childhood (Plomin et al. 1997b). The consistency in these findings is likely due to the fact that verbal ability, as measured by Vocabulary or similar subtests, and visuo-spatial ability, as measured by Block Design or similar subtests, often are moderately to highly predictive of full scale IQ (e.g., Sattler 1992).
Univariate twin studies of brain volumes
Twin studies of brain volumes indicate that heritability is relatively high and age-specific (for review, see Peper et al. 2007; Schmitt et al. 2007). Many of these studies have examined total brain volumes, often quantifying gray matter and white matter separately, but to date, only three have examined lobar-level structures (Carmelli et al. 2002; Geschwind et al. 2002; Wallace et al. 2006). Two of these studies analyzed results from 72 monozygotic (MZ) pairs and 67 dizygotic (DZ) pairs of older adult twins (Carmelli et al. 2002; Geschwind et al. 2002) and one analyzed results from a pediatric sample including 90 MZ twin pairs and 38 DZ twin pairs (Wallace et al. 2006). Across all three studies, heritability values for lobar-level structures were generally more variable and lower than those for global indices of brain volume. When comparing brain structures common to these studies (frontal, temporal, and parietal), lobar-level volumes were more heritable in the pediatric (additive genetic influences = .77–.88) than in the older adult (additive genetic influences = .40–.75) samples.
Phenotypic correlation between intelligence and brain volumes
Several studies have investigated the correlation between total brain volume and intelligence. McDaniel (2005) completed a meta-analysis of 37 samples, comprising 440 child and 1,090 adult participants, and reported an average correlation of .33 between intelligence and total brain volume. This correlation was reported to be higher for females than males and higher for adults than children. To the best of our knowledge, no study has reported correlations between brain volumes and verbal or visuo-spatial skills in a sample of typically developing children and adolescents. However, a few studies have examined these relations in adults. For example, Posthuma et al. (2003) reported no significant correlations between total gray matter, total white matter, or cerebellar volumes and the Verbal Comprehension Index (of which Vocabulary is one of three subtests) of the Wechsler Adult Intelligence Scale-III (r = .06, .01, and .03, respectively); however, they did find small, significant correlations between the Perceptual Organization Index (of which Block Design is one of three subtests) and both total gray matter and cerebellar volumes (r = .20 and .18, respectively). These findings are consistent with earlier research (Flashman et al. 1998) in which small, non-significant correlations between Verbal IQ and lobar volumes (r from .06 to .19) and somewhat larger, significant correlations between Performance IQ and lobar volumes (r from .15 to .28) were found. In this latter study, no significant correlations between Vocabulary and any brain measure (frontal, temporal, occipital, parietal, cerebellar, and whole brain volumes) were reported; however, small significant correlations were reported between Block Design and all of the aforementioned brain volumes except frontal lobar volumes (r from .21 to .28).
Bivariate twin studies of brain structure and intelligence
We are aware of four previous reports (based on two adult samples) that have used twin modeling to investigate the shared genetic and environmental relations between brain structure and intelligence. These include both brain volume (Posthuma et al. 2002, 2003) and voxel-based methods (Hulshoff Pol et al. 2006; Thompson et al. 2001). Consistent with investigations of singletons, a small to moderate phenotypic relationship has been observed between brain structure and IQ. The limited data available suggest that a substantial portion of this is due to shared genetic influences. In a study of adults, the phenotypic relationship between cerebellar volume and performance on the Perceptual Organization index was underpinned by both genetic and unique environmental influences. In contrast, significant genetic and environmental correlations between brain volume and Verbal Comprehension were not found. The first bivariate study to utilize voxel-based methods, focusing on gray matter densities and general intellectual ability, demonstrated significant shared genetic contributions (Thompson et al. 2001). Extending these findings, a subsequent investigation (Hulshoff Pol et al. 2006) showed significant genetic effects on the phenotypic relationship between nonverbal intellectual skills (Performance IQ) and localized gray and white matter densities. Shared genetic effects between brain structure and verbal ability (Verbal IQ) were also present, but to a lesser extent.
Because childhood and adolescence are periods of dynamic structural brain development (e.g., Lenroot et al. 2007) and explosive growth in educational attainment and cognitive skill acquisition, the examination of shared genetic and environmental covariances between brain structure and intellectual functioning during this age range is particularly important. Thus, the current study investigates these covariances in a young typically developing sample using finer-grained measurements of both brain volume and intellectual functioning than have been used previously.
Methods
Participants
A total of 649 typically developing individual twins, siblings of twins, and singletons (see Table 1 for demographic characteristics) were recruited as part of an ongoing longitudinal brain imaging project being conducted at the Child Psychiatry Branch of the National Institute of Mental Health (NIMH; Giedd et al. 2009). The same-sex twins comprised 107 MZ pairs, 53 DZ pairs and 23 unmatched individual twins. BRT Laboratories and Proactive Genetics determined zygosity through DNA analysis of buccal cheek swabs using 9–21 unlinked short tandem repeat loci for a minimum certainty of 99%. Prospective participants were excluded if they had ever required special services in school, taken psychiatric medications, received mental health treatment, or had any condition known to affect gross brain development. Inclusionary criteria were a gestational age of at least 29 weeks, a minimum birth weight of 1,500 g, and a summary IQ score of 70 or greater. The groups were comprised of predominantly Caucasian participants (88%). We obtained verbal or written assent from the child and written consent from the parents or adult participants. The NIMH Institutional Review Board approved the protocol.
Table 1.
Demographic characteristics
| MZ |
DZ |
Singletons |
Siblings of twins |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | p | |
| Sex | ||||||||||
| Male | 103 | 54.0 | 58 | 51.3 | 96 | 55.8 | 52 | 41.6 | 309 | 0.14 |
| Female | 121 | 46.0 | 61 | 48.7 | 121 | 44.2 | 37 | 58.4 | 340 | |
| Ethnicity | ||||||||||
| White | 212 | 94.6 | 114 | 95.8 | 160 | 73.7# | 87 | 97.8 | 573 | <0.001 |
| Other | 12 | 5.4 | 5 | 4.2 | 57 | 26.3 | 2 | 2.2 | 76 | |
| Handednessa | ||||||||||
| Right | 198 | 88.4 | 94 | 79.0^ | 194 | 89.4 | 72 | 80.9^ | 558 | 0.02 |
| Non-right | 26 | 11.6 | 25 | 21.0 | 23 | 10.6 | 17 | 19.1 | 91 | |
|
| ||||||||||
| M (SD) | M (SD) | M (SD) | M (SD) | |||||||
|
| ||||||||||
| Age | 12.1 (3.7) | 10.1 (3.7)* | 12.5 (3.8)* | 11.8 (3.8) | <0.0001 | |||||
| Range | 5.5–19.5 | 5.3–18.2 | 4.4–19.5 | 5.5–19.6 | ||||||
| SESb | 43.8 (18.2) | 44.5 (15.7) | 40.7 (21.3) | 41.3 (16.5) | 0.25 | |||||
| Range | 20–89 | 20–82 | 20–95 | 20–82 | ||||||
For all study participants, handedness was assessed by using the Physical and Neurological Examination for Soft Signs (PANESS; Denckla 1985)
SES (Socioeconomic Status) was measured by using the Hollingshead Two-Factor Index (Hollingshead and Redlich 1958). Note that lower scores denote higher SES
Groups carrying significant differences (p < 0.05) for a measure compared to the total sample
Singletons have a lower proportion of white participants than all other groups as indicated by pair-wise chi-square analyses (p < 0.001)
Pair-wise chi-square analyses indicate that DZs and Siblings of twins have a lower proportion of right-handed participants than Singletons and that DZs also have a lower proportion of right-handed participants than MZs (p < 0.05)
Measures of verbal and nonverbal intellectual skills
Vocabulary and Block Design subtest scores were obtained using the Wechsler Scales. Given the age range of the participants and that study enrollment has been ongoing for 19 years, different versions of the Wechsler Scales had been administered. The majority of participants (n = 551) completed the Vocabulary and Block Design subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999); the remainder completed these subtests from the Wechsler Intelligence Scale for Children (Wechsler 1974, 1991; n = 78), the Wechsler Preschool and Primary Scale of Intelligence (Wechsler 1967, 1989, 2002; n = 17), or the Wechsler Adult Intelligence Scale (Wechsler 1981; n = 3). Administration procedures for the two subtests are very similar across instruments. For the Vocabulary sub-test, participants are asked to provide the definitions of words of increasing difficulty (for younger and less able participants, in some cases, picture vocabulary items precede oral vocabulary items). For the Block Design subtest, participants copy a series of geometric designs utilizing colored blocks and completion is timed.
Although different versions of the Wechsler scales were used, performance on these tests is thought to be comparable. Based on data reported in the Wechsler Abbreviated Scale of Intelligence manual (Wechsler 1999), typically developing children who completed both the Wechsler Abbreviated Scale of Intelligence and the Wechsler Intelligence Scale for Children—Third Edition obtained very similar mean subtest scores (within 1 point of each other). Furthermore, these subtest scores were highly correlated, with a magnitude (r = .72–.74) similar to reported test–retest reliability values (r = .80–.84). These data provide support for our including subtest scores from various versions of the Wechsler scales. While one study raised questions about the comparability of the abbreviated and full versions of the adult Wechsler scales (Axelrod 2002), it used very different participants—a heterogeneous clinical sample recruited from a veteran's hospital. Given that our participants were typically developing children/adolescents and that very few of them completed the full adult version of the Wechsler scale, we do not believe that the use of different versions of the Wechsler scales is problematic in our study.
Magnetic resonance imaging (MRI) acquisition
All MRI scans were acquired on the same General Electric 1.5 Tesla Signa Scanner located at the National Institutes of Health Clinical Center in Bethesda, Maryland. A three-dimensional spoiled gradient recalled echo sequence in the steady state, designed to optimize discrimination between gray matter, white matter, and cerebrospinal fluid, was used to acquire 124 contiguous 1.5-mm thick slices in the axial plane (TE/TR = 5/24 ms; flip angle = 45 degrees, matrix = 256 × 192, NEX = 1, FOV = 24 cm, acquisition time 9.9 min).
Image analysis
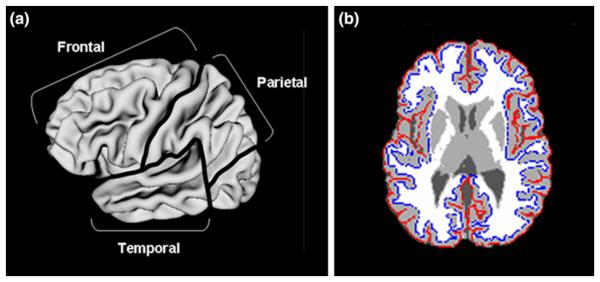
The native MRI scans were registered into standardized stereotaxic space using a linear transformation (Collins et al. 1994) and corrected for nonuniformity artifacts (Sled et al. 1998). The registered and corrected volumes were segmented into gray matter, white matter, cerebrospinal fluid and background using a neural net classifier (Zijdenbos et al. 2002). The tissue classification information was combined with a probabilistic atlas to provide volumetric measures (Collins et al. 1995) of gray and white matter volumes of the total cerebrum, frontal, temporal, and parietal lobes, the lateral ventricles (see Fig. 1), and the caudate nucleus (not shown). These measures have shown high agreement with conventional hand tracing measures.
Fig. 1.
a Lateral view of lobar-level brain regions quantified for this study (occipital lobe not labeled); b Axial view of gray matter (in light gray) and white matter (in white) segmentation with lateral ventricles depicted in dark gray
Statistical analysis
Univariate variance component analyses were conducted on Vocabulary and Block Design scores and volumetric measures in order to characterize the relative influence of genetic and environmental factors prior to conducting the bivariate analyses (Neale and Cardon 1992). These heritability estimates were obtained using all available twin, sibling, and singleton data. In these analyses the total variance () was partitioned into additive genetic (), shared environmental effects (), and non-shared or unique environmental () effects. The total variance was thus parameterized as: , while the covariance terms were parameterized as: and . Univariate ACE, CE, AE and E only models were fitted to test the significance of additive genetic and shared environmental effects. Optimization of this data used maximum likelihood (Edwards 1984) by calculating twice the negative log-likelihood of the raw data for each twin pair and summing across all pairs. The use of maximum likelihood in measuring model fit allows for hypothesis testing between an original model (ACE) and its nested models (AE, CE and E only). Because the variance component estimates are zero-bounded, the difference between an original model and its respective submodels follows a 50:50 mixture of zero and a χ2 distribution with degrees of freedom equal to the difference in model parameters (df = 1).
Two series of analyses were conducted to examine the genetic and environmental covariation between brain volume and (1) Vocabulary and (2) Block Design performance. The statistical significance of genetic and environmental correlations was assessed by comparing twice the log-likelihood of the full Cholesky model to that of a submodel in which the genetic or environmental correlation between morphometric measures and Wechsler subtest scores was set to zero. Under certain regularity conditions, such differences are asymptotically distributed as chi-squared with one degree of freedom. A third sub-model tested the significance of the phenotypic correlation by setting all three genetic and environmental correlations between the Wechsler subtests and brain volumes to zero. Genetic and environmental covariances were used to estimate both the total covariance and phenotypic correlations (Posthuma et al. 2003) as well as to determine relative and absolute contributions of each factor to the relationship between brain volumes and Vocabulary and Block Design subtest scores.
A Cholesky factorization allows for the decomposition of the genetic and environmental covariance matrices for measures of brain structure and Wechsler subtest performance. The diagonal elements of either an A or E matrix produces the variances due to that specific effect for each of the individual measures while the off-diagonal element produces the covariances due to either of the effects. The Cholesky decomposition calculates A and E matrices as the product between a lower triangular matrix and its transpose. The production of separate genetic and environmental variance/covariance matrices are positive semi-definite which is consistent with the idea that variation is caused by factors that operate in a linear additive fashion.
The genetic and environmental covariances between measures of brain structure and Wechsler subtest scores were used to calculate genetic correlations to indicate the degree to which genetic effects are shared between the two measures. The genetic correlation between two measures is defined as.
where Axy is the genetic covariance between each measure of brain structure (x) and a subtest score (y) and Ax and Ay represent the heritability of x and y. The environmental correlation is similarly defined using measures of environmental variance and covariance. Shared environmental effects were non-significant in univariate analyses of Wechsler subtest performance and measures of brain volume (Table 3). Therefore, bivariate genetic models decomposed the covariance between these measures into genetic and unique environmental variance.
Table 3.
Univariate model fit summary
| Full model | No A | No C | No AC | A | 95% CI | C | 95% CI | E | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vocabulary | 3137.27 | <0.0001 | 1.00 | <0.0001 | 0.64 | (0.42; 0.78) | 0.08 | (0; 0.25) | 0.28 | (0.22; 0.38) |
| Block design | 3118.95 | <0.0001 | 1.00 | <0.0001 | 0.75 | (0.55; 0.81) | 0 | (0; 0.18) | 0.25 | (0.19; 0.33) |
| Gray matter + white matter | 3118.95 | <0.0001 | 0.90 | <0.0001 | 0.91 | (0.74; 0.94) | 0 | (0; 0.18) | 0.08 | (0.06; 0.1) |
| Gray matter | 3169.00 | <0.0001 | 0.49 | <0.0001 | 0.83 | (0.65; 0.92) | 0.06 | (0; 0.24) | 0.11 | (0.08; 0.15) |
| White matter | 3032.88 | <0.0001 | 1.00 | <0.0001 | 0.89 | (0.75; 0.92) | 0 | (0; 0.14) | 0.11 | (0.08; 0.15) |
| Frontal gray matter | 3205.08 | <0.0001 | 0.49 | <0.0001 | 0.82 | (0.64; 0.91) | 0.05 | (0; 0.24) | 0.11 | (0.08; 0.15) |
| Frontal white matter | 3006.51 | <0.0001 | 1.00 | <0.0001 | 0.92 | (0.76; 0.94) | 0 | (0; 0.16) | 0.08 | (0.06; 0.1) |
| Parietal gray matter | 3156.09 | <0.0001 | 0.34 | <0.0001 | 0.61 | (0.4; 0.82) | 0.18 | (0; 0.36) | 0.21 | (0.16; 0.29) |
| Parietal white matter | 3073.19 | <0.0001 | 0.94 | <0.0001 | 0.74 | (0.55; 0.88) | 0.1 | (0; 0.28) | 0.15 | (0.11; 0.2) |
| Temporal gray matter | 3208.84 | <0.0001 | 0.97 | <0.0001 | 0.92 | (0.78; 0.94) | 0 | (0; 0.13) | 0.08 | (0.06; 0.11) |
| Temporal white matter | 3062.08 | <0.0001 | 1.00 | <0.0001 | 0.89 | (0.77; 0.92) | 0 | (0; 0.12) | 0.11 | (0.08; 0.15) |
| Lateral ventricles | 3399.24 | <0.0001 | 1.00 | <0.0001 | 0.65 | (0.5; 0.73) | 0 | (0; 0.09) | 0.35 | (0.27; 0.46) |
| Caudate nucleus | 3340.93 | <0.0001 | 1.00 | <0.0001 | 0.85 | (0.71; 0.89) | 0 | (0; 0.12) | 0.15 | (0.11; 0.21) |
The false discovery rate (FDR), set at q = .05, was utilized to control for multiple comparisons (Benjamini and Hochberg 1995). Because previous analyses of brain volume measures have shown significant effects of age and sex (Giedd et al. 1999; Lenroot et al. 2007), all analyses included sex, and the linear, quadratic, and cubic effects of age as fixed effects in the means models. Maximum likelihood analyses of individual observations (as implemented in Mx 1.66; Neale et al. 2006) were used for all analyses.
Results
Descriptive statistics
Prior to variance component estimation, group differences in Vocabulary and Block Design scores and brain volumes were examined. Performance on the Vocabulary and Block Design subtests differed significantly between groups (see Table 2). For Vocabulary, MZ twins had significantly lower scores and singletons had significantly higher scores compared to the entire sample. For Block Design, MZ twins had significantly lower scores. These group differences were not expected to bias estimates of genetic and environmental effects since there were no differences in group means or variances between MZ and DZ twins. There were no significant differences in brain volume means or variances between MZ twins, DZ twins, singletons, and siblings of twins, with the exception of the lateral ventricles (see Table 2). Mean differences were assessed using PROC MIXED in SAS (SAS version 9.1.3; SAS Institute, Cary, NC) to account for the random residual effects of twin and family resemblance on the simplifying assumption of constant correlation between measures within families and sibling groups.
Table 2.
Means (SDs) for vocabulary, block design, and brain volumes
| MZ (N = 224) |
DZ (N = 119) |
Singletons (N = 217) |
Siblings of twins (N = 89) |
p | |
|---|---|---|---|---|---|
| Vocabulary | 11.4 (2.8)* | 11.5 (2.6) | 12.3 (3.0)* | 12.0 (3.0) | 0.03 |
| Range | 4–18 | 5–18 | 2–19 | 5–18 | |
| Block design | 11.3 (2.8)* | 11.9 (3.0) | 12.2 (2.9) | 12.3 (3.0) | 0.02 |
| Range | 4–19 | 3–19 | 4–19 | 5–19 | |
| Gray matter + white matter | 1172.5 (106.5) | 1146.1 (105.9) | 1164 (117.5) | 1165.8 (118.6) | 0.41 |
| Range | 894.7–1470.4 | 816.3–1417.7 | 891.3–1473.1 | 920.4–1495.6 | |
| Gray matter | 736.5 (66.2) | 725 (70.1) | 728 (78.1) | 732.6 (75.1) | 0.68 |
| Range | 430.5–910.3 | 492.9–904.5 | 563.1–935.8 | 583.3–981.6 | |
| White matter | 435.9 (55.8) | 421.1 (53.3) | 435.9 (54.4) | 433.2 (55.9) | 0.22 |
| Range | 322.3–586.4 | 286.7–576.9 | 302–612.9 | 329.4–605.4 | |
| Frontal gray matter | 232.3 (21.3) | 228.8 (22.6) | 230.5 (25.2) | 231.2 (24.6) | 0.78 |
| Range | 143.2–288.8 | 155.9–286.6 | 173–298.7 | 178.8–310.3 | |
| Frontal white matter | 167.3 (22.6) | 161.6 (20.3) | 167.4 (21) | 167.1 (22.3) | 0.21 |
| Range | 125.4–227.5 | 112.8–217.9 | 113.1–238.2 | 125.7–234.4 | |
| Parietal gray matter | 123.1 (13) | 124.2 (13.2) | 122.6 (15.4) | 124 (14.8) | 0.81 |
| Range | 89.8–154.8 | 95.1–167.9 | 91.6–169.6 | 93.7–177 | |
| Parietal white matter | 83.9 (11.8) | 82.9 (11.3) | 85 (11.2) | 84.5 (11.9) | 0.57 |
| Range | 58.2–115.1 | 56.9–114.6 | 57.6–120.6 | 62.1–125.6 | |
| Temporal gray matter | 190.5 (18.5) | 186.5 (20.2) | 187.8 (20.4) | 189.7 (20) | 0.52 |
| Range | 88.4–245.2 | 117.8–232.5 | 138.6–246.1 | 150.7–264.5 | |
| Temporal white matter | 94 (12.3) | 91 (12.7) | 93.9 (12.4) | 93.6 (12.9) | 0.35 |
| Range | 69.1–131 | 65.9–130.6 | 64.7–134.8 | 67.4–129.3 | |
| Lateral ventricles | 11.5 (6.6)* | 10.2 (5.1) | 10.6 (5.9) | 8.4 (4.1)* | 0.0001 |
| Range | 2.9–41.5 | 3–31.6 | 2.8–35.9 | 3.3–27.2 | |
| Caudate nucleus | 9.9 (1.1) | 9.8 (1.1) | 9.8 (1) | 9.9 (1.1) | 0.96 |
| Range | 7.7–13.7 | 7.3–12.3 | 7–12.6 | 7.4–13 |
Groups with significant mean differences (p < 0.05) for a measure compared to the total sample
Univariate analyses
For Vocabulary and Block Design scores and all brain volumes, there were no significant differences in model fit between the full ACE model and models where shared environmental effects were removed (see Table 3). There was a significant decrease in model fit for models excluding additive genetic effects. Therefore, all subsequent model fitting was conducted using a model including both additive genetic and unique environmental effects.
Bivariate analyses
Vocabulary and brain volumes
Table 4 displays the standardized genetic and environmental covariance estimates and the phenotypic correlations between Vocabulary scores and total/regional brain volumes. Most phenotypic correlations were significant but small. In general, the unique environmental correlations between gray matter brain volumes and performance on the Vocabulary subtest were significant. However, the contribution of the unique environment to the total covariance was less than the genetic contribution. A significant genetic correlation was also detected between the caudate nucleus and Vocabulary scores; however, after FDR correction for multiple comparisons (q = .05), only unique environmental correlations for frontal gray matter, gray matter ? white matter, and total gray matter remained significant.
Table 4.
Model fitting summary for vocabulary and brain volumes
| Model comparison p-values |
Standardized covariance estimates |
Correlation estimate |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full model | No rA | No rE | No rP | A | 95% CI | E | 95% CI | rP | 95% CI | |
| GMWM | 3138.53 | 0.048 | 0.003* | 0.0001* | 0.09 | (0; 0.18) | 0.04 | (0.01; 0.07) | 0.13 | (0.05; 0.22) |
| GM | 3188.54 | 0.066 | 0.003* | <0.0001* | 0.09 | (−0.01; 0.18) | 0.05 | (0.02; 0.09) | 0.14 | (0.05; 0.22) |
| WM | 3060.48 | 0.086 | 0.285 | 0.042* | 0.08 | (−0.01; 0.17) | 0.02 | (−0.02; 0.05) | 0.10 | (0.01; 0.18) |
| FGM | 3219.21 | 0.055 | 0.002* | <0.0001* | 0.09 | (0; 0.18) | 0.05 | (0.02; 0.09) | 0.14 | (0.06; 0.23) |
| FWM | 3029.50 | 0.023 | 0.221 | 0.009* | 0.10 | (0.01; 0.19) | 0.02 | (−0.01; 0.05) | 0.13 | (0.04; 0.21) |
| PGM | 3178.07 | 0.117 | 0.031 | 0.002* | 0.08 | (−0.02; 0.17) | 0.05 | (0; 0.1) | 0.13 | (0.04; 0.21) |
| PWM | 3104.55 | 0.018 | 0.469 | 0.06 | 0.11 | (0.02; 0.2) | −0.01 | (−0.05; 0.02) | 0.10 | (0.01; 0.18) |
| TGM | 3237.09 | 0.028 | 0.027 | 0.001* | 0.1 | (0.01; 0.19) | 0.03 | (0; 0.06) | 0.13 | (0.05; 0.22) |
| TWM | 3089.76 | 0.015 | 0.259 | 0.004* | 0.12 | (0.02; 0.21) | 0.02 | (−0.01; 0.06) | 0.14 | (0.05; 0.22) |
| LATVENT | 3421.22 | 0.93 | 0.567 | 0.821 | 0 | (−0.1; 0.1) | −0.02 | (−0.07; 0.04) | −0.02 | (−0.1; 0.07) |
| CAUD | 3363.82 | 0.009 | 0.377 | 0.003* | 0.13 | (0.03; 0.22) | 0.02 | (−0.02; 0.06) | 0.15 | (0.06; 0.23) |
rA-Genetic Correlation; rE-Unique Environmental Correlation; rP-Phenotypic Correlation; GMWM gray matter + white matter, GM gray matter, WM white matter, FGM frontal gray matter, FWM frontal white matter, PGM parietal gray matter, PWM parietal white matter, TGM temporal gray matter, TWM temporal white matter, LATVENT lateral ventricles, CAUD caudate nucleus
Correlations significant after adjustment for multiple testing (q = .05)
Block design and brain volumes
Table 5 displays the standardized genetic and environmental covariance estimates and the phenotypic correlations between Block Design scores and total/regional brain volumes. Phenotypic correlations between most brain volumes and Block Design scores were significant but weak. There were significant genetic correlations between Block Design performance and all brain volume measures except the lateral ventricles; the environmental correlations were non-significant. All of these genetic correlations remained significant after FDR correction for multiple comparisons. Furthermore, just as with Vocabulary, there was greater genetic contribution to the total covariance between brain volumes and Block Design performance compared to the contribution of environmental effects.
Table 5.
Model fitting summary for block design and brain volumes
| Model comparison p-values |
Standardized covariance estimates |
Correlation estimate |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full model | No rA | No rE | No rP | A | 95% CI | E | 95% CI | rP | 95% CI | |
| GMWM | 3118.96 | 0.002* | 0.748 | 0.004* | 0.14 | (0.05; 0.23) | 0 | (−0.02; 0.03) | 0.14 | (0.06; 0.23) |
| GM | 3169.54 | 0.006* | 0.302 | 0.002* | 0.13 | (0.04; 0.22) | 0.02 | (−0.02; 0.05) | 0.15 | (0.06; 0.23) |
| WM | 3032.88 | 0.007* | 0.351 | 0.027* | 0.13 | (0.03; 0.22) | −0.01 | (−0.05; 0.02) | 0.12 | (0.03; 0.19) |
| FGM | 3205.64 | 0.015* | 0.235 | 0.004* | 0.11 | (0.02; 0.2) | 0.02 | (−0.01; 0.05) | 0.13 | (0.05; 0.22) |
| FWM | 3006.51 | 0.016* | 0.411 | 0.054 | 0.11 | (0.02; 0.2) | −0.01 | (−0.04; 0.02) | 0.10 | (0.01; 0.18) |
| PGM | 3159.37 | 0.038* | 0.491 | 0.024* | 0.10 | (0.01; 0.19) | 0.01 | (−0.03; 0.06) | 0.11 | (0.03; 0.2) |
| PWM | 3074.20 | 0.007* | 0.581 | 0.006* | 0.13 | (0.03; 0.22) | 0.01 | (−0.03; 0.05) | 0.14 | (0.05; 0.22) |
| TGM | 3208.84 | 0.001* | 0.259 | <0.0001* | 0.16 | (0.07; 0.25) | 0.02 | (−0.01; 0.05) | 0.18 | (0.09; 0.26) |
| TWM | 3062.08 | 0.0003* | 0.226 | 0.002* | 0.17 | (0.08; 0.26) | −0.02 | (−0.05; 0.01) | 0.15 | (0.06; 0.23) |
| LATVENT | 3399.24 | 0.715 | 0.97 | 0.902 | −0.02 | (−0.12; 0.08) | 0 | (−0.06; 0.05) | −0.02 | (−0.1; 0.07) |
| CAUD | 3340.93 | 0.04* | 0.094 | 0.002* | 0.10 | (0; 0.19) | 0.03 | (−0.01; 0.07) | 0.13 | (0.05; 0.21) |
rA-Genetic Correlation; rE-Unique Environmental Correlation; rP-Phenotypic Correlation; GMWM gray matter + white matter, GM gray matter, WM white matter, FGM frontal gray matter, FWM frontal white matter, PGM parietal gray matter, PWM parietal white matter, TGM temporal gray matter, TWM temporal white matter, LATVENT lateral ventricles, CAUD caudate nucleus
Correlations significant after adjustment for multiple testing (q = .05)
Discussion
In this article we report the bivariate relationship between verbal (word knowledge) and nonverbal (visuo-spatial) intellectual skills and brain volume in a typical pediatric sample of twins, their siblings, and singletons. Several unique features of this study include the focus on (a) children and adolescents in an extended twin design, (b) regional brain volumes, and (c) separate consideration of verbal and nonverbal intellectual skills, as measured by the Vocabulary and Block Design subtests of the Wechsler scales. Small phenotypic correlations between brain volumes (overall gray and white matter as well as regional volumes) and subtest performance were found. Modest but significant unique environmental correlations were found between gray matter volumes and Vocabulary subtest performance. Results for the Block Design subtest indicated small genetic correlations with both gray and white matter volumes.
Our findings of high heritability of word knowledge, visuo-spatial abilities and brain volumes are consistent with previous univariate analyses (for reviews, see Plomin et al. 1997a; Peper et al. 2007; Schmitt et al. 2007). Also observed were small but consistent phenotypic correlations between both verbal and visuo-spatial intellectual skills and brain volumes (e.g., Flashman et al. 1998; Posthuma et al. 2003).
Although the phenotypic correlations between verbal/visuo-spatial skills and brain volumes were consistently low and positive, the genetic and environmental contributions to these relationships varied. The covariance between Block Design performance and both gray and white matter volumes appears to be due to additive genetic factors. These findings are consistent with at least one previous study (Hulshoff Pol et al. 2006) which showed significant genetic correlations between Performance/nonverbal IQ, including the Block Design subtest, and discrete (voxel-level) gray and white matter measurements (densities). However, another study (Posthuma et al. 2003) did not find significant genetic correlations between Perceptual Organization (which also includes the Block Design subtest) and total gray or white matter volumes in an adult sample, but instead reported this association with cerebellar volumes, which were not quantified here.
In contrast, unique environmental influences appear to underlie the association between Vocabulary performance and lobar gray matter volumes. This was unexpected because prior studies have reported that variation in verbal ability is more strongly influenced by common rather than unique environmental factors during childhood (e.g., Byrne et al. 2006). Unique environmental influences may be playing a greater role because our sample included older adolescents and unique environmental effects on verbal ability become more prominent later in development (see Plomin et al. 1997a). Lastly, it is noteworthy that our failure to find a genetic correlation between verbal ability and brain structure is consistent with the two adult studies previously testing this association (Hulshoff Pol et al. 2006; Posthuma et al. 2003).
Limitations and future directions
Brain lobar volumes are likely not the optimal level of anatomic resolution for description of structural and functional relationships, and this may have contributed to the relatively small magnitude of the correlations we observed between brain structures and cognitive ability. Future research should investigate the genetic and environmental relationships between intellectual skills and more functionally relevant measures of brain anatomy, such as cortical thickness in specific regions of interest (Shaw et al. 2006) or measures of connectivity (Lerch et al. 2006; Nagy et al. 2004; Schmitt et al. 2008). Furthermore, based on our findings and previous studies with adults in which relations between brain volumes and verbal/visuo-spatial skills were examined (Flashman et al. 1998; Posthuma et al. 2003), it appears that a ‘bigger is better’ conceptualization of brain structure may be an oversimplification, particularly during childhood and adolescence when brain structure undergoes both linear (mostly white matter) and nonlinear (mostly gray matter) changes (Lenroot et al. 2007). Because this phenotypic relationship is dynamic and captured best via longitudinal designs (Shaw et al. 2006), we may have been limited in our ability to detect genetic and environmental correlations between brain structure and intellectual skills in this sample spanning early childhood through late adolescence. Thus, future research should examine their contributions to brain developmental trajectories associated with differing levels of intellectual functioning in a longitudinal framework.
While our findings suggest that different genetic and environmental influences underpin the phenotypic relationship between brain volumes and verbal versus visuo-spatial skills, the present study did not directly model their unique and shared contributions (e.g., in the context of a trivariate model). Given that both Vocabulary and Block Design performance are highly predictive of general intellectual ability (Sattler 1992) but only moderately correlated with one another (r = .33 in the present study), we would anticipate both independent and common genetic and environmental correlations with regional brain volumes. We plan to assess these relationships further in future analyses.
Future research should seek to extend these findings beyond twin modeling in the search for shared molecular markers. It may be that genes associated with general intellectual functioning, such as SSADH and CHRM2 (for review, see Payton 2006; Shaw 2007) also shape brain structure. However, thus far, genes associated with cognitive functioning have proven to explain only a very small proportion of variance in cognitive test performance (for review, see Payton 2006), and at least one study failed to find a significant association of genes influencing brain size with general cognitive and language abilities (Bates et al. 2008). Similar to existing twin research, these studies may be limited by cross-sectional designs that obscure the often nonlinear nature of brain development and its relationship with cognitive abilities. Thus, studies of molecular markers may benefit from examining longitudinal trajectories of relationships between brain structure and intelligence.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health (GW, NRL, LSC, RL, JG), extramural grant funding from the NIH (MH-20030; EP-W, SEM, JES, MCN), and an NHMRC Australia, Sidney Sax Public Fellowship (443036; SEM). We would like to express our gratitude to the individuals who volunteered their time to contribute to this research.
References
- Axelrod BN. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9(1):17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- Bates TC, Luciano M, Lind PA, Wright MJ, Montgomery GW, Martin NG. Recently-derived variants of brain-size genes ASPM, MCPH1, CDK5RAP and BRCA1 not associated with general cognition, reading or language. Intelligence. 2008;36(6):689–693. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- Byrne B, Olson RK, Samuelsson S, Wadsworth S, Corley R, DeFries JC, Willcutt E. Genetic and environmental influences on early literacy. J Res Reading. 2006;29(1):33–49. [Google Scholar]
- Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biol Psychol. 2002;61(1–2):139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3(3):190–208. [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- Edwards AWF. Likelihood. Cambridge University Press; Cambridge: 1984. [Google Scholar]
- Flashman LA, Andreasen NC, Flaum M, Swayze VW. Intelligence and regional brain volumes in normal controls. Intelligence. 1998;25(3):149–160. [Google Scholar]
- Galton F. Hereditary genius: an inquiry into its laws and consequences. Macmillan; London: 1869. [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA. 2002;99(5):3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FN, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad of Child Adolesc Psychiatry. 2009;48(5):465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.55. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: a community study. Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins DL, Evans AC, Amunts K, Burgel U, Zilles K, de Geus E, Boomsma DI, Kahn RS. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006;26(40):10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33(4):337–346. [Google Scholar]
- McGue M, Bouchard TJ Jr, Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: a life-span perspective. American Psychological Association; Washington, DC: 1993. [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Neale M, Cardon L. Methodology for genetic studies of twins and families. Kluwer; Dordrecht: 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 6th edn. Department of Psychiatry; Richmond, VA: 2006. [Google Scholar]
- Payton A. Investigating cognitive genetics and its implications for the treatment of cognitive deficit. Genes Brain Behav. 2006;5:144–153. doi: 10.1111/j.1601-183X.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28(6):464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Craig I. Human behavioral genetics of cognitive abilities and disabilities. BioEssays. 1997;19(12):1117–1124. doi: 10.1002/bies.950191211. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, Rutter M. Behavioral genetics. 3rd edn. W.H. Freeman and Company; New York: 1997a. [Google Scholar]
- Plomin R, Fulker DW, Corley R, DeFries JC. Nature, nurture, and cognitive development from 1 to 16 years: a parent-offspring adoption study. Psychol Sci. 1997b;8(6):442–447. [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5(2):83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Res. 2003;6(2):131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: revised and updated. 3rd edn. Jerome M. Sattler Publisher, Inc.; San Diego, CA: 1992. [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007;10(5):683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, Giedd JN. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb Cortex. 2008;18(8):1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. Intelligence and the developing human brain. Bioessays. 2007;29(10):962–973. doi: 10.1002/bies.20641. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler preschool and primary scale of intelligence. The Psychological Corporation; San Antonio: 1967. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-R. The Psychological Corporation; San Antonio: 1974. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Wechsler D. Wechsler preschool and primary scale of intelligence-revised. The Psychological Corporation; San Antonio: 1989. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3rd edn. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- Wechsler D. Wechsler preschool and primary scale of intelligence. 3rd edn. The Psychological Corporation; San Antonio: 2002. [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]