Abstract
Music making (playing an instrument or singing) is a multimodal activity that involves the integration of auditory and sensorimotor processes. The ability to sing in humans is evident from infancy, and does not depend on formal vocal training but can be enhanced by training. Given the behavioral similarities between singing and speaking, as well as the shared and distinct neural correlates of both, researchers have begun to examine whether singing can be used to treat some of the speech-motor abnormalities associated with various neurological conditions. This paper reviews recent evidence on the therapeutic effects of singing, and how it can potentially ameliorate some of the speech deficits associated with conditions such as stuttering, Parkinson's disease, acquired brain lesions, and autism. By reviewing the status quo, it is hoped that future research can help to disentangle the relative contribution of factors to why singing works. This may ultimately lead to the development of specialized or “gold-standard” treatments for these disorders, and to an improvement in the quality of life for patients.
Keywords: singing, treatment, stuttering, Parkinson's disease, aphasia, autism
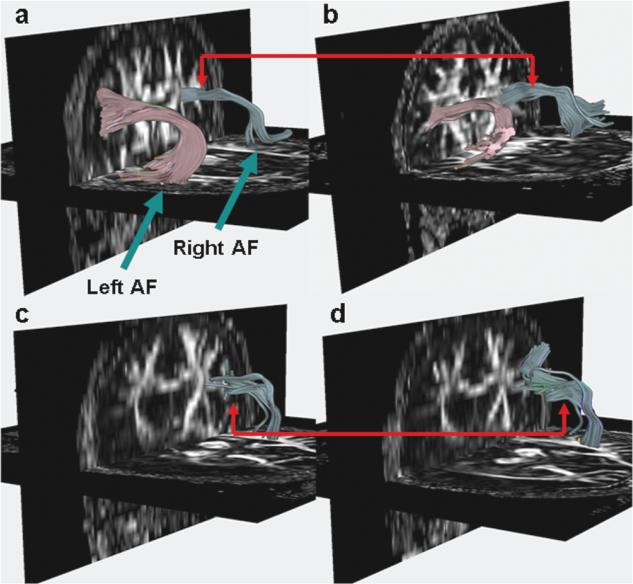
Over the past few decades, there has been growing evidence supporting the potential utility of music in medicine. Many studies have shown that music listening can enhance the emotional and cognitive functioning of patients affected by various neurological conditions (e.g., Chan, Chan, Mok, Kwan, & Tse, 2009; Forsblom, Laitinen, Sarkamo, & Tervaniemi, 2009). Unlike music listening, active music making places additional demands on the nervous system, leading to a strong coupling of perception and action; processes that are mediated by sensory, motor, and multimodal integrative regions distributed throughout the brain. This integrative fronto-temporoparietal network overlaps with components of the putative mirror neuron system, which is important in the perception and execution of actions. Deficits in the execution of motor and articulatory actions are symptoms of many neurological disorders. Indeed, it is known that long-term music making (practicing an instrument or singing) can induce plastic changes in the brain (e.g., Gaser & Schlaug, 2003; Kleber, Veit, Birbaumer, Gruzelier, & Lotze, 2009; Schlaug, 2001). Figure 1, an example of such an adaptation, shows a pronounced structural difference in the size of a right-hemispheric fiber tract that connects auditory with motor regions (the arcuate fasciculus) in a professional singer (Figure 1b) compared to a healthy control participant who may only occasionally sing (Figure 1a). Music making activities may also facilitate the establishment of alternative pathways, which could have the ability to circumvent dysfunctional brain regions caused by focal lesions and neurodevelopmental/neurodegenerative disorders.
FIGURE 1.
The arcuate fasciculus (AF) fiber bundles of: a healthy nonmusician (a); a healthy professional singer (b); a patient with Broca's aphasia before (c) and after (d) intensive melodic intonation therapy. This patient has no left AF due to his left hemispheric stroke.
Singing in particular can serve as a valuable therapeutic tool because it is a universal form of musical expression that is as natural as speaking. Moreover, singing engages an auditory-motor feedback loop in the brain more intensely than other music making activities such as instrumental playing (e.g., Bangert et al., 2006; Kleber et al., 2009). From a developmental perspective, babies produce vocalizations that can be regarded as precursors for music and speech intonation (Welch, 2006). By kindergarten age, children can sing a fairly large repertoire of songs, and their performance level is similar to that of adults (Dowling, 1999). Some children exhibit “intermediate vocalizations,” a type of vocal behavior that lies at the boundary between speech and song (Mang, 2001). This blurring of boundaries is reinforced by a shared network in the brain that underlies both singing and speaking (e.g., Kleber et al., 2009; Ozdemir, Norton, & Schlaug, 2006). The goal of this paper is to summarize recent evidence on the therapeutic effects of singing, and how it can modify the speech-motor symptoms of several neurological disorders. Because of the overlap between the expressive components of the music and language systems, the focus of this review will be on the use of singing in the treatment of speech-motor abnormalities associated with neurological conditions.
General Physiological Effects of Singing
Singing, or the act of producing musical sounds with the voice, has the potential to treat speech abnormalities because it directly stimulates the musculature associated with respiration, phonation, articulation, and resonance. The act of singing involves relatively strong and fast inspirations, followed by extended, regulated expirations. Singing requires breathing to be regulated in order to sustain the notes. It also results in a higher vocal intensity (Tonkinson, 1994) and vocal control (Natke, Donath, & Kalveram, 2003) than does speaking. Moreover, it has been suggested that singing increases respiratory muscle strength (Wiens, Reimer, & Guyn, 1999).
Research has shown that intensive singing practice can lead to long-lasting changes in both the cardiovascular and pulmonary systems. Grape, Sandgren, Hansson, Ericson, and Theorell, (2003) compared professional and amateur singers on heart rate variability before and after their singing lessons. Their rationale for examining this variable rests on the assumption that the more the heart is able to vary its rate, the better trained it is. Across the two time points (before and after singing lessons), heart rate variability increased significantly in the professional group but not in the amateur group. This finding indicates that professional singers have better cardio-physiological fitness, compared to amateur singers, thus providing evidence for the potential long-term health benefits of singing.
Using a longitudinal design, Sabol, Lee, and Stemple (1995) examined whether vocal function exercises would improve physiological parameters of vocal production in singers. These exercises, which combined isotonic and isometric elements, were designed to strengthen the laryngeal musculature and to facilitate efficient vocal fold vibration. Participants underwent assessments before and after four weeks of daily exercises. The primary physiological effects observed were higher phonation volumes and maximum phonation times, as well as a reduction in airflow. These findings were taken to reflect improved coordination of laryngeal function and vocal fold vibration.
Recently, the therapeutic effect of singing on pulmonary functions of chronically ill patients has been investigated. Bonilha et al. (2009) examined whether singing could have an effect on pulmonary function parameters in patients with chronic obstructive pulmonary disease. These patients were randomly assigned to weekly classes consisting of either singing or handicraft (control) activities. Within the singing group, increases in dyspnea were reported after two minutes of vocal exercises. An elevated level of arterial oxygen saturation also was found during singing. While the singing group showed increased inspiratory capacity and decreased expiratory reserve volumes, the opposite patterns were observed in the control group on these two measures. More importantly, the singing group showed improvement in maximal expiratory pressure, while the control group showed deterioration on this measure. Because the act of singing requires long, repeated contractions of various respiratory muscles, this type of training may help to preserve the maximal expiratory pressure of patients with chronic obstructive pulmonary disease.
Examples of Speech-Motor Deficits Associated with Neurological Disorders Treated by Singing
Stuttering
Stuttering is a largely developmental condition that affects the fluency of speech. It is characterized by repetition of words or parts of words, as well as prolongations of speech sounds, resulting in disruptions in the normal flow of speech. This condition occurs most often in young children, while they are developing their speech and language skills. Stuttering may persist into adulthood: About 1% of adults continue to be affected by this condition (Prasse & Kikano, 2008). It has been suggested that stuttering may be linked with deficits in complex isochronous timing (Max & Yudman, 2003).
Most existing treatments have focused on teaching individuals who stutter ways to produce more fluent speech, by instilling “fluency-enhancing” conditions. Singing, in particular, has been identified as having important therapeutic potential, and research has provided evidence in favor of this approach for enhancing fluency among individuals who stutter. For example, Healey, Mallard, and Adams (1976) examined whether singing could reduce stuttering and if so, whether familiarity with the lyrics played a role in producing such an effect. Participants were asked to read or sing the lyrics of well-known songs with original (familiar) or altered (unfamiliar) text. The reduction of stuttering was greater in the singing than in the reading condition. The greatest reduction was observed when familiar lyrics were sung. Thus, increased phonation duration, intonation, as well as familiarity may all contribute to the fluency-enhancing effect of singing.
In another study, Andrews, Howier, Dozsa, and Guitar (1982) examined the effects of 15 different fluency-enhancing methods (including singing) on a number of stuttering measures. In the singing condition, participants were asked to sing songs of their choice for 10 minutes. Results showed that singing reduced the frequency of stuttering by over 90%, presumably due to the increased duration of phonation. Further evidence for the benefit of singing in increasing fluency has been shown by Colcord and Adams (1979), and most recently by Davidow, Bothe, Andreatta, and Ye (2009).
To investigate whether the rate of production affected fluency, Glover, Kalinowski, Rastatter, and Stuart (1996) compared singing and reading under fast and slow conditions using a repeated-measures design. Replicating previous findings, singing generated more fluent speech than did reading. However, there was no significant difference between the two rates of reading production. The authors concluded that the rate of production alone is unlikely to account for the fluency-enhancing effect of singing. Irrespective of the underlying mechanism, however, it is clear that singing is an effective method for generating more fluent speech in individuals who stutter.
The fluency-enhancing effect of singing also has been investigated in a neuroimaging study comparing stuttering individuals with controls. Using positron emission tomography, Stager, Jeffries, and Braun (2003) examined brain activations associated with tasks that induce fluency (singing and paced speech using a metronome), versus tasks that typically induce dysfluent speech (sentence construction and unpaced event narration). A rest condition served as a baseline. The brain regions that were significantly more active during fluency-inducing tasks compared to dysfluent speech tasks included auditory areas that process speech and receive sensory feedback as well as motor and premotor regions that are involved in articulatory motor actions. This suggests that a common auditory-motor mechanism might underlie fluency in stutterers, which allows individuals to participate in self-monitoring of speech, resulting in more effective control of oral articulators. Furthermore, the fluency-inducing tasks produced more robust activation within the left hemisphere of stuttering participants (compared with controls), suggesting compensatory processes that facilitate fluency.
Parkinson's Disease
In people with Parkinson's disease, abnormalities of voice and speech (beyond those associated with aging) are overwhelmingly common. It has been estimated that over 80% of patients with Parkinson's disease develop voice and speech problems at some point (Ramig, Fox, & Sapir, 2008). Some of these problems are significant enough to impair communication and quality of life (e.g., Streifler & Hofman, 1984). Pharmacological interventions and traditional speech therapy techniques have not been consistently effective in treating these abnormalities (e.g., Weiner & Singer, 1989). As a result, speech intelligibility and oral communication skills in patients with Parkinson's disease remain poor.
Some common characteristics of Parkinsonian speech include: decreased loudness, breathy vocal quality, and short phonation time, which is associated with decreased glottal closure and respiratory function. Decreased mouth opening and velopharyngeal port function contribute to decreased vocal resonance. Other problems that are often noted include difficulty initiating speech movements, abnormal speaking rate and rhythm, and short rushes of speech. Furthermore, the decrease in ranges of pitch and loudness often limit the ability of patients with Parkinson's disease to convey emotions in their speech.
Research has shown that an intensive voice therapy program (known as the “Lee Silverman Voice Treatment” [LSVT] named after the first patient treated) can be effective in reducing some of the speech abnormalities experienced by patients with Parkinson's disease, compared to a placebo therapy (Ramig, Countryman, O'Brien, Hoehn, & Thompson, 1996). LSVT emphasizes the use of loud phonation and high intensity vocal exercises to improve respiratory, laryngeal, and articulatory functions during speech. Studies on LSVT have reported positive and long-term effects, with improvements in vocal production parameters such as duration of sustained vowel phonation and fundamental frequency range. These improvements were maintained even 12 months after the termination of treatment (e.g., Ramig et al., 2001). A recent study added a low pitch component LSVT, and found that voicing in the low register helped to minimize strains on the laryngeal muscles (DeStewart, Willemse, Maassen, & Horstink, 2003).
Some voice therapy techniques have used singing as an intervention, and these preliminary results appear promising. For example, Haneishi (2001) used a music-based voice protocol consisting of vocal warm-ups and singing exercises, with an emphasis on phonation and breathing. After 12-14 sessions, patients with Parkinson's disease showed significant increases in speech intelligibility and vocal intensity. A more recent study by Di Benedetto et al. (2008) used group choral singing as an intervention. The protocol involved singing chants with a piano accompaniment, to enhance auditory rhythmic stimulation. The protocol also involved a series of prosodic, respiratory, and laryngeal exercises. After 13 sessions of singing, patients with Parkinson's disease showed improvements in vowel phonation and reading. Despite the relatively small sample sizes and the uncontrolled nature of these studies, the results of these two studies indicate that singing may help to ameliorate some of the speech deficits associated with Parkinson's disease. For future research, dose-effects need to be determined, and the efficacy of these interventions needs to be tested in a randomized controlled fashion.
Aphasia
Aphasia is a common and devastating complication of stroke or other brain injuries that results in the loss of ability to produce and/or comprehend language. It has been estimated that between 24-52% of acute stroke patients have some form of aphasia if tested within 7 days of their stroke; 12% of survivors still have significant aphasia at 6 months after stroke (Wade, Hewer, David, & Enderby, 1986). The nature and severity of language dysfunction depends on the location and extent of the brain lesion. Accordingly, aphasia can be classified broadly into fluent or nonfluent (Beeson & Rapcsak, 2005). Fluent aphasia often results from a lesion involving the posterior superior temporal lobe known as Wernicke's area. Patients who are fluent exhibit articulated speech with relatively normal utterance length. However, their speech may be completely meaningless to the listener, and littered with jargon, as well as violations to syntactic and grammatical rules. These patients also have severe speech comprehension deficits. In contrast, nonfluent aphasia results most commonly from a lesion in the left frontal lobe, involving the left posterior inferior frontal region known as Broca's area. Patients who are nonfluent tend to have relatively intact comprehension for conversational speech, but have marked impairments in articulation and speech production. In Figure 1 we show diffusion tensor images and reconstructions of the arcuate fasciculus (AF), a fiber bundle that connects the temporal lobe with the frontal lobe motor regions. The AF plays an important role in auditory-motor mapping, and the left bundle, in particular, underlies language processing. Figure 1a shows the AF of a healthy nonmusician, with the typical left-hemisphere dominance for language (i.e., larger left AF). Figure 1b shows the AF of a healthy professional singer. The right AF of the singer is more developed compared to that of the nonmusician (Fig. 1a), presumably because of years of voice training. Figure 1c shows the AF of a patient with chronic nonfluent aphasia before commencing the singing (melodic intonation) therapy. In this patient, the lesion is so large that there is no AF on the left hemisphere, resulting in significant speech deficits. Figure 1d shows the AF of the same patient after intensive therapy. Singing engages auditory-motor regions of the right hemisphere, and intense therapy over a long period of time may lead to structural adaptations in gray and white matter. In this patient, the right AF showed structural adaptations when diffusion tensor images were compared before and after therapy.
Research has shown that singing or intoning spoken words and phrases can help facilitate speech output in patients with nonfluent aphasia. In particular, a technique termed melodic intonation therapy (MIT) has been shown in a case series to produce improvements in expressive language beyond the limitations of either natural recovery or traditional non-intonation-based speech therapies (Schlaug, Marchina, & Norton, 2008). Inspired by the clinical observation that patients with nonfluent aphasia often can sing the lyrics of a song better than they can speak the same words, MIT emphasizes the prosody of speech through the use of slow, pitched vocalization or singing in combination with rhythmic tapping of the left hand (Albert, Sparks, & Helm, 1973; Norton, Zipse, Marchina, & Schlaug, 2009). Specifically, MIT is an intensive intervention that involves practicing a series of words or phrases, using melodic intonation and continuous voicing, as well as left hand rhythmic tapping to provide cueing for syllable production (see Norton, et al., 2009 for details).
To date, studies using MIT have produced positive outcomes in patients with nonfluent aphasia. These outcomes range from improvements on the Boston Diagnostic Aphasia Examination (BDAE; Goodglass & Kaplan, 1983; see also Bonakdarpour, Eftekharzadeh, & Ashayeri, 2000; Sparks, Helm, & Albert, 1974), to improvements in articulation and phrase production (Wilson, Parsons, & Reutens, 2006) after treatment. The effectiveness of this intervention is further demonstrated in a recent study that examined transfer of language skills to untrained contexts. Schlaug et al. (2008) compared the effects of MIT with a control intervention (speech repetition) on picture naming performance and measures of propositional speech. After 40 daily sessions, both therapy techniques resulted in significant improvement on all outcome measures, but the extent of this improvement was far greater for the patient who underwent MIT compared to the one who underwent the control therapy.
The therapeutic effect of MIT is also evident in neuroimaging studies that show reorganization of brain functions. MIT resulted in increased activation in a right-hemisphere network involving the premotor, inferior frontal, and temporal lobes (Schlaug, Marchina, & Norton, 2008), as well as increased fiber number and volume of the arcuate fasciculus in the right hemisphere (Schlaug et al., 2009). A patient treated with a non-intonation-based speech therapy showed less right hemisphere changes and more left hemisphere changes. These findings demonstrate that intensive experimental therapies such as MIT—when applied over a longer period of time in chronic stroke patients—can induce functional and structural brain changes, and these changes are related to speech output improvements. A large-scale randomized controlled study assessing the efficacy of MIT compared to a non-intonation based control therapy is currently underway (for more details, see Clinical Trial Registry Number: NCT00903266).
Autism
Another condition whose symptoms can potentially be helped by singing is autism. It has been estimated that this condition affects about 1% of the population (Williams, Higgins, & Brayne, 2006). Autism is characterized by impairments in expressive language and communication, with some affected individuals completely lacking functional speech (Tager-Flusberg, 1997). Individuals with autism have superior auditory processing abilities (e.g., Heaton, 2003; Heaton, Hermelin, & Pring, 1998) and often exhibit strong interests in learning and making music (e.g., Hairston, 1990; Trevarthen, Aitken, Paoudi, & Robarts, 1996).
To date, only two case studies have described the positive effects of singing on the development of speech in children with autism. One study used an adapted version of MIT involving intoned questions and statements (Miller & Toca, 1979). Another study reported using pitch matching and singing to encourage vocalizations, which eventually led to the articulation of words (Hoelzley, 1993). Although the results of these single case studies are encouraging, the efficacy of these methods have to be tested in a controlled design that would allow us to determine whether these approaches can be generalized to a broader population of affected individuals, and whether effects in the trained words/phrases transfer to untrained items. Further research testing the efficacy of singing in autism is therefore warranted.
An intervention that is specifically designed to help children with autism to develop expressive language is currently being tested (Wan et al., 2009; Wan, Demaine, Zipse, Norton, & Schlaug, 2010). Known as auditory-motor mapping training (AMMT), this intervention involves three main components—singing, motor activity, and imitation—that engage a presumed dysfunctional human mirror neuron system that is believed to underlie some of the communication deficits in autism (Wan et al., 2010). First, singing engages a bilateral fronto-temporal network more prominently than speaking does, and this network contains some components of the mirror neuron system (Brown, Martinez, Hodges, Fox, & Parsons, 2004; Ozdemir et al., 2006). Second, motor activity through playing a percussion instrument not only captures the child's interest, but also engages a sensorimotor network that controls oro-facial and articulatory movements (Meister et al., 2003; Meister, Buelte, Staedtgen, Borooierdi, & Sparing, 2009). Moreover, the sound produced by the percussion instrument may facilitate the auditory-motor mapping that is critical to meaningful vocal communication (Lahav, Saltzman, & Schlaug, 2007). Finally, imitation through repetitive training facilitates learning and alters the responses in the mirror neuron system (Catmur, Walsh, & Heyes, 2007). Because AMMT enhances interactions between the auditory and motor systems, it may represent an effective therapeutic strategy through which individuals with autism can develop their communication skills.
Concluding Remarks
As illustrated in this paper, singing represents a promising therapeutic tool in a variety of neurological disorders. Singing is particularly useful in ameliorating some of the associated speech-motor difficulties because of features such as continuous voicing, decreased production rate, and increased awareness of individual phonemes. Although the precise mechanisms underlying the efficacy of singing remain largely unexplored, a number of hypotheses have been proposed. First, continuous voicing helps to increase the connectedness between syllables and words. This idea is consistent with the explanations of increased phonation time, syllable lengthening, and intelligibility provided by the stuttering (e.g., Andrews et al., 1982) and acquired brain lesion (e.g., Norton et al., 2009; Schlaug et al., 2008; Tamplin, 2008) studies, respectively. Because the impaired language system is often lateralized to the left hemisphere, singing or intoned speaking engages a larger bihemispheric network than does speaking (Ozdemir et al., 2006). Furthermore, the right hemisphere may be particularly engaged by prosodic and slowly-modulated signals over a defined period of time. Second, the decreased production rate also reduces the dependence on the left hemisphere, according to an explanation put forward by some aphasia studies (e.g., Schlaug et al., 2008). Rate of production in singing, however, does not appear to affect the performance of stuttering individuals (Glover et al., 1996). Third, increased awareness of individual phonemes facilitates articulation (e.g., Auriemmo et al., 2009). When words are sung, the phonemes are isolated, allowing an opportunity for self-correction. Finally, because there is an articulatory-motor component associated with most of the disorders described above, singing may help to engage a brain network that facilitates sound-motor mapping (e.g., Lahav et al., 2007; Meister et al., 2003). Indeed, some of the existing singing techniques have already incorporated the use of hand tapping (e.g., Schlaug et al., 2008) in the intervention. The use of hand-tapping or external auditory cues has been demonstrated to facilitate vocal output (e.g., Pilon, McIntosh, & Thaut, 1998; Thaut, McIntosh, McIntosh, & Hoemberg, 2001; Wambaugh & Martinez, 2000). Taken together, there appears to be a number of possible mechanisms underlying the efficacy of singing in ameliorating the symptoms of various neurological conditions. Although it may be difficult to test the contribution of all of the variables to an improvement in speech motor output, it is important to test the efficacy of any new experimental intervention versus a controlled or established intervention in a randomized controlled trial. Equally important are the neural mechanisms underlying singing or auditory-motor mapping training and their therapeutic effects. Elucidating these mechanisms will enable us to tailor the interventions, to select the most appropriate patients for particular interventions, and to make predictions about recovery.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (DC008796, DC009823) and the Nancy Lurie Marks Family Foundation.
References
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Archives of Neurology. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Andrews G, Howie PM, Dozsa M, Guitar BE. Stuttering: Speech pattern characteristics under fluency-inducing conditions. Journal of Speech and Hearing Research. 1982;25:208–216. [PubMed] [Google Scholar]
- Auriemmo J, Kuk F, Lau C, Marshall S, Thiele N, Pikora M, et al. Effect of linear frequency transposition on speech recognition and production of school-age children. Journal of the American Academy of Audiology. 2009;20:289–305. doi: 10.3766/jaaa.20.5.2. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, et al. Shared networks for auditory and motor processing in professional pianists: Evidence from fMri conjunction. Neuroimage. 2006;30:917–926. doi: 10.1016/j.neuroimage.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Beesson PM, Rapcsak SZ. The aphasias. In: Synder P, Nussbaum P, Robins D, editors. Clinical neuropsychology: A pocket handbook for assessment. American Psychological Association; Washington, DC: 2005. pp. 436–459. [Google Scholar]
- Bonakdarpour B, Eftekharzadeh A, Ashayeri H. Preliminary report on the effects of melodic intonation therapy in the rehabilitation of Persian aphasic patients. Iranian Journal of Medical Sciences. 2000;25:156–160. [Google Scholar]
- Bonilha AG, Onofre F, Vieira ML, Prado MYA, Martinex JAB. Effects of singing classes on pulmonary function and quality of life of COPD patients. International Journal of Chronic Obstructive Pulmonary Disease. 2009;4:1–8. [PMC free article] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Hodges DA, Fox PT, Parsons LM. The song system of the human brain. Brain Research Cognitive Brain Research. 2004;20:363–375. doi: 10.1016/j.cogbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Current Biology. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chan MF, Chan EA, Mok E, Kwan, Tse FY. Effect of music on depression levels and physiological responses in community-based older adults. International Journal of Mental Health Nursing. 2009;18:285–294. doi: 10.1111/j.1447-0349.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- Colcord RD, Adams MR. Voicing duration and vocal SPL changes associated with stuttering reduction during singing. Journal of Speech and Hearing Research. 1979;22:468–479. doi: 10.1044/jshr.2203.468. [DOI] [PubMed] [Google Scholar]
- Davidow JH, Bothe AK, Andreatta RD, Ye J. Measurement of phonated intervals during for fluency-inducing conditions. Journal Speech and Language Hearing Research. 2009;52:188–205. doi: 10.1044/1092-4388(2008/07-0040). [DOI] [PubMed] [Google Scholar]
- DeStewart BJ, Willemse SC, Maassen BAM, Horstink MWIM. Improvement of voicing in patients with Parkinson's disease with speech therapy. Neurology. 2003;60:498–500. doi: 10.1212/01.wnl.0000044480.95458.56. [DOI] [PubMed] [Google Scholar]
- Di Benedetto P, Cavazzon M, Mondolo F, Rugiu G, Peratoner A, Biasutti E. Voice and choral singing treatment: A new approach for speech and voice disorders in Parkinson's disease. European Journal of Physical Rehabilitative Medicine. 2008;44:1–7. [PubMed] [Google Scholar]
- Dowling WJ. The development of music perception and cognition. In: Deutsch D, editor. The psychology of music. Academic Press; San Diego, CA: 1999. pp. 603–625. [Google Scholar]
- Forsblom A, Laitinen S, Sarkamo T, Tervaniemi M. Therapeutic role of music listening in stroke rehabilitation. Annals of the New York Academy of Sciences. 2009;1169:426–430. doi: 10.1111/j.1749-6632.2009.04776.x. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. Journal of Neuroscience. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover H, Kalinowski J, Rastatter M, Stuart A. Effect of instruction to sing on stuttering frequency at normal and fast rates. Perceptual and Motor Skills. 1996;832:511–522. doi: 10.2466/pms.1996.83.2.511. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston diagnostic aphasia examination. 2nd ed. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Grape C, Sandgren M, Hansson LO, Ericson M, Theorell T. Does singing promote well-being? An empirical study of professional and amateur singers during a singing lesson. Integrative Physiological and Behavioral Science. 2003;38:65–74. doi: 10.1007/BF02734261. [DOI] [PubMed] [Google Scholar]
- Hairston M. Analyses of responses of mentally retarded autistic and mentally retarded nonautistic children to art therapy and music therapy. Journal of Music Therapy. 1990;27:137–150. [Google Scholar]
- Haneishi E. Effects of a music therapy voice protocol on speech intelligibility, vocal acoustic measures, and mood of individuals with Parkinson's disease. Journal of Music Therapy. 2001;38:273–290. doi: 10.1093/jmt/38.4.273. [DOI] [PubMed] [Google Scholar]
- Healey EC, Mallard AR, III, Adams MR. Factors contributing to the reduction of stuttering during singing. Journal of Speech and Hearing Research. 1976;19:475–480. doi: 10.1044/jshr.1903.475. [DOI] [PubMed] [Google Scholar]
- Heaton P. Pitch memory, labelling and disembedding in autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:543–551. doi: 10.1111/1469-7610.00143. [DOI] [PubMed] [Google Scholar]
- Heaton P, Hermelin B, Pring L. Autism and pitch processing: A precursor for savant musical ability? Music Perception. 1998;15:291–305. [Google Scholar]
- Hoelzley PD. Communication potentiating sounds: Developing channels of communication with autistic children through psychobiological responses to novel sound stimuli. Canadian Journal of Music Therapy. 1993;1:54–76. [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M. The brain of opera singers: Experience-dependent changes in functional activation. Cerebral Cortex. 2009 August 19; doi: 10.1093/cercor/bhp177. DOI:10.1093/cercor/bhp177. [DOI] [PubMed] [Google Scholar]
- Lahab A, Saltzman E, Schlaug G. Action representation of sound: Audiomotor recognition network while listening to newly acquired actions. Journal of Neuroscience. 2007;27:208–214. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang E. Intermediate vocalisations: An investigation of the boundary between speech and songs in young children's vocalisations. Bulletin of the Council for Research in Music Education. 2001;147:116–121. [Google Scholar]
- Max L, Yudman EA. Accuracy and variability of isochronous rhythmic timing across motor systems in stuttering vs nonstuttering individuals. Journal of Speech Language and Hearing Research. 2003;46:146–163. doi: 10.1044/1092-4388(2003/012). [DOI] [PubMed] [Google Scholar]
- Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, Topper R. Motor cortex hand area and speech: Implications for the development of language. Neuropsychologia. 2003;41:401–406. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- Meister IG, Buelte D, Staedtgen M, Borooierdi B, Sparing R. The dorsal premotor cortex orchestrates concurrent speech and fingertapping movements. The European Journal of Neuroscience. 2009;29:2074–2084. doi: 10.1111/j.1460-9568.2009.06729.x. [DOI] [PubMed] [Google Scholar]
- Miller SB, Toca JM. Adapted melodic intonation therapy: A case-study of an experimental language program for an autistic child. Journal of Clinical Psychiatry. 1979;40:201–203. [PubMed] [Google Scholar]
- Natke U, Donath TM, Kalveram KT. Control of voice fundamental frequency in speaking vs singing. Journal of the Acoustical Society of America. 2003;113:1587–1593. doi: 10.1121/1.1543928. [DOI] [PubMed] [Google Scholar]
- Norton A, Zipse L, Marchina S, Schlaug G. Melodic intonation therapy: How it is done and why it might work. Annals of New York Academy of Sciences. 2009;1169:431–436. doi: 10.1111/j.1749-6632.2009.04859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Pilon MA, McIntosh KW, Thaut MH. Auditory vs visual speech timing cues as external rate control to enhance verbal intelligibility in mixed spastic-ataxic dysarthric speakers. Brain Injury. 1998;12:793–803. doi: 10.1080/026990598122188. [DOI] [PubMed] [Google Scholar]
- Prasse JE, Kikano GE. Stuttering: An overview. American Family Physician. 2008;77:1271–1276. [PubMed] [Google Scholar]
- Ramig LO, Countryman S, O'Brien C, Hoehn M, Thompson L. Intensive speech treatment for patients with Parkinson's disease: Short- and long-term comparison of two techniques. Neurology. 1996;47:1496–1504. doi: 10.1212/wnl.47.6.1496. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson's disease. Expert Review of Neurotherapeutics. 2008;8:297–309. doi: 10.1586/14737175.8.2.297. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O'Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol JW, Lee L, Stemple JC. The value of vocal function exercises in the practice regimen of singers. Journal of Voice. 1994;8:271–278. doi: 10.1016/s0892-1997(05)80220-6. [DOI] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians: A model for functional and structural plasticity. Annals of New York Academy of Sciences. 2001;930:281–299. [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. From singing to speaking: Why patients with Broca's aphasia can sing and how that may lead to recovery of expressive language functions. Music Perception. 2008;25:315–323. doi: 10.1525/MP.2008.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Annals of New York Academy of Sciences. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks R, Helm N, Albert M. Aphasia rehabilitation resulting from melodic intonation therapy. Cortex. 1974;10:303–316. doi: 10.1016/s0010-9452(74)80024-9. [DOI] [PubMed] [Google Scholar]
- Stager SV, Jeffries KJ, Braun AR. Common features of fluency-evoking conditions studied in stuttering subjects and controls: An H(2)15O PET study. Journal of Fluency Disorders. 2003;28:319–335. doi: 10.1016/j.jfludis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Streifler M, Hofman S. Disorders of verbal expression in Parkinsonism. In: Hassler R, Christ J, editors. Advances in neurology. Raven Press; New York: 1984. pp. 385–393. [PubMed] [Google Scholar]
- Tager-Flusberg H. Language acquisition and theory of mind: Contributions from the study of autism. In: Adamson LB, Romski MA, editors. Research on communication and language disorders: Contributions to theories of language development. Pauk Brookes Publishing; Baltimore, MD: 1997. pp. 133–158. [Google Scholar]
- Tamplin J. A pilot study into the effect of of vocal exercises and singing on dysarthric speech. Neurorehabilitation. 2008;23:207–216. [PubMed] [Google Scholar]
- Thaut MH, McIntosh KW, McIntosh GC, Hoemberg V. Auditory rhythmicity enhances movement in speech motor control in patients with Parkinson's disease. Functional Neurology. 2001;16:163–172. [PubMed] [Google Scholar]
- Tonkinson S. The Lombard effect in choral singing. Journal of Voice. 1994;8:24–29. doi: 10.1016/s0892-1997(05)80316-9. [DOI] [PubMed] [Google Scholar]
- Trevarthen C, Aitken K, Paoudi D, Robarts J. Children with autism. Jessica Kingsley Publishers; London: 1996. [Google Scholar]
- Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: Natural history and associated deficits. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JL, Martinez AL. Effects of rate and rhythm control treatment on consonant production accuracy in apraxia of speech. Aphasiology. 2000;14:851–871. [Google Scholar]
- Wan C, Zipse L, Norton A, Demaine K, Baars R, Zuk J, et al. Using an auditory-motor mapping therapy to improve expressive language abilities in nonverbal children with autism; Poster session presented at the 8th Annual Auditory Perception, Cognition, and Action Meeting; Boston, MA. Nov, 2009. [Google Scholar]
- Wan C, Demaine K, Zipse L, Norton A, Schlaug G. From music making to speaking: Engaging the mirror neuron system in autism. 2010. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- Weiner W, Singer C. Parkinson's disease and nonpharmacological treatment programs. Journal of American Geriatric Society. 1989;37:359–363. doi: 10.1111/j.1532-5415.1989.tb05505.x. [DOI] [PubMed] [Google Scholar]
- Welch GF. Singing and vocal development. In: McPherson G, editor. The child as musician: A handbook of musical development. Oxford University Press; New York: 2006. pp. 311–329. [Google Scholar]
- Wiens ME, Reimer MA, Guyn HL. Music therapy as treatment method for improving respiratory muscle strength in patients with advanced multiple sclerosis. Rehabilitation Nursing. 1999;24:74–80. doi: 10.1002/j.2048-7940.1999.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood. 2006;91:8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Parsons K, Reutens DC. Preserved singing in aphasia: A case study of the efficacy of the Melodic Intonation Therapy. Music Perception. 2006;24:23–36. [Google Scholar]