Abstract
Objective
To study WM development in youth at high familial risk for BD. White matter (WM) alterations are reported in youth and adults with bipolar disorder (BD). WM undergoes important maturational changes in adolescence. The authors compared age-related changes in WM microstructure using diffusion tensor imaging (DTI) with tract-based spatial statistics in healthy offspring having a parent with BD and healthy controls.
Method
A total of 45 offspring participated, including 20 healthy offspring with a parent diagnosed with BD (HBO) and 25 healthy control offspring of healthy parents (CONT). All were free of medical and psychiatric disorders. Mean fractional anisotropy (FA), radial diffusivity (RD), and longitudinal diffusivity (L1) were examined using wholebrain analyses, covarying for age.
Results
Group by age interactions revealed a linear increase in FA, and linear decrease in RD, in CONT in left corpus callosum (CC) and right inferior longitudinal fasciculus (ILF). In HBO, there was a linear decrease in FA and increase in RD, with age in left CC, and no relationship between FA, or RD, and age in right ILF. Curve fitting confirmed linear, and revealed non-linear, relationships between FA, and RD, with age in the above regions in CONT and HBO.
Conclusions
This is the first study to examine WM in healthy offspring at high familial risk for BD. Results from this cross-sectional study suggest altered development of WM in HBO relative to CONT in CC and temporal associative tracts, which may represent vulnerability markers for future BD and other psychiatric disorders in HBO.
Keywords: bipolar disorder, familial risk, white matter, diffusion tensor imaging, neurodevelopment
Introduction
Bipolar disorder (BD) is a serious psychiatric illness affecting 1–3% of the adult population and remains a leading cause of morbidity, functional impairment, and completed suicide.1 BD is characterized by difficulties in the regulation of emotions and behavior, as indicated by episodes of mania and depression. BD is highly heritable: the risk of BD is much greater in first-degree relatives of individuals diagnosed with BD.2, 3 Recent evidence indicates that offspring of parents with BD are at increased risk for BD and other psychiatric disorders, including BD spectrum disorder, anxiety and depression disorders.2 Although genetic and environmental factors and their interactions are important in the development of BD, abnormalities of brain structure and function that most likely mediate these effects have yet to be elucidated. Converging evidence from epidemiological, genetic, and neuroimaging studies suggest that abnormalities in the development of white matter (WM) may play an important role in the neuropathophysiology of BD.4 However, the extent to which WM development may be altered in offspring at high familial risk of BD has yet to be determined.
Diffusion tensor imaging (DTI) is a noninvasive neuroimaging technique that uses the diffusion of water to investigate subtle changes in WM microstructural organization. DTI employs fractional anisotropy (FA), an index of the ratio of diffusional anisotropy in longitudinally-aligned versus transverse directions of WM tracts. That is, voxels containing water moving predominantly along the principal diffusion direction, rather than the transverse direction, have higher FA. Findings from previous DTI studies suggest that adults with BD have WM abnormalities in prefrontal and subcortical regions implicated in emotional processing and emotion regulation. The majority of these studies, using a region-of-interest (ROI) approach, reported WM abnormalities in BD adults in prefrontal regions,5–12 including fronto-temporal WM abnormalities in the uncinate fasciculus, a major WM tract connecting anterior temporal and orbitomedial prefrontal cortices.7, 10, 11 Wholebrain studies in adults with BD confirmed WM abnormalities, including increases and decreases in FA, in fronto-temporal regions. 6, 13, 14 They also reported abnormalities in fibers projecting to temporal13 and occipital cortices.6, 13 Similarly, the few recent studies in pediatric BD also reported abnormally lower FA in prefrontal regions.15–18 Furthermore, lower FA,15, 19 abnormal signal intensity,20 and abnormal curvature shape 21 of the corpus callosum (CC) have been shown in youth with BD relative to healthy controls. FA reductions in fibers projecting to temporal16, frontal,19 and occipital cortices15, 16 have also been reported in wholebrain studies in youth with BD relative to healthy controls.
One methodological issue in DTI is the interpretation of changes in FA. Greater FA could reflect greater myelination of WM fibers, greater number of myelinated fibers, or greater longitudinal versus oblique directional alignment of fibers. In order to improve the interpretation of changes in FA, including measures of radial and longitudinal diffusivities (RD and L1, respectively)22 has been recommended. L1 is an index of the principal - longitudinal - diffusion direction whereas RD is an average of the transverse directions and an index of the diffusivity in directions that are perpendicular to the principal axis of diffusion. For example, changes in RD in the absence of changes in L1 have been associated with changes in myelin,23 while changes in L1 in the absence of changes in RD have been associated with increases in axon diameter.24 In a recent study with BD versus healthy adults,6 we included RD and L1, which contributed to a better interpretation of group differences in FA.
Abnormalities in WM have been observed in individuals at risk for BD.2, 25, 26 For example, one study reported decreased left hemispheric WM volume in both adult BD patients and their unaffected twin relative to age-matched healthy individuals.27 We are aware of only one study to date that, using voxel-based DTI, examined WM tracts in offspring (4–12 years old) of parents with BD (n=7) relative to children with BD (n=10) and age-matched healthy controls (n=8).19 This study reported lower FA in bilateral superior longitudinal fascicule (SLF) in both the offspring of parents with BD and children with BD, relative to healthy controls. However, like the children with BD, the majority of the offspring of parents with BD met criteria for another Axis I disorder (e.g., ADHD), which may have had an effect on the DTI findings in this group. Although offspring of parents with BD do exhibit higher levels of psychopathology than community controls,2 focusing on healthy offspring with a parent diagnosed with BD is an important first step to improve the ability to identify potential neurodevelopmental vulnerability markers of BD while eliminating the possible confounding effects of psychopathology and/or psychotropic medication in BD offspring on DTI measures.
Another important issue is the extent to which WM tracts develop normally in youth at high familial risk of BD. A growing number of studies in healthy youth suggest important maturational changes in WM during adolescence.28, 29 A recent DTI study reported age-related increases in FA (decreases in RD) in specific WM tracts across adolescence.28 Given that adolescence is a vulnerable developmental window for the onset of mood disorders such as BD,30 examining age-related changes in WM tracts in healthy offspring having a parent with BD across adolescence may therefore help elucidate specific neurodevelopmental vulnerability markers of BD.
In this study, we used DTI to examine the development of WM microstructure in healthy offspring having a parent with BD (Healthy Bipolar Offspring: HBO) versus healthy control offspring of healthy parents (Healthy Control: CONT). We conducted wholebrain analysis using tract-based spatial statistics (TBSS). Our main focus was on age-related changes in FA across groups. We also explored age-related changes in RD and L1, as complementary measures to FA, and examined main effects of group. Given evidence of abnormal WM microstructure associated with BD, in this cross-sectional study, we tested the hypothesis that HBO would exhibit alterations in the development of WM and as such, unlike CONT, would not show the normative increases in FA (and decreases in RD) in wholebrain WM with age. We further hypothesized that these alterations in WM in HBO would be particularly evident in the corpus callosum and fronto-temporal regions (i.e., uncinate fasciculus).
Method
Participants
The study was approved by the University of Pittsburgh Institutional Review Board. Parents signed consent forms, and youths signed assent forms. A total of 45 healthy offspring participated in the study (see Table 1). Of these, 20 were HBO having at least one biological parent diagnosed with BD (13 bipolar disorder type I, 6 bipolar disorder type II, 1 bipolar disorder not otherwise specified), and 25 were age-matched CONT. Parents of the CONT did not have any current Axis I disorder or history of mood disorder or psychotic disorder. Also, first-degree relatives of the CONT did not have any current or history of BD. Participants in HBO and CONT groups were matched on age (e.g., participants in the CONT group were matched using a maximum of one year older or younger than their HBO counterparts). Participants were recruited from an ongoing longitudinal study on the psychiatric symptomatology in offspring of parents with BD (MH#060952-06, PI: B.B.).
Table 1.
Demographic and Clinical Characteristics of Healthy Offspring Having a Parent with Bipolar Disorder and Age-Matched Control Offspring of Healthy Parents
| Group | Statistic | df | p Value | ||
|---|---|---|---|---|---|
| HBO (n=20) | CONT (n = 25) | ||||
| Age at Scan (years), mean (SD) | 13.2 (2.5) | 13.9 (2.6) | t = −.94 | 43 | .36 |
| Sex (M/F) | 9/11 | 7/18 | χ2=.76 | 1 | .35 |
| Socio-economic Status, mean (SD) | 41 (14.9) | 47 (9.7) | t = −1.4 | 33 | .17 |
| Full Scale IQ, mean (SD) | 114.7 (13.6) | 114.6 (10.1) | t = .01 | 21 | .99 |
| Handedness, % right-handed | 82 | 82 | χ2= .001 | 1 | .97 |
| MFQ – parent version, mean (SD) | 3.8 (3.7) | 2.1 (4.5) | t = 1.33 | 42 | .19 |
| MFQ – child version, mean (SD) | 5.5 (5.7) | 5.4 (5.4) | t = .07 | 41 | .95 |
| SCARED – parent version, mean (SD) | 6.0 (4.7) | 4.1 (5.2) | t = 1.2 | 42 | .23 |
| SCARED – child version, mean (SD) | 9.2 (7.2) | 8.6 (5.5) | t = .31 | 42 | .76 |
| CALS, mean (SD) | 5.0 (4.4) | 2.8 (3.9) | t = 1.69 | 42 | .10 |
Note: CALS = Child Affect Lability Scale (range, 0–80); CONT = healthy control offspring of healthy parents; HBO = healthy offspring having a parent diagnosed with bipolar disorder; MFQ = Mood and Feelings Questionnaire (range, 0–68); SCARED = Screen for Childhood Anxiety and Related Disorders (range, 0–82).
As part of their participation in the above study, diagnostic interviews were conducted with the offspring and their parents using semi-structured diagnostic instruments: The Structural Clinical Interview for DSM-IV (SCID I, II) was used to ascertain lifetime psychopathology for all parents and the Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (K-SADS-PL) was used to interview parents about their children and children about themselves for the presence of current and lifetime psychiatric disorders. To date, diagnostic reliability on the K-SADS-PL has been high (κ = .90). Final diagnoses were assigned by consensus using best-estimate procedures. The family history–research diagnostic criteria method31 was used to ascertain the psychiatric history of biological coparents not seen for direct interview.
Participants in the above study who were between 8–17 years old and who did not endorse any current DSM-IV Axis I diagnosis or history of depression or BD on the K-SADS-PL were invited to participate in the current neuroimaging study. Eligible participants were sent a letter and contacted by phone for initial screening. Participants and their parents completed the questionnaires noted below on the day of the neuroimaging scan to ensure that all participants were free of any current DSM-IV Axis I psychiatric diagnoses immediately before the neuroimaging evaluation. Parents completed the following questionnaires about their children: the Stony Brook Child or Adolescent Symptom Inventory-4, to assess for DSM-IV Axis I diagnoses; the Mood and Feelings Questionnaire (MFQ), to assess for symptoms of depression; the Child Affect Lability Scale (CALS), to assess for mood lability, and the Screen for Childhood Anxiety and Related Disorders (SCARED) to assess for symptoms of anxiety. Offspring completed the child self-report version of the MFQ and SCARED. Socio-economic status (SES) was measured with the Hollingshead Four-Factor Index. Handedness was determined using the Edinburgh Handedness Inventory. IQ was determined using the Wechsler Abbreviated Scale of Intelligence (WASI). Exclusion criteria included: IQ<70, history of head trauma, neurological disorder, unstable medical illness, presence of metal objects in their body, use of drug and alcohol, and pregnancy.
DTI Acquisition Parameters
MRI images were acquired on a 3T Siemens Magnetom Allegra syngo MR-2004A. Diffusion tensor data were acquired using a coronal diffusion-weighted single-shot spin-echo planar imaging sequence, parallel to the AC–PC line (TR=4400 ms, TE=76 ms, bandwidth 1860[Hz/Px], flip angle=90, [FOV]=200×200, thirty-four 3 mm-thick slices, no-gaps, matrix size = 80×128, EPI factor = 128; acquisition:6′:16″). Two b values were used: one b=0 (no-diffusion weighting) image and six non-coplanar b=850s/mm2 (diffusion-weighting b-value) images were acquired with parameters similar to those employed in recent DTI studies.6, 32 Fat saturation was used to remove scalp signal (to control for chemical shift or ghosting artifacts).
DTI Preprocessing
Diffusion-weighted images were processed using the FMRIB [Functional Magnetic Resonance Imaging of the Brain] Software Library (FSL) (v.4.1). They were first inspected for motion artifacts and then registered to the b=0 image, as a reference, by affine transformations to minimize distortions due to eddy currents and reduce simple head motion, using Eddy Current Correction. Images were extracted using the Brain Extraction Tool (BET).33, 34 A diffusion tensor model was fitted at each voxel, providing a voxelwise calculation of FA and main diffusion scalar vectors.35
DTI Data Analyses
Diffusion-weighted images were analyzed using the FMRIB [Functional Magnetic Resonance Imaging of the Brain] Software Library (FSL; v. 4.1). Using a non-linear registration algorithm, we employed tract-based spatial statistics (TBSS) (v. 1.2) in FSL to align FA images. Each subject’s FA-image was first aligned to a higher-resolution FA standard space (voxel size=1 mm3) (Montreal Neurological Institute atlas, MNI).36 Then, the derived mean FA image was minimized to generate a template-skeleton embodying the center of all tracts derived from the entire group. Finally, an FA≥0.20 threshold was set in order to exclude peripheral tracts that might lead to erroneous interpretations due to anatomic inter-subject variability and/or partial volume effects with gray matter. To examine the group × age interaction and main effects of group upon FA, each subject’s aligned FA data was projected onto this template-skeleton mask (volume = 138, 148 mm3) and entered into a wholebrain voxelwise analysis of FA. We used randomize (v. 2.1) (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html), which is a permutation program enabling modeling and inference using standard ‘general linear model’ design, and non-parametric independent t-tests (permutation method, n=5000, no smoothing factor). Age was modeled as a covariate. To control for multiple statistical testing, we maintained a false positive detection rate at p<0.05. The number of contiguous voxels needed to maintain this false positive detection rate was empirically determined by Monte Carlo simulations implemented in AlphaSim37, which was conducted on uncorrected t maps (p≤0.001) in FA, employing the white matter skeleton mask described above; a dual thresholding of both type I error and cluster-size thresholding (CST) were obtained. To help interpret findings in FA, wholebrain voxelwise analyses of RD and L138 were performed along with AlphaSim correction (p<.05) as described above.
Our main analyses were based on a general linear model. We therefore report linear relationships between FA (RD, L1) and age. Given that some studies in normative samples have also reported non-linear relationships, we also explored non-linear functions (See Table S1, available online). Because these analyses were exploratory, we used p<0.05 uncorrected. We also explored whether there were any group by sex differences.
Results
Demographic and Clinical Characteristics
There were no significant group differences in age, sex, socio-economic status, IQ, handedness, and mean total scores on the MFQ, SCARED, and CALS (Table 1).
Wholebrain Analyses of FA and Corresponding RD and L1
Group by age interactions for FA were AlphaSim corrected (p<0.05), with a resulting cluster-size threshold (CST) of ≥16 voxels. AlphaSim correction for RD and L1 required a CST of ≥12 voxels. All linear relationships between diffusivity measures and age are reported in Table 2A-C. All non-linear curve fitting statistics are reported in Table S1, available online.
Table 2A.
Means, standard deviations, and non-parametric independent t-test examining group by age interaction (age as covariate) and main effect of group upon fractional anisotropy in wholebrain level analysis.
| Fractional Anisotropy (FA) | Descriptives | GROUP * AGE Interaction a,b | Main Effect of GROUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Mean FA | SD | N of voxels | tmax | P value | N of voxels | tmax | P value | |
| Left Corpus Callosum [body division] | HBO | 0.52 | 0.04 | 20 | 3.8 | .050 | 35 | 4.0 | .050 |
| MNI x, y, z: −18, −30, 34 | CONT | 0.50 | 0.05 | ||||||
| Right Inferior Longitudinal Fasciculus [temporal cortex] | HBO | 0.46 | 0.07 | 15 | 4.3 | .050 | 13 | 4.1 | .050 |
| MNI x, y, z: 49, −23, −22 | CONT | 0.45 | 0.10 | ||||||
Note: MNI = Montreal Neurological Institute atlas.
Monte Carlo simulation with AlphaSim correction was run on uncorrected f statistical maps (p<0.001), obtaining a dual thresholding of both type I error (AlphaSim p<0.05, corrected) and cluster-size thresholding (t-tests CST >16 voxels) to control for multiple voxel-level comparisons.
Between FA and age, in the left corpus callosum there was a positive relationship in healthy offspring of healthy parents (CONT) (R2linear=.3, p=.011) and a negative relationship in healthy offspring having a parent diagnosed with bipolar (HBO) (R2 linear = .4, p=.003) and in the right inferior longitudinal fasciculus in CONT (R2 linear =.3, p=.004), but no relationship in HBO (R2linear=.0, p=.369).
Table 2C.
Means, standard deviations, and non-parametric independent t-test examining group by age interaction (age as covariate) and main effect of group upon longitudinal diffusivity in white matter (WM) regions showing differences in fractional anisotropy between healthy offspring having a parent diagnosed with bipolar disorder (HBO) and healthy control offspring of healthy parents (CONT).
| Longitudinal Diffusivity (L1) | Descriptives | GROUP * AGE Interaction a,b | Main Effect of GROUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Mean L1 | SD | N of voxels | tmax | P value | N of voxels | tmax | P value | |
| Right Inferior Longitudinal Fasciculus [visual cortex] | HBO | 1.19 | 0.25 | 28 | 4.5 | .050 | 26 | 4.4 | .050 |
| MNI x, y, z: 24, −59, −1 | CONT | 1.19 | 0.13 | ||||||
Note: Mean and SD are reported with a scaling factor of 1000. Non-parametric independent t-test=permutation method (n of permutations=5000, no smoothing factor), including age as a covariate and group by age interaction; t max= maximal value of t in the reported cluster; HBO (n=20) and CONT (n=25). MNI = Montreal Neurological Institute atlas.
Monte Carlo simulation with AlphaSim correction was run on uncorrected f statistical maps (p<0.001), obtaining a dual thresholding of both type I error (AlphaSim p<0.05, corrected) and cluster-size thresholding (t-tests CST>12 voxels) to control for multiple voxel-level comparisons.
Between L1 and age, in the right inferior longitudinal fasciculus there was a positive relationship in CONT (R2 linear =.2, p=.023) and a negative relationship in HBO (R2 linear =.4, p=.002).
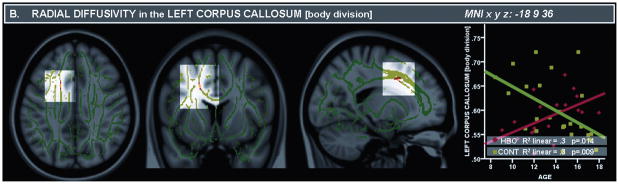
There was a significant group by age interaction in the region of the body of the left corpus callosum (CC) (tmax=3.8; Table 2A). This interaction resulted from a positive relationship between FA and age in the left CC in CONT (R2 linear=.3, p=.011) and a negative relationship in HBO (R2 linear= .4, p=.003). Furthermore, in a nearby region in the body of the left CC, there was a significant interaction in RD (tmax=3.7; Table 2B), which resulted from a negative relationship between age and RD in CONT (R2 linear =.3, p<.009) and a positive relationship in HBO (R2 linear =.3 p=.014) (Figure 1).
Table 2B.
Means, standard deviations, and non-parametric independent t-test examining group by age interaction (age as covariate) and main effect of group upon radial diffusivity in WM regions showing differences in fractional anisotropy between healthy offspring having a parent diagnosed with bipolar disorder (HBO) and healthy control offspring of healthy parents (CONT).
| Radial Diffusivity (RD) | Descriptives | GROUP * AGE Interaction a,b | Main Effect of GROUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Mean RD | SD | N of voxels | tmax | P value | N of voxels | tmax | P value | |
| Left Corpus Callosum [body division] | HBO | 0.54 | 0.05 | 12 | 3.7 | .050 | 12 | 4.3 | .050 |
| MNI x, y, z: −18, 9, 36 | CONT | 0.53 | 0.07 | ||||||
| Right Inferior Longitudinal Fasciculus [temporal cortex] | HBO | 0.56 | 0.05 | 14 | 4.2 | .050 | 14 | 3.5 | .050 |
| MNI x, y, z: 49, −20, −22 | CONT | 0.55 | 0.07 | ||||||
Note: Mean and SD are reported with a scaling factor of 1000. MNI = Montreal Neurological Institute atlas.
Monte Carlo simulation with AlphaSim correction was run on uncorrected f statistical maps (p<0.001), obtaining a dual thresholding of both type I error (AlphaSim p<0.05, corrected) and cluster-size thresholding (t-tests CST>12 voxels) to control for multiple voxel-level comparisons.
Between RD and age, in the left corpus callosum there was a negative relationship in CONT (R2 linear =.3, p=.009) and a positive relationship in HBO (R2 linear =.3 p=.014) and in the right inferior longitudinal fasciculus in CONT (R2 linear =.5, p<.001), but no relationship in HBO (R2linear=.1, p=.214).
Figure 1.
Panel A. Fractional Anisotropy (FA) maps depicting (from left to right) coronal, axial, and sagittal views (above) of the left corpus callosum, body division [Montreal Neurological Institute atlas (MNI) x, y, z: −18, −30, 34]. Panel B. Radial Diffusivity (RD) maps depicting (from left to right) coronal, axial, and sagittal views (above) of the left corpus callosum, body division [MNI x, y, z: −18, 9, 36]. Note: RD values are reported on the Y axis with a scaling factor of 1000. The template is the standard MNI-152 1 mm brain template. Colored voxels in red-yellow represent findings significantly different between healthy offspring having a parent with bipolar disorder (HBO) and age-matched control offspring of healthy parents (CONT). Red-yellow indicates higher FA and decreased RD in HBO than CONT (t ≥ 3; p ≥ 0.05, corrected: scale ranging from red to yellow). We determined the most probable anatomical localization of each cluster with the FSL atlas tool51, using all anatomical templates.
There was also a significant group by age interaction for FA in the region of the right inferior longitudinal fasciculus (ILF) in the temporal pole (tmax= 4.3; Table 2A), that just failed to meet our CST (clustersize=15). This interaction resulted from a positive relationship between age and FA in the right ILF in CONT (R2 linear =.3, p=.004), but no relationship between age and FA in this region in HBO (R2 linear=.0, p=.369). In the same region, there was a significant interaction in RD (tmax=4.2; Table 2B), which resulted from a negative relationship between age and RD in CONT (R2 linear =.5, p<.001) and no relationship in HBO (R2 linear =.1, p=.214) (Figure 2).
Figure 2.
Panel A. Fractional Anisotropy (FA) maps depicting (from left to right) coronal, axial, and sagittal views (above) of the right inferior longitudinal fasciculus in the temporal cortex [Montreal Neurological Institute atlas (MNI) x, y, z: 49, −23, −22]. Panel B. Radial Diffusivity (RD) maps depicting (from left to right) coronal, axial, and sagittal views (above) of the right inferior longitudinal fasciculus in the temporal cortex [MNI x, y, z: 49, −20, −22]. RD values are reported on the Y axis with a scaling factor of 1000. Panel C. Longitudinal Diffusivity (L1) maps depicting (from left to right) coronal, axial, and sagittal views (above) of the right inferior longitudinal fasciculus in the visual cortex [MNI x, y, z: 24, −59, −1]. Note: L1 values are reported on the Y axis with a scaling factor of 1000. The template is the standard MNI-152 1 mm brain template. Colored voxels in red-yellow represent findings significantly different between healthy offspring having a parent with bipolar disorder (HBO) and age-matched control offspring of healthy parents (CONT). Red-yellow indicates higher FA, L1 and decreased RD in HBO than CONT (t ≥ 3; p ≥ 0.05, corrected: scale ranging from red to yellow). We determined the most probable anatomical localization of each cluster with the FSL atlas tool51, using all anatomical templates.
There was a significant group by age interaction in L1 for a cluster in the region of right ILF in the visual cortex (tmax= 4.5; Table 2C), which resulted from a positive relationship between age and L1 in CONT (R2 linear =.2, p=.023), and a negative relationship between age and L1 in HBO (R2 linear =.4, p=.002; Figure 2).
In the region of the CC, there was also a significant main effect of group for FA and RD (FA: tmax=4.0 and RD: tmax=4.2; Table 2A), indicating greater FA (and reduced RD) in HBO relative to CONT. In the region of the right ILF in the temporal pole, there was a significant main effect of group for RD (tmax= 3.5; Table 2A), indicating reduced RD in HBO relative to CONT. FA failed to meet our CST (cluster-size=13; tmax= 4.1; Table 2A). In the region of the right ILF in the visual cortex, there was also a significant main effect of group for L1 (tmax=4.4; Table 2C), which resulted from significantly greater L1 in HBO relative to CONT.
Curve fitting confirmed linear, but also revealed non-linear, best curve fits for relationships between FA, RD, and L1 with age in left CC and right ILF in CONT, and FA and L1 with age in left CC and right ILF (visual cortex) in HBO (See Table S1, available online).
Exploratory non-parametric independent-samples Mann-Whitney U tests did not reveal any significant sex differences in either group in FA and RD in the region of the body of the left CC, in the right ILF (temporal pole), and in L1 in the region of the right ILF in the visual cortex.
Discussion
This is the first study to examine WM microstructure in healthy offspring having a parent diagnosed with BD. The significant group by age interactions in this cross-sectional study supported our hypotheses that HBO would not show the same patterns of greater FA (and lower RD) with increasing age observed in CONT and that these alterations would be particularly evident in the corpus callosum and fronto-temporal regions. In particular, our results show increases in FA (decreases in RD) in CONT in left CC and right ILF, supporting previously documented age-related changes in normative samples. In HBO, however, we observed decreases in FA (increases in RD) with age in left CC, and no relationship between FA (RD) and age in right ILF. We next discuss how our findings relate to previous findings in BD and familial risk for BD.
We found opposite patterns of age-related changes between HBO and CONT in the left CC. The CC is a major midline WM tract that is involved in integrating, between-hemispheres, sensory-motor functions, attention, language, memory, and emotional states.39 Developmental studies indicate that the CC matures through adolescence into adulthood with a posterior-anterior axonal maturation most likely due to increased myelination.28 Our findings of normative increases with age in FA in the CC in CONT are consistent with findings from larger cross-sectional studies indicating normative increases in FA (and decrease in RD) with age in adolescence.28 Thus, our findings of decrease in FA (and increase in RD) with age in the CC suggests possible alterations in the development of WM in the CC in HBO. This would support the idea that altered development of the CC may represent a marker for BD.40
Previous studies in individuals with BD reported abnormalities in volume, signal intensity, shape and microstructure of the CC.40 Most studies generally reported lower FA in individuals with BD,40 with the exception of one study that reported greater FA in the anterior portion of the CC in adult BD patients versus controls.41 A recent study in adolescents with familial BD reported lower FA throughout the CC, including the genu and splenium.15 Few studies have examined CC WM in individuals at high familial risk for BD. One study using voxel-based morphometry reported that genetic liability BD was associated with WM deficits in, among other regions, the anterior CC.26 This study involved adult BD patients, however, and as such, does not evaluate developmental changes in CC as possible vulnerability markers for BD. Longitudinal studies are needed to determine the nature of the developmental changes in CC WM during adolescence that may precede the onset of BD or other psychiatric disorder in youth at risk for BD.
Our findings also indicate differences in the pattern of age-related changes between HBO and CONT in the right ILF. The ILF is a major WM associative tract connecting occipital and temporal cortices that runs laterally and inferiorly to the lateral wall of the temporal horn. This regions is considered part of a “ventral semantic network” with an important role in the visual processing of emotionally salient information, as ILF projections feed information regarding the emotional valence of visual stimuli back to early visual processing cortical regions, thereby enhancing the visual processing of emotionally salient stimuli.42 Our findings of normative increases with age in FA in right ILF in CONT are consistent with findings from cross-sectional studies.28 The absence of such age-related changes in FA (and RD) in right ILF in HBO suggests alterations in the development of this WM tract in HBO.
Recent findings show abnormalities, predominantly lower FA, in ILF FA in adults with BD relative to healthy controls.13 However, other studies did not report abnormalities in ILF FA in pediatric BD.15, 17 With regard to individuals at high familial risk for BD, previous studies did not report any differences in ILF between at-risk for BD groups and healthy controls,19, 43 although one study reported that increasing genetic liability for BD in genetically at-risk adults was significantly associated with lower FA in bilateral portions of the ILF44. In light of the potential role of ILF in emotional information processing, along with findings of important developmental changes in adolescence in the processing of emotionally salient information,45 and evidence of abnormalities in emotional information processing in BD,46, 47 it is possible that alterations in the development of this WM tract may also be implicated in vulnerability for BD48. For instance, recent evidence of altered emotional information processing in unaffected offspring having a parent with BD48 suggests that further research focusing on the ILF and emotional information processing in youth at high familial risk for BD may be warranted.
Unlike the one previous DTI study in children at risk for BD,19 we did not show any significant alteration in WM in the SLF in HBO. The discrepancy between our findings and those from this previous study may be attributed to differences in age range (4–12 years vs. 8–17 years). Another factor may be that most of the at-risk youth in the previous study met criteria for an Axis I diagnosis whereas in our study all were healthy. Given evidence of reduced SLF FA in children with ADHD49, it is unclear to what extent the presence of psychopathology in the at-risk youth affected findings in the previous study.19 Further study is required to determine the extent to which age-related changes in FA in this region predispose to BD or other psychiatric disorders in offspring at high familial risk for BD.
A major strength of our study was that by including age in our statistical model, we were able to document differences in age-related changes in WM microstructure across groups. Another strength was including RD and L1 to help interpret relationships between FA and age in HBO relative to CONT. Our findings indicated that there were significant group by age interactions in RD. The pattern of RD changes with age in left CC and right ILF was opposite to those for FA in CONT, which is what has been showed in previous studies with normative samples. However, in HBO, the pattern was opposite in CC and there was no relationship between FA and RD with age in ILF. There were no significant group by age interactions in L1 in these regions, although there was one significant group by age interaction in L1 in another region of right ILF in the visual cortex, resulting from a positive relationship between age and L1 in CONT and a negative relationship between age and L1 in HBO. Studies in animals38 and humans50 indicate that age-related increases in FA, with decreases in RD, is considered to reflect developmental changes associated with myelination. Thus, one possible interpretation of our main findings in left CC and right ILF may be that HBO show alterations in the myelination of these WM tracts. Increases in L1 in the absence of changes in RD have been associated with increases in axon diameter/density.27 One interpretation of age-related changes in LI in right ILF in visual cortex may be that HBO may show an abnormal pattern of axonal growth in this region with age. Altered development of these WM tracts may then confer increased vulnerability for subsequent development of BD or other psychiatric disorders observed in offspring having a parent with BD.2
There were limitations to our study. First, we used a cross-sectional design to examine age-related changes in WM across groups. Although such designs have been used to understand age-related changes in brain development,28 longitudinal examination with a larger sample will provide a much more in-depth understanding of the developmental trajectories of these WM tracts in HBO, including examination of the role of other developmental factors such as pubertal maturation and sex. Second, we decided to focus initially on healthy offspring having a parent with BD in order to control for potential confounds related to pathology and/or medication. On the one hand, because certain psychiatric disorders are highly prevalent in this population, this led to a targeted sampling that may have limited the ability to generalize our findings to all offspring of parents having BD. On the other hand, because HBO were healthy, our findings are more closely related to genetic-risk for BD and not presence of psychopathology. Nevertheless, the current study represents an initial step toward the identification of altered WM development as a possible vulnerability marker for BD. Future studies will address these issues through longitudinal designs and comparison groups matched on age, sex, and psychiatric disorder (e.g., anxiety disorder, ADHD).
In summary, this is the first DTI study using TBSS to document, in healthy offspring having a parent diagnosed with BD relative to age-matched healthy offspring of healthy parents, age-related changes in key WM tracts supporting interhemispheric integration (CC),39 and semantic and visually-salient information processing (ILF),42 previously reported to be abnormal in BD. We show that, unlike in CONT, HBO exhibit decreases in FA (increases in RD) with increasing age in the CC and no changes with increasing age in ILF. Our findings suggest altered developmental patterns of WM with age, which may be indicative of potential vulnerability markers for future BD or other psychiatric disorders, in healthy offspring having a parent with BD.
Supplementary Material
Acknowledgments
This study was supported in part by the National Alliance for Research on Schizophrenia and Depression (NARSAD): Rebecca Ehlrich Taking Strides Against Mental Illness Investigator Young Investigator Award (Dr. Versace), Young Investigator Award (Dr. Ladouceur), Independent Investigator Award to MLP, and by the National Institute of Mental Health (NIMH): grant to Dr. Birmaher (MH 060952-06).
The authors gratefully acknowledge the children and their families for participating in this study. The authors also thank Jacqueline Rosenstern (research data coordinator), Sharon Nau (research assistant) and Emily Belleau (research assistant), University of Pittsburgh employees on this project, for their assistance in research procedures, as well as Dr. KJ Jung, Scott Kurdilla and Debbie Vizslay, employed by the University of Pittsburgh and Carnegie Mellon’s Brain Imaging Research Center (BIRC), for their help acquiring the neuroimaging data.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Birmaher receives research support from the National Institute of Mental Health. He has served as a consultant to Schering Plough. He has participated in a forum sponsored by Forest Laboratories Inc. He receives royalties from Random House and Lippincott Williams and Wilkins. Drs. Versace, Ladouceur, Romero, Axelson, Kupfer, and Phillips report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merikangas KR, Akiskal H, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009 Mar;66(3):287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001 Dec;3(6):325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 4.Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disorders. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 5.Beyer JL, Taylor WD, MacFall JR, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 6.Versace A, Almeida J, Hassel S, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1061. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houenou J, Wessa M, Douaud G, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between subgenual cingulate and amygdalo-hippocampal complex. Mol Psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 8.Adler CM, Holland SK, Schmithorst V, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion imaging study. Bipolar Disorders. 2004;6(3):197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 9.Regenold WT, D’Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP. Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: A pilot study. Bipolar Disorders. 2006;9:504–512. doi: 10.1111/j.1399-5618.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Sussmann JE, Lymer GK, McKirdy J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009 Feb;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AM, Maniega SM, Lymer GK, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64(12):1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Haznedar MM, Roversi F, Pallanti S, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Bruno S, Cercignani M, Ron MA. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disorders. 2008;10(5):657–657. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahon K, Wu J, Malhotra AK, et al. A Voxel-Based Diffusion Tensor Imaging Study of White Matter in Bipolar Disorder. Neuropsychopharmacology. 2009 Jan 14; doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: A tract-based spatial statistics analysis. Biol Psychiatry. 2009;66:238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Kafantaris V, Kingsley P, Ardekani B, et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavuluri MN, Yang S, Kamineni K, et al. Diffusion tensor imaging study of white matter tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler CM, Adams J, DelBello MP, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163(2):322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 19.Frazier JA, Breeze JL, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007 Dec;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 20.Caetano SC, Silveira CM, Kaur S, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. Journal of Affective Disorders. 2008 Jun;108(3):297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasar AS, Monkul ES, Sassi RB, et al. MRI study of corpus callosum in children and adolescents with bipolar disorder. Psychiatry Research-Neuroimaging. 2006 Jan 30;146(1):83–85. doi: 10.1016/j.pscychresns.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Hasan KM. Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: fractional anisotropy alone is not sufficient. Radiology. 2006 May;239(2):611–612. doi: 10.1148/radiol.2392051172. author reply 612–613. [DOI] [PubMed] [Google Scholar]
- 23.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 24.Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox G, Wandell B. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academic of Sciences USA. 2005;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh A, Job D, Moorhead T, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;56(8):544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 26.McDonald C, Bullmore ET, Sham P, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 27.Kieseppa T, van Erp T, Haukka J, et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 28.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 29.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 30.Perlis R, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder. Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 32.Anjari M, Srinivasan L, Allsop JM, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007 Apr 15;35(3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, et al. Adcances in functional and structural MR image and implementation as FSL. Neuroimage. 2004;23 (suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 Jul 15;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Ward D. [Accessed June, 2009.];Simultaneous Inference for fMRI Data. http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf.
- 38.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002 Nov;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 39.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition. Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 40.Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37:1096–1098. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- 41.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimental PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disorders. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 42.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003 Sep;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 43.Frazier J, Breeze J, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disorders. 2007;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 44.Chaddock CA, Barker GJ, Marshall N, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. The British Journal of Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 45.Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003 Sep;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 46.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation:implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich B, Fromm S, Berghorst L, et al. Neural connectivity in children with bipolar disorder: impairment in the face of emotion processing circuit. Journal of Child Psychology and Psychiatry. 2008;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton LS, Levitt JG, O’Neill J, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuro Report. 2008;19:1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giorgio A, Watkins KE, Douaud G, et al. Developmental changes in white matter microstructure in adolescence. J Neurol Neurosurg Psychiatry. 2007;78:1019–1020. [Google Scholar]
- 51.FSL Atlas Tool. [Accessed April 2009.]; ( http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.