Abstract
Upper abdominal exenteration for upper abdominal malignancies was carried out in 15 patients with removal of the liver, spleen, pancreas, duodenum, all or part of the stomach, proximal jejunum and ascending and transverse colon. Organ replacement was with the liver, pancreas and duodenum plus, in some cases, a short segment of jejunum. Eleven of the 15 patients survived for more than 4 months; 2 died, after 6½ and 10 months, of recurrent tumor. Of the 9 patients who are surviving after 6½ to 14 months, recurrent tumor is suspected in only 1 and proven in none. Four patients with sarcomas and carcinoid tumors (2 each) have had no recurrences. The other 5 survivors had duct cell cancers (3 examples), a cholangiocarcinoma (1 example), and a hepatoma (1 example). The experience so far supports further cautious trials with this drastic cancer operation.
Keywords: liver transplantation, pancreas transplantation, multiorgan transplantation
Between 22 July 1988 and 24 March 1989, 15 patients with otherwise unbeatable intraabdominal malignancies underwent upper abdominal exenteration, followed by transplantation of the liver in continuity with the pancreas and duodenum. The first 10 of these cases have been reported previously (1). The purpose of the present report is to provide an accounting of all 15 patients who now have potential survival ranging from 6 to 14 months.
Methods
Case material
The patients were 27 to 49 years old. There were 9 men and 6 women (Table 1). Three of the 15 patients had liver failure and were originally sent to us for consideration of transplantation for that reason. Jaundice and weight loss were common.
Table 1.
Recipient patients
| No | Age | Sex | Race | Preop Liver Function |
Date of Operation | ||
|---|---|---|---|---|---|---|---|
| Bilirubin | Albumin | Prothrombin Time | |||||
| 1 | 27 | M | W | 2.3 | 3.6 | 11.3 | 7/22/88 |
| 2 | 31 | F | W | 0.6 | 3.8 | 12.9 | 7/28/88 |
| 3 | 35 | F | W | 3.2 | 4.2 | 13.0 | 8/26/88 |
| 4 | 43 | M | W | 0.6 | 3.6 | 10.7 | 9/17/88 |
| 5 | 37 | F | W | 0.3 | 3.8 | 11.9 | 10/27/88 |
| 6 | 27 | M | W | 0.3 | 1.9 | 13.9 | 11/23/88 11/26/88 |
| 7 | 29 | M | W | 9.3 | 2.9 | 14.0 | 11/25/88 |
| 8 | 30 | M | W | 6.0 | 4.8 | 15.3 | 12/1/89 |
| 9 | 47 | F | W | 14.0 | 3.1 | 13.2 | 1/1/89 |
| 10 | 40 | F | W | 8.9 | 3.6 | 10.5 | 1/12/89 |
| 11 | 49 | M | W | 1.0 | 3.9 | 11.8 | 1/18/89 |
| 12 | 39 | M | W | 9.3 | 3.5 | 15.5 | 2/7/89 |
| 13 | 49 | M | W | 0.9 | 4.2 | 12.2 | 2/24/89 |
| 14 | 33 | M | 0 | 0.7 | 4.1 | 12.4 | 2/27/89 3/03/89 |
| 15 | 41 | F | W | 0.5 | 2.9 | 12.0 | 3/24/89 |
The previous operations in these patients are summarized in Table 2. A previous hepatic resection had been carried out in 2 patients. One had had a Klatskin tumor with Roux-y biliary reconstruction 2.5 yr earlier, and the other had had a left hepatic trisegmentectomy 8 months previously for treatment of a cholangiocarcinoma. Both of these patients with previous resections were physicians.
Table 2.
Characteristics of tumors
| Case | Histopathology | Primary |
Metastases |
Previous Treatment | |||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Size | Liver | Pancreas | Bowel | Lymph Nodes | Other | |||
| 1 | duct cell carcinoma sclerosing cholangitis | common duct | diffuse | minor | no | no | 0/9 | no | none |
| 2 | spindle cell sarcoma | duodenum | 5 cm | extensive | no | no | 0/38 | no | none |
| 3 | duct cell carcinoma sclerosing cholangitis | hilum | 9.5 cm | extensive | no | no | 12/50 | lungs* | none |
| 4 | spindle cell sarcoma | duodenum (4th) | 8 cm | extensive | no | no | 0/57 | no | none |
| 5 | carcinoid | duodenum (2nd) | 1.8 cm | extensive | no | no | 5/35 | no | adriamycin and tumor embolization |
| 6 | carcinoid | stomach | 3 cm | extensive | no | no | 2/27 | no | open liver biopsy |
| 7 | recurrent duct cell | intrahepatic | 11 cm | extensive | no | extensive | 0/28 | no | resection of klatskin tumor hepatico-jejunostomy on 3/26/86 followed by 4500 RADS |
| 8 | duct cell carcinoma | liver hilum | 7.5 cm | regional | no | no | 0/94 | no | exploratory laparotomy |
| 9 | duct cell carcinoma | common duct | 10 cm | extensive | head and body | no | 6/30 | portal vein, skull* | none |
| 10 | duct cell carcinoma | liver hilum | 11.5 cm | direct invasion | 0 | 0 | 0/70 | no | percutaneous transhepatic stent |
| 11 | cholangiocarcinoma | liver | 14 cm | extensive | head | no | 0/28 | no | none |
| 12 | duct cell carcinoma sclerosing cholangitis | common duct | 6 cm | extensive | no | no | 2/21 | positive margin at the hepatic vein | total colectomy and ileostomy |
| 13 | recurrent cholangiocarcinoma | liver | multiple lesions up to 5 cm | extensive | no | no | 0/34 | no | left triseg-mentectomy 8 months previously |
| 14 | hepatocellular carcinoma, PNCB** | liver | 11 cm | extensive | no | no | 0/25 | portal vein invasion | exploratory laparotomy |
| 15 | carcinoid | pancreas | 4.5 cm | extensive | – | stomach colon | 0/38 | no | open liver biopsy |
PNCB = Hepatitis B postnecrotic cirrhosis;
Diagnosed postoperatively upon retrospective review of X-ray films.
The tumor pathology in the 15 cases is summarized in Table 2. There were 7 duct cell carcinomas, 2 cholangiocarcinomas of the liver, 2 small bowel sarcomas, 3 neuroendocrine tumors, and 1 hepatocellular carcinoma. In most cases, there were metastases with as many as 4 organs involved by tumor. Particular attention was paid to the lymph nodes in the specimen. Five of the 15 patients had positive nodes.
The essence of the operation is shown in Fig. 1. The organs removed included the liver, stomach, pancreas, spleen, duodenum, proximal jejunum, and ascending plus transverse colon. Although the operation had several variations (1), the usual recipient procedure and gastrointestinal reconstruction is that in Fig. 1. A veno-venous bypass was used during the recipient organ removal, permitting bypass of systemic venous blood from the vena cava and of splanchnic blood from the superior mesenteric vein during a 1- to 3-hour interval. In 2 patients (Cases 6 and 14) retransplantation was necessary due to primary non-function of the initial organ cluster.
Fig. 1.
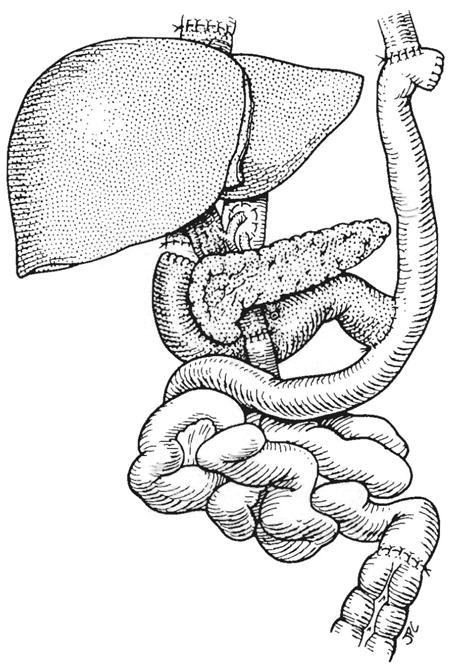
Usual gastrointestinal reconstructions in patients with upper abdominal exenteration and organ cluster transplantation.
Postoperatively, the patients received immunosuppression with cyclosporine and prednisone to which azathioprine was added when and if the white blood count was more than 5000 mm3. OKT3 was used with the indication of rejection or with the suspicion of graft-versus-host disease.
Adjuvant cancer therapy was not given. Diet was resumed when requested by the patient and advanced quickly to multiple small feedings. All 15 patients were treated during operation and for 7 to 21 days afterward with the synthetic somatostatin, sandozstatin®, which has been shown to minimize experimentally caused pancreatitis.
Results
Mortality
The causes of death are tabulated in Table 3. In the patients who died early (9 to 112 d), there was no evidence of tumor detected clinically or at autopsy, although Patient 12 had a positive margin in the original specimen. In 2 instances, the deaths were due to slough or fistula of the pancreas. Primary graft non-function in a 3rd could not be rectified with retransplantation. The 4th patient died 57 d postoperatively of massive gastrointestinal bleeding caused by CMV gastroenteritis.
Table 3.
Causes of death
| Case Number | Primary Diagnosis | Cause of Death | Autopsy Findings | Survival in Days |
|---|---|---|---|---|
| 6 | carcinoid | Candida sepsis and multiorgan failure after retransplantation | invasive systemic candidiasis – no evidence of residual disease | 9 |
| 11 | cholangiocarcinoma | acute myocardial infarction secondary to massive Gl bleeding | acute myocardial infarction, massive Gl bleeding, multiple ulcerations of the gastrointestinal tract secondary to disseminated CMV* – no evidence of residual disease | 57 |
| 12 | duct cell carcinoma and sclerosing cholangitis | sepsis, disseminated CMV* and multiorgan failure secondary to pancreatic fistula | not done | 72 |
| 1 | duct cell carcinoma and sclerosing cholangitis | bacterial septicemia and multiple organ failure secondary to pancreatic fistula | not done | 112 |
| 9 | duct cell carcinoma | metastasic disease (lungs, bones and brain) | not done | 197 |
| 3 | duct cell carcinoma and sclerosing cholangitis | metastasic disease (lungs and bones) | not done | 304 |
Cytomegalovirus.
Two late deaths were caused by widespread tumor recurrence after 10 and 6 months. Both patients (Cases 3 and 9), had metastatic disease present at the time of operation, although this was not realized until the preoperative X-rays were reviewed later.
Surviving patients
Nine of the 15 patients are still living with survival of 6 to 14 months (Table 4). Only one is suspected of having recurrence. This is a young physician who had a thoracic CT scan 7 months postoperatively which revealed multiple nodules in the lower lung fields. These lesions have been stable for 3 months. The decision was made not to biopsy the lung nodules since it was not thought justified in any event to use chemotherapy. The patient is stable clinically with completely normal liver and pancreas graft function 10 months after resection for a duct cell carcinoma.
Table 4.
Current status of the surviving patients
| Patient Number | Latest Postop. Liver Function | Insulin or Enzyme Replacement | Weight | Early Satiety and/or Pain with meals | Number of Meals per day | Stools/day | Follow-up (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilirubin (mg%) | Albumin (mg%) | Protime (sec) | Preop | Lowest | Now | ≤ 6 months Postop. | > 6 months Postop. | ≤ 6 months Postop. | > 6 months Postop. | ≤ 6 months Postop. | > 6 months Postop. | |||
| 2 | 0.9 | 4.2 | 11.2 | 0 | 109 | 95 | 95 | only with big meals | no | 6 | 3–5 | 1* | 0–1* | 416 |
| 4 | 0.2 | 3.6 | 11.3 | 0 | 140 | 112 | 125 | no | no | 6 | 3 | 2* | 1–2 | 364 |
| 5 | 1.0 | 4.5 | 12.4 | 0 | 159 | 137 | 122 | occasionally | no | 6 | 3–5 | 3–4* | 1* | 325 |
| 7 | 0.5 | 4.0 | 12.7 | 0 | 141 | 99 | 99 | no | no | 6 | 3–5 | 2–3* | 2–3* | 296 |
| 8 | 0.4 | 4.1 | 11.8 | 0 | 140 | 95 | 124 | yes | no | 6 | 3 | 3* | 3* | 290 |
| 10 | 0.5 | 4.0 | 11.5 | 0 | 168 | 126 | 126 | occasionally | no | 6 | 3–4 | 3* | 3* | 247 |
| 13 | 0.6 | 3.8 | 12.0 | 0 | 121 | 110 | 110 | no | – | 6 | – | 4* | – | 205 |
| 14 | 0.8 | 3.1 | 10.6 | 0 | 120 | 83 | 86 | yes | – | 3 | – | 3* | – | 202 |
| 15 | 0.5 | 3.9 | 12.7 | 0 | 145 | 90 | 98 | only with big meals | – | 6 | – | 2–3* | – | 177 |
Treatment for diarrhea (with dypnoxylate HCL (patients 2, 5, 14, 15) loperamide HCL (patients 7, 10) propantheline bromide (patient 8) and atropine (patient 13)).
Two patients with carcinoid tumors and massive liver metastases, as well as the 2 with sarcoma in multiple organs have no evidence of recurrence. None of the sarcoma patients had lymph node involvement, while in 1 (Case 5) of the 2 surviving patients with a carcinoid there were metastases in 5 lymph nodes. All of these patients had clean resection margins in spite of the fact that the tumors were truly massive.
The other 4 patients who are free of malignancy had hepatoma (1 example), recurrent cholangiocarcinoma after a left trisegmentectomy 9 months previously (1 example), and duct cell carcinoma (2 examples).
Quality of life
The 9 surviving patients have had a very acceptable quality of life which has improved with the passage of time. At first, all of the patients had very severe weight loss and it was feared that they might become nutritional cripples. However, the weight loss was reversed in 2 of the patients and maintained at a stable level in 7 others (Table 4). Some of the patients seemingly have enjoyed their new weight status, having previously been self-classified as obese.
Patient 5 was of special interest because of the use of her entire duodenum and proximal jejunum as a segment in continuity of the gastrointestinal tract (Fig. 2). This piece of small bowel which has now functioned as a true intestinal homograft for 11 months has continued to be complication-free. This patient was explored for a small bowel intestinal obstruction 6 months after her transplantation (by Dr. Wallis Marsh of St. Louis) and was found to be completely free of intraabdominal tumor.
Fig. 2.
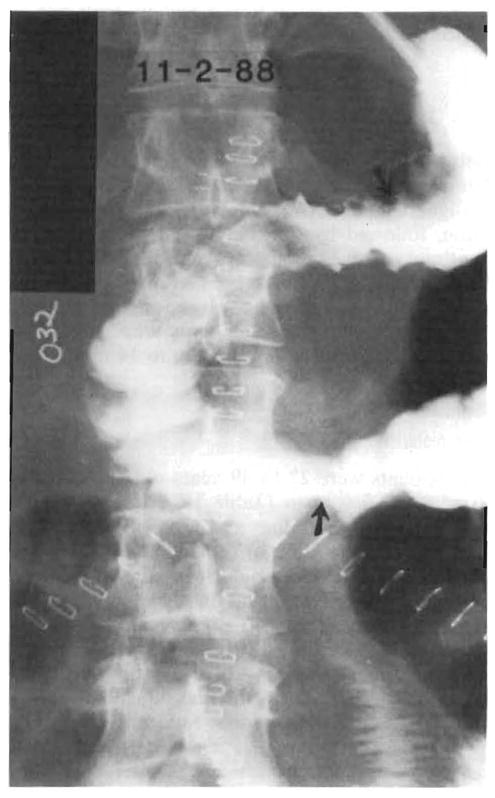
Gastrointestinal series obtained in Patient 5, showing homograft duodenum and jejunum in continuity with patient’s own stomach and jejunum. This segment of intestine has function as a true intestinal homograft for 11 months.
The gastrointestinal tract function of the surviving patients is summarized in Table 4. In patients with more than 6 months survival, frequent small feedings have been reduced (Table 4). The diarrhea which was universal early after operation has changed to more formed and less frequent bowel movements.
Discussion
The concept upon which this operation was based was discussed extensively in an earlier publication (1). What was done was to excise the adult equivalent of most of the embryonal foregut, including the stomach, liver, pancreas, duodenum and spleen. In addition, the proximal jejunum and the ascending and transverse colon which derive from the upper and lower portions of the midgut were also removed. The philosophic question with such an operation is whether the mere extension of the surgical resection limits would permit extended tumor-free survival of patients whose malignancies were so extensive as to preclude conventional therapy.
With each passing month, the answer to this question has seemed to be affirmative. Although the operation was dangerous, with 4 of the 15 patients dying within the first 4 postoperative months, survival beyond this time has been acceptable. Only 2 patients have developed recurrences. In both patients, the diagnosis was duct cell carcinomas with lymph node metastases, and in both there was evidence upon retrospective review of the X-ray studies that metastases beyond the surgical boundaries were already present at the time of operation. There has been no recurrence in the sarcomas and carcinoid tumors which were exceptionally bulky, and there have been examples of long tumor-free state in patients with duct cell carcinomas, a cholangiocarcinoma, and a hepatoma.
The evolution in Case 13 was particularly gratifying. A surgeon who was the Chairman of a department in a major European university underwent a left hepatic trisegmentectomy for a cholangiocarcinoma on 5 July 1988. He developed recurrences in the residual posterior segment and had a cluster operation 8 months later. Since then, he has been appointed as Departmental Chairman at another University and works full time.
Thus, there is reason to continue further trials of the upper abdominal exentreration procedure, and to consider cautious exploration of further indications for this operation. For example, patients with carcinomas of the right and transverse colon with multiple hepatic metastases might be appropriate candidates for the procedure. Acinar carinomas of the pancreas with liver metastases have not yet been treated. The only discouraging observation that can be made from the experience so far is the poor survival of patients with bile duct cell carcinomas, particularly those with multiple lymph node metastases at the time of their operations.
Follow-up periods of only 6 to 14 months are still inadequate to permit more than tentative conclusions, but in this period many of the survivors have achieved virtually complete rehabilitation. Whether or not the full cluster replacement graft is necessary is under study. The upper abdominal exenteration has been performed by Tzakis et al. (2) with transplantation of the liver only. The penalty for the more limited procedure is diabetes mellitus and exocrine pancreatic insufficiency. Eighteen such procedures have been carried out, but with follow-ups of a few days to more than 6 months, it is too early to speculate about which is the preferred operation. The cluster replacement permits freedom from endocrine and exocrine pancreatic insufficiency, and it avoids biliary tract reconstruction. On the negative side, the pancreas component of the organ cluster homograft was responsible for the death of at least 2 patients. In addition, it is much more difficult to find organ cluster donors than it is to find donors of livers alone.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Presented at the 4th International Symposium on Organ Procurement and Preservation, Minneapolis, Minnesota, September 20, 1989.
References
- Starzl TE, Sodo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzakis AG, Todo S, Starzl TE. Upper abdominal exenteration with liver replacement: A modification of the “cluster” procedure. Transplant Proc. (in press) [PMC free article] [PubMed] [Google Scholar]


