Abstract
Worldwide, diabetes is a rapidly growing problem that is managed at the individual level by monitoring and controlling blood glucose levels to minimize the negative effects of the disease. Because of limitations in diagnostic methods, significant research efforts are focused on developing improved methods to measure glucose. Nanotechnology has impacted these efforts by increasing the surface area of sensors, improving the catalytic properties of electrodes and providing nanoscale sensors. Herein, we discuss developments in the past several years on both nanosensors that directly measure glucose as well as nanomaterials that improve glucose sensor function. Finally, we discuss challenges that must be overcome to apply these developments in the clinic.
Keywords: Biosensor, Glucose Oxidase, Direct Oxidation, Quantum Dot, Nanoparticle, Nanosensor, Carbon nanotube, Smart tattoo, Continuous monitoring
Diabetes and Blood Glucose Monitoring
Diabetes is a rapidly growing problem, currently affecting 24 million people in the US alone [1]. This number could increase to 44.1 million by 2034 with treatment costs approaching $336 billion (in 2007 dollars) [2]. Diabetes can lead to complications such as serious as lower-limb amputations, blindness, and cardiovascular disease [1]. Although there is no cure for diabetes, patients can reduce disease-associated complications through the tight control of blood glucose levels [1].
In order to attain optimal control, patients must monitor their blood glucose levels. Currently, this requires a patient to obtain a small sample of blood, usually via a finger prick. Blood is placed onto a sensor test strip that is then read by a handheld electronic reader, which reports the blood glucose concentration. These sensors are based on electrochemical enzymatic measurements (Figure 1) with screen printed electrodes [3] and provide rapid and accurate measurements of blood glucose without the need for laboratory analysis. However, there are limitations to this approach including painful sampling, analyses cannot be performed if the patient is otherwise occupied (e.g. sleeping) and large fluctuations between sampling time points are missed [4, 5]. To help overcome the problems with discrete blood glucose measurement, new commercial products focus on continuous glucose measurement (Box 1). Early stage nanotechnology research involving nanosensors and nanomaterials is also directed toward continuous monitoring (Box 2).
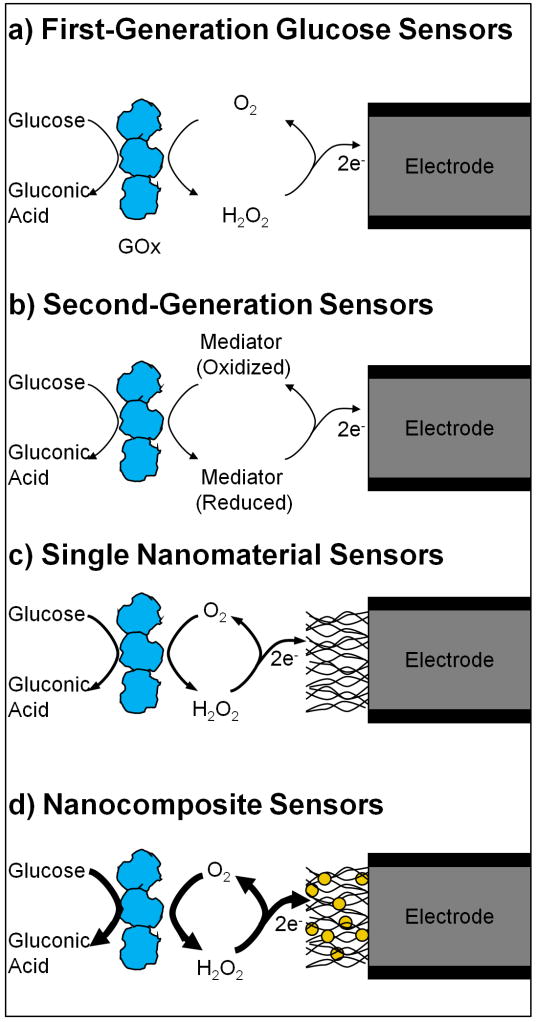
Figure 1.
Nanostructured materials used in glucose sensors. Standard glucose oxidase (GOx) based electrochemical biosensors (a, b) utilize a GOx layer to recognize glucose and generate an electrochemical signal. This signal is transferred from the enzyme through O2 reduction to H2O2 (a) or reduction of another chemical mediator (b). Nanomaterials can be incorporated into these sensors in order to increase surface area, improve catalytic action, modify operating parameters, and improve electron transfer from the enzyme to the electrode. This can be accomplished through the use of single types of nanomaterials (c) such as CNTs, or nanocomposites consisting of multiple nanomaterials working together (d).
Box 1. Continuous glucose monitoring.
Traditional monitoring of blood glucose uses discrete blood sampling time points during the course of a day. For many diabetics this provides satisfactory data for the control of blood glucose levels. However, inherent in this approach lays the risk of overlooking hypo- and hyperglycemic excursions between sampling points as well as limiting predictive value. The figure presents a hypothetical case, demonstrating the limitations of discrete blood sampling. Three of the four measurements are near 100 mg/dL (the optimal range), with no hypoglycemic events. However, continuous monitoring data indicates a hypoglycemic event occurred between the first two sampling points, which was not captured with discrete measurements. Capturing this event would allow the patient to intercede and avoid complications, highlighting the advantages of an increased frequency of measurement.
Another advantage of continuous measurements is the estimation of future blood glucose levels. In the figure, the patient’s blood glucose level is trending lower at the last sample point. Thus, the patient could take action to limit a major excursion from the desired blood glucose concentration. This is especially important if the blood glucose trend for the patient is changing rapidly. Finally, this approach also allows monitoring without patient intervention, which can be extremely advantageous during sleep when blood glucose can drop dangerously low [4].
Current technology for continuous glucose monitoring has some disadvantages that have prevented widespread adoption for the management of diabetes. All FDA approved devices are implanted sensors, which have a maximum useful lifetime of several days to a week (partially because the immune system responds to the sensor as a foreign body). As they are implanted in subcutaneous tissue, these sensors do not directly sample blood, and this can lead to a time lag in measurements taken during periods of rapid concentration changes. This lag has been estimated from several minutes to nearly 30 minutes [4]. Additionally, current sensors must be calibrated and checked against standards as they are only approved for tracking trends in blood glucose levels. Finally, current sensors are expensive and are not always covered by health insurance plans, so this technology has not been widely adopted.
Figure I.
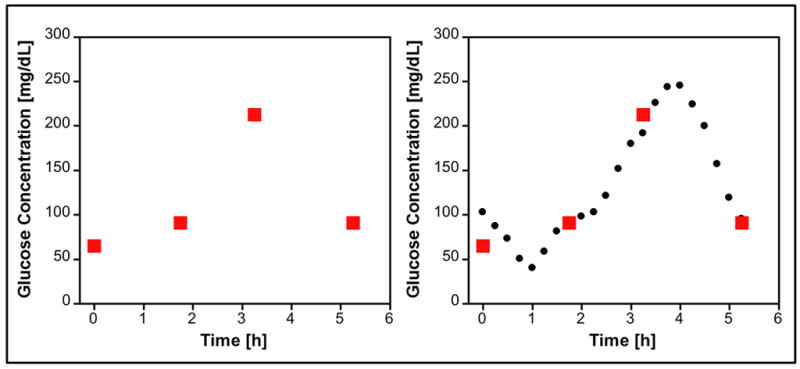
Hypothetical discrete versus continuous glucose monitoring. Discrete samples (red boxes) are taken every few hours. Continuous monitoring (black circles) provides more frequent measurements of blood-glucose concentrations.
Box 2. Nanotechnology for glucose sensors.
Nanotechnology has been incorporated into glucose sensors using two primary approaches. First, sensors can be designed using macro- or microscale components (such as electrodes, membranes and supporting hardware) but incorporate either a nanostructured surface or a nanomaterial into this design. The nanoscale properties of these modified systems have several advantages, including higher surface areas (yielding larger currents and faster responses) and improved catalytic activities. These sensors, owing to their size, would be implanted similar to current technology if used for continuous monitoring. Accordingly, these sensors could experience the same drawbacks as current sensors, including sensor fouling and decreased sensor life as a result of immune foreign body response.
Secondly, nanofabrication techniques can generate glucose sensors that are nanoscale in all dimensions. These sensors offer some advantages over traditional sensors for continuous monitoring: these sensors would be injectable, which could lead to more facile administration of the sensing system than the current implantation approach. Additionally, because of the small size of these sensors, they could potentially avoid the foreign body response of the immune system and, therefore, have longer useful lives. However, these sensors are a radical change from current continuous monitoring sensors and there is little clinical data on these systems, so further research is needed before these nanosensors can be of use to patients.
A defining characteristic of nanomaterials is that they have at least one structural dimension on the order of 100 nanometers or less [6]. Nanomaterials and nanosensors offer some significant advantages owing to their small size. High surface area/volume ratios (allowing larger signals, better catalysis and more rapid movement of analyte through sensors) as well as enhanced optical properties [quantum dot fluorescence, gold nanoparticle quenching, surface enhanced raman scattering (SERS)] represent significant benefits over macroscale materials. Researchers have used these properties to improve the accuracy, size, lifetime and usability of sensors for the treatment of diabetes. Nanosensors are finally nearing the stages of commercial and clinical implementation, which will hopefully allow better treatment for patients suffering from diabetes in the future.
Here, we review recent developments in the field of nanomaterials and nanosensors for diabetes care. A review by Wang provides an overview of work on electrochemical glucose biosensors (including nanomaterials [3]); another excellent source is a review by Pickup concerning nanomedicine for diabetes care and includes a section on nanosensors [5]. We limit our discussion to sensors that include either nonbiological nanomaterials or that are on the nanoscale (i.e. we exclude sensors that comprise only chemicals or mutated proteins). Additionally, we focus our discussion of electrochemical biosensors on those that have been applied to clinical samples. For additional discussion of electrochemical biosensors, see [7]. Finally, although nanotechnology has been applied to other diabetes targets (e.g. autoantibodies in type 1 diabetes [8] or acetone as a possible diagnostic in diabetes [9]), we focus on glucose detection.
Nanomaterials to supplement current sensors
The most common application of nanotechnology for sensors in diabetes is the use of nanomaterials to assist standard enzymatic electrochemical detection of glucose (Figure 1). Incorporation of nanomaterials into these sensors provides a variety of advantages including increased surface area, more efficient electron transfer from enzyme to electrode and the ability to include additional catalytic steps. While a detailed discussion of all possible modifications to the standard electrode would be prohibitively long, we highlight recent advances that demonstrate the range of options for nanomaterials in glucose sensors.
Carbon nanotube (CNT) incorporation is a heavily investigated modification to enzymatic electrode detection of glucose, partly because of the electron transfer capabilities of CNTs as well as the large surface area. The electrode can be replaced with a highly porous nanofiber onto which glucose oxidase is immobilized [10]. This structure has a much higher electronic surface area than bulk metal electrodes, and accordingly can immobilize more enzyme and generate larger signals. Another approach is to modify the nanotubes with an electrochemical mediator such as ferrocene to improve the electron transfer between the enzyme and the electrode [11].
CNTs can be coupled with other nanomaterials or polymers to form nanocomposites for glucose detection. Combining CNTs with additional nanomaterials improves aspects such as catalytic activity. Nanocomposite membranes have recently been fabricated with layer-by-layer assembly of CNTs and gold nanoparticles [12]. Similar approaches have also coupled CNTs with metal nanoparticles of silver [13], platinum [14], or gold/platinum [15] as well as nonmetals such as silica [16] or composites of silica/iron oxide [17]. CNT membranes can be deposited on nanostructured electrodes, such as alumina-coated-silica modified electrodes [18] or titanium dioxide (TiO2) nanotube arrays [19]. Another form of nanostructured carbon, graphene nanosheets, was utilized as a platform to support platinum-gold or gold nanoparticles [20]. Nanocomposites made from nanotubes and polymers such as cellulose can serve as a matrix for entrapment of glucose oxidase (GOx) onto an electrode surface [21].
A variety of nanostructured electrodes provide improvements over conventional macrostructured electrodes. Zinc oxide deployed as nanowires [22] and nanotube arrays [23, 24] has been used for glucose detection. Nanowire arrays fabricated from ruthenium [25] and gold [26] have increased surface area and improved electrochemical interrogation compared with conventional electrodes. In addition to creating nanoscale features on the surface of the electrode, nanostructure can be generated with nanoparticles. Gold [27], platinum [28] and palladium [29] nanoparticles have been utilized in membranes to assist electron transfer and to increase the surface area of the sensor.
Magnetic nanoparticles, commonly made from iron oxide, have also been used for glucose sensors. These particles can be combined with other systems such as CNTs [17] or used on their own [30, 31]. The magnetic nature of these nanoparticles simplifies the assembly of GOx-labeled nanoparticles onto the electrode [32] as well as enabling the formation of nanoparticle conductive wires on the electrode surface [33]. In both of these examples, the particles were attracted to the electrode surface using magnetic fields, which highlights one advantage in utilizing magnetic nanoparticles in fabrication of nanoparticle electrode assemblies.
Nanostructured polymers can improve the development of glucose sensors. Hollow spheres of conductive polymer can be used to transfer electrons from GOx to the electrode [34]. Conductive polymer electrodes can be used in a method similar to other nanostructured surfaces, where GOx is immobilized directly on the modified electrode. In one example, the electrode surface was covered with highly ordered polyaniline nanotubes, which have GOx immobilized within the tubes [35]. The use of polymers introduces a range of different electrochemical properties, including operation at varying potentials. The use of different potentials helps to minimize electrochemical interference from common electroactive compounds in blood (e.g. acetaminophen, ascorbic acid, and uric acid) which can cause nonspecific signals with standard electrochemical detection approaches.
As the goal of research to improve sensors is to assist patients with diabetes, an important factor to consider when assessing nanomaterial sensors is whether they work in clinical samples. All of the sensors discussed have at minimum been tested in a buffered system with glucose; many have been tested with interferants; and most perform better than standard sensors as a result of the nanomaterials used. However, surprisingly few have been tested in clinical samples (i.e. blood or serum). The research groups reporting tests in clinical samples describe comparable sensitivity to conventional testing techniques [12, 13, 15, 16, 28–30, 32, 35], which is promising for future applications of these sensors (see Table 1 for further details on these sensors). Testing in clinical samples is only the first step in proving the utility of nanomaterials application, and very little research has been performed to demonstrate cost-effective increases in positive attributes when compared with the standard approach of glucose detection.
Table 1.
Electronic sensors used In biological samples
| Detection | Nano-component | Response Time | Linear Rangea | Detection Limit | Sample Type | Sample Treatment | Ref. |
|---|---|---|---|---|---|---|---|
| GOx | CNT, Au NP | <6s | 6 μM-5 mM | 3 μM | Plasma | Diluted (1:4) PBS, pH 7 |
[12] |
| GOx | CNT, Ag NP | <10s | 0.5–50 μM | 0.1 μM | Serum | Diluted (1:500) 0.1 M BRB, pH 6 |
[13] |
| GOx | CNT, AuPt NP | 3s | 0.01–9.49 mM | 0.01 mM | Spiked Serum | Diluted (1:6.25) PBS, pH 7 |
[15] |
| GOx | Polyaniline grafted CNTs | ~6s | 1–10 mM | 0.1 μM | Spiked Serum | Diluted (UNK) PBS, pH 7 |
[16] |
| GOx | Pt NP | ND | 0.1–10 mM | ND | Cerebrospinal Fluid | Untreated | [28] |
| GOx | Pd NP, PEDOT Nanofibers | ND | 0.5–30 mM | 75 μM | Serum | Diluted (1:1) PBS, pH 7 |
[29] |
| GOx | Fe3O4 NP | ND | 6 μM–2.2 mM | 6 μM | Serum | Diluted (1:10) PBS, pH 6.8 |
[30] |
| GOx | Fe3O4 NP | 10s | 0.5–80 μM | 0.1 μM | Spiked Blood | Diluted (1:250) PBS, pH 6.5 |
[32] |
| GOx | Polyaniline Nanotubes | 3s | 0.01–5.5 mM | 0.3 μM | Urine | Diluted (UNK) PBS, pH 5.5 |
[35] |
| Direct Oxidation | CNT (coated with NiO) | ND | 0.2–12 mM | 0.16 mM | Serum | Diluted (1:200) 0.1M NaOH |
[36] |
| Direct Oxidation | CuO/CuOX NS | <1s | 2 μM–15 mM | 0.05 μM | Serum | Diluted (1:50) 0.1M, NaOH |
[40] |
| Direct Oxidation | Au NP | ND | 0.4–10.7 mM | 0.37 mM | Serum | Diluted (1:25) 0.1M, NaOH |
[42] |
| Direct Oxidation | Ni(OH)2 NS | ND | 0.05–23 mM | 6 μM | Serum | Diluted (1:200) 0.5M, NaOH |
[46] |
| Direct Oxidation | CNT, Pd NP | 3s | 0.5–17 mM | 0.2 μM | Blood | Diluted (UNK) PBS, pH 7.4 |
[51] |
| Direct Oxidation | CNT, CuO NP | <1s | 0.4 μM–1.2 mM | 0.2 μM | Serum | Diluted (1:250) 0.1M, NaOH |
[52] |
| Direct Oxidation | CNT, Pd NP | ND | 0–46 mM | ND | Spiked Urine | pH 13 | [53] |
| FET & Con A | CNT | >1 min | 1 pM–1 nM | 1 pM | Spiked Plasma | Untreated | [60] |
Normal physiological range for blood or serum glucose concentration is 4.4 to 6.6 mM [3]
Abbreviations: Au, gold; BRB, Britton-Robinson buffer; CuO, Copper oxide; CuOx, copper oxalate; Fe3O4, iron oxide; NaOH, sodium hydroxide; ND, not determined; NP, nanoparticle; NS, nanostructures; Pd, Lead; PEDOT, poly(3,4-ethylenedioxythiophene); PBS, phosphate buffered saline; Pt, Platinum; UNK, unknown dilution ratio;
Nanomaterials for the direct oxidation of glucose
Coupling biological recognition elements with electrochemistry increases the selectivity and sensitivity of sensors and explains both the popularity of this approach and the commercial success of sensors based on proteins. Despite these advantages, there are several drawbacks to sensors based on biological recognition including the intrinsically poorer stability when compared with nonbiological systems. As a result of this limitation, many research groups have focused on the development of glucose detection assays that do not rely on a protein for recognition and, as a result, could have longer storage lifetimes.
One of the most heavily researched areas in nonenzymatic glucose sensors is detection of glucose oxidation directly at an electrode. This method also has several limitations such as slow reaction kinetics and the need for a large applied potential, which decreases specificity [36]. Nanomaterials have helped to overcome these limitations and thereby have allowed the development of direct-oxidation glucose sensors as replacements for biological recognition sensors.
Recent progress in this field can be roughly categorized based on the nanomaterial used in the sensors. Glucose detection has been demonstrated using copper and copper oxide nanowires [37], porous films [38] as well as nanoflowers and nanorods [39]. Nanostructured copper oxide/copper oxalate has also been employed [40]. Detecting the direct oxidation of glucose, however, does not require copper. Nanoparticles composed of silver [41], gold [42], nickel [43] and nickel/palladium [44] and other nanostructures such as gold nanowires [45], nickel hydroxide nanocomposites [46], boron-doped diamond nanorods [47] and platinum/lead nanoporous networks [48] have been reported. Finally, the inclusion of carbon nanomaterials in sensor constructs improves sensor performance; metal nanoparticles incorporated with carbon nanofibers [49, 50] or nanotubes [36, 50–54], fluorine-doped nanotubes [55] and boronic acid functionalized nanotubes [56] all have improved oxidation characteristics (e.g. working potential or sensitivity) when compared with direct oxidation systems with an unmodified electrode.
Several of the direct glucose oxidation sensors perform in biological samples [36, 40, 42, 46, 51, 52, 55] (see Table 1 for further details on these sensors). Many of these sensors work at high pH, likely precluding in vivo applications; however, Meng and colleagues [51] have shown that a palladium nanoparticle/CNT system can work in phosphate buffered saline at pH 7.4 as well as in clinical samples diluted with this buffer, showing the potential for future in vivo applications. The nanomaterials employed in these sensors have enabled significantly improved electrochemical properties such as lower working potentials, improved sensitivity and improved limits of detection. However, they will likely not see much utility in clinical settings without significant work to improve their ability to function in undiluted samples like those obtained routinely by patients.
Other electrochemical methods of detection
Nanomaterial-based sensors can also be designed to detect glucose through changes in pH or charge, often through a field effect transistor (FET). These devices measure a property of the nanomaterial (such as conductance) that is affected by charges near the surface of the sensor or the pH of the solvent. As the concentration of glucose changes, the charge near the surface or the pH changes either as a result of an enzymatic reaction or competitive binding, causing the sensor to register a change in the measured property. This allows indirect quantification of glucose concentration, although pH changes in the bulk solution can affect the measured response.
The breakdown of glucose catalyzed by GOx decreases solution pH by liberating hydrogen ions, and generates negative charges by producing the gluconate ion. Risveden and colleagues used a region selective ion sensitive field effect transistor (RISFET) to detect gluconate generation in order to quantify glucose concentrations [57]. The RISFET focuses the gluconate between the sensing electrodes, and the increase in current is proportional to the amount of glucose present. Layer-by-layer assembly of CNTs with GOx allows the change in pH generated by glucose degradation to be monitored by measuring the conductance changes in the CNT layer [58]. Modified nanoparticles can also improve the sensitivity of capacitive electrolyte-insulator-semiconductor (EIS) structures. The use of gold nanoparticles modified with both GOx and ferrocene improved sensitivity nearly two-fold over nanoparticles modified with only GOx [59]. In addition to using GOx to recognize glucose, other proteins such as concanavalin A (ConA), a plant lectin that binds polysaccharide, can be used in FET detection platforms. A CNT-based FET labeled with the polysaccharide dextran detected a change in resistance upon binding, or in the presence of glucose, displacement of ConA with picomolar detection limits [60]. Although unsuitable for glucose detection in blood or interstitial fluid, this could be valuable for testing in alternate scenarios or fluids where glucose concentration is very low and has been demonstrated in spiked plasma (Table 1).
In addition to resistance and conductance based methods, other electronic measurements can be used for glucose detection. In the presence of glucose, dextran displacement from ConA, immobilized onto an electrode coated with gold nanoparticles, changes capacitance across the electrode [61]. Ion-selective electrodes can potentiometrically measure free silver ions (Ag+), which are released from silver nanoparticles in the presence of hydrogen peroxide generated by GOx [62]. Finally, glucose can be detected by electrochemiluminescence after glucose oxidation on palladium nanoparticle functionalized nanotubes, although with a linear range of detection several orders of magnitude below physiological blood glucose concentration [63].
The nanomaterials and nanofabrication techniques employed in these sensor architectures improve sensitivity as well as yield extremely low limits of detection. Although far too low for applications in direct clinical samples, these might be of utility in other testing scenarios or when coupled with methods to increase the working range to physiological levels. Although one sensor was demonstrated in human plasma doped with extremely low concentrations of glucose [60], there is little other research on the clinical applicability of these sensors or improvement over commercial sensors for the care of patients with diabetes.
Fluorescent polymeric nanosensors
Electrochemical detection technologies represent a large portion of research into glucose detection and dominate the field of commercially available sensors. However, for in vivo continuous monitoring, fluorescence-based sensors offer several advantages. Chief among them is the ability to optically interrogate the sensors through the skin rather than having an electrode system implanted. This approach often involves a “smart tattoo” for the patient, as sensors would be implanted into the skin of the patient similar to regular tattoos (Figure 2). However, unlike regular tattoos, these smart tattoos would be only temporary and would need to be replaced on the time scale of weeks to months to account for sensor migration and loss of signal owing to degradation. The sensors would change fluorescence properties in response to blood glucose, and this change could be read out using optical interrogation through the skin. This method would eliminate or reduce the need for patients to take blood samples while allowing data to be collected in a more continuous manner. This also minimizes the chances for infection at the implantation site and avoids other complications of implanted devices such as capsule formation and the accompanying decreases in glucose transport [64].
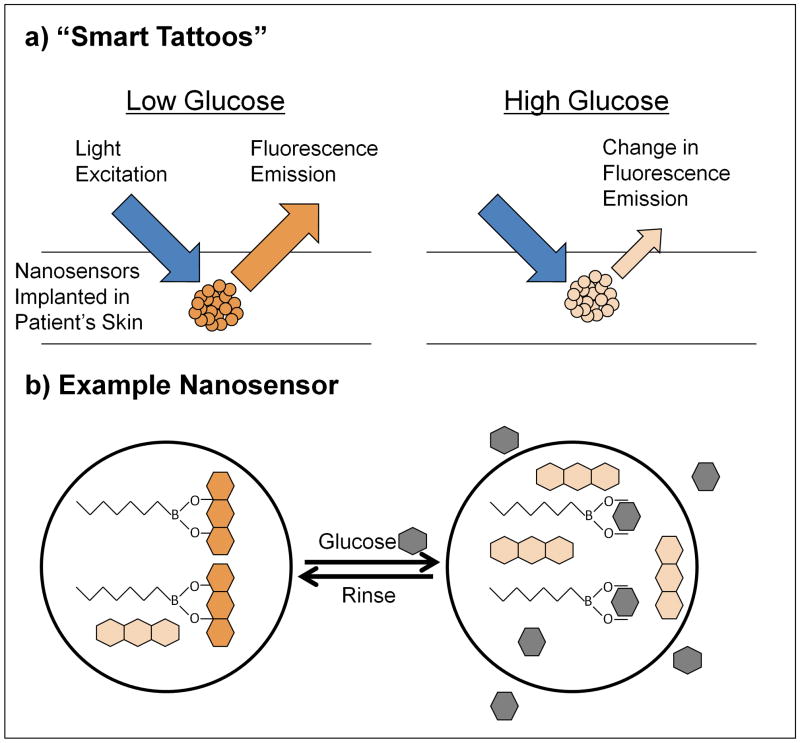
Figure 2.
Polymeric nanosensors for “smart tattoos.” Smart tattoos (a) are implanted in the skin of a patient to assist with continuous monitoring of glucose levels. The sensors change their fluorescent properties with glucose concentration, which allows optical interrogation of glucose levels without blood draws. One example of this class of sensors (b) is nanosensors composed of a hydrophobic polymer containing lipophilic glucose recognition elements and fluorescent reporters. In the absence of glucose (left), the boronic acid-based recognition element is bound to the reporter alizarin (orange), forming a fluorescent complex. In the presence of glucose (right, gray), the boronic acid binds to glucose, extracts it into the sensor core, displacing the fluorophore alizarin in the process, rendering it nonfluorescent (light orange). This causes a decrease in fluorescence, signaling detection of glucose through optical interrogation [67].
With this goal in mind, a variety of nanosensor technologies have been developed using fluorescence signals. Several such sensors are based on polymeric nanosensors incorporating boronic acid derivatives to recognize glucose. Nanospheres based on N-isopropylacrylamide containing a covalently bound phenyl-boronic acid derivative as well as two attached fluorophores have been synthesized [65]. In the absence of sugar, the nanospheres are small, holding the fluorophores close together and allowing efficient Förster resonance energy transfer (FRET). Upon sugar binding to the boronic acid, the polymer swells, increasing the average distance between the fluorophores. This decreases FRET, which increases the donor fluorescence and decreases the acceptor fluorescence. This approach was subsequently improved (faster response times, large and reversible signal changes in physiological concentration ranges) by using multiple boronic acid derivatives and altering the concentrations of fluorophores used [66].
Fluorescent nanosensors have also been developed based on highly plasticized hydrophobic polymers [67] (Figure 2b, Table 2). A hydrophobic boronic acid capable of extracting glucose was incorporated into the core of the nanosensor. The diol-containing dye, alizarin, was used as the reporting group. In the absence of glucose, boronic acid binds the nonfluorescent alizarin, generating a fluorescent complex. In the presence of glucose, boronic acid binds glucose, releasing alizarin and decreasing overall fluorescence. As all of the components are hydrophobic, they remain in the core of the sensor, making the sensors reversible, which is essential for continuous glucose monitoring. Importantly, these sensors have been used to monitor the blood glucose of mice in vivo, and can track blood glucose throughout the physiological range (from 66 mg/deciliter (dL) or 3.7 mM to 427 mg/dL or 23.7 mM)[67]. Thus, these nanosensors represent a step towards the development of a “smart tattoo” for glucose monitoring.
Table 2.
Fluorescence-based sensors used in biological samples
| Detection | Nanocomponent | Response Time | Linear Range | Detection Limit | Sample Type | Sample Treatment | Ref. |
|---|---|---|---|---|---|---|---|
| Boronic acid | Nanosized Sensor | <1 min | 0.35–347 mMa | ND | in vivo (mouse) | SC injection | [67] |
| GOx | QD | 5 min | 0.5–16 mM | 0.5 mM | Serum | Untreated | [77] |
| GOx | QD | 15 min | 10–100 μMb 0.1–1 mM |
3 μM | Serum | Diluted (1:100) PBS and water |
[78] |
| GOx | TiO2/SiO2 nanocomposite | ND | 1 nM–10 mM | 0.12 nM | Serum | Untreated | [79] |
| Con A | QD | 15 min | 0.1–50 μM | 50 nM | Serum | Diluted (1:1000) PBS, pH 7.4 and water |
[80] |
| GOx | QD | 5 min | 0–0.6 mM (Fluorescence) 0–3 mM (Colorometric)c |
ND | Serum | Diluted (1:5) PBS, pH 7 |
[82] |
| Europium Complex | Silica NP | 20 min | 0–1 mM | 4.4 μM | Serum | Diluted (1:10) PBS, pH 6.5 |
[84] |
Dynamic range, no linear range reported
This sensor demonstrated two linear response regimes
This sensor can function in two modes with different linear ranges
Abbreviations: SC subcutaneous
Nanotechnology can also facilitate the development of “smart tattoos” using microspheres in addition to nanoscale sensors. For example, glucose/galactose binding proteins can be incorporated on the surface of a microsphere using layer-by-layer assembly of a nanofilm to encapsulate the components [68]. The fluorescence intensity and lifetime of the fluorescent protein and the spheres change in response to glucose, although this has been demonstrated in concentration range lower than the clinically relevant range. Future work should be directed to translate the sensitivity of this technology towards the physiological range. Catalytically inactive GOx has been used as a recognition element inside layer-by-layer assembled nanofilms [69–70]. These films were assembled on dissolvable manganese carbonate microspheres and then the interior microsphere was dissolved, leaving the nanofilm behind. The modified GOx, labeled with a dye, and dextran, labeled with a quencher, were then loaded through the nanofilm. Upon exposure to glucose, the dextran and GOx separate and fluorescence increases. Upon removal of glucose, this process is reversed as both components are contained inside the nanofilm; thus, this is a reversible sensor. Later work expanded this concept to alginate microspheres [71]. Importantly, this work makes use of near-infrared fluorophores, which offers an advantage for in vivo application, as background signal from tissue and biological fluids is minimized, allowing easier imaging through the skin. Layer-by-layer assembly has also been used to fabricate GOx-molecular wire shells around gold nanoparticles [72]. By using osmium molecular wires, Scodeller and colleagues demonstrated glucose dependant changes in the resonant Raman signals, which were amplified by the presence of the gold nanoparticles through SERS [72]. Barone and colleagues utilized nanomaterials to generate a “smart tattoo”. They fabricated a hydrogel containing a modified GOx, which caused the hydrogel to change internal conformation. This change was measured through the fluorescence of embedded CNTs, yielding excellent signal changes with response to glucose in buffered systems. They also demonstrated excellent signal to noise imaging of the hydrogels (without the glucose responsive element) in mice using a standard microscope [73]. Nanotubes as fluorophores have two important attributes for in vivo application: they have near-infrared fluorescence and they do not photobleach (allowing easier imaging and longer useful lifetimes). The same group also demonstrated a sensor approach based on glucose controlled aggregation of CNT (labeled with a glucose analogue) onto ConA. As the aggregates have different fluorescence than free CNTs, detection of glucose is possible through measuring the CNT fluorescence change [74]. This system could be encapsulated in a microdialysis capillary for implantation and imaging, although a wider dynamic range is needed before clinical application [75]. Of note, this group also discusses the future potential cost of manufacture for these sensors, finding that costs will likely be similar to current devices [75].
These “smart tattoo” approaches are a large departure from both current electrochemical glucose sensors as well as from the nanomaterial electrochemical approaches. They offer the ability to measure glucose through the skin, allowing continuous monitoring with a less invasive approach. Additionally, many of these sensors would not be possible without nanomaterials or nanofabrication techniques. The nanosize of some of the sensors also might avoid the immune system effects as well as ease eventual injection, although both of these attributes will require future laboratory and clinical studies. This category of sensors demonstrates the possibilities in advancing glucose sensors through the use of nanotechnology.
Quantum dots in glucose sensors
In addition to utilizing nanomaterials to encapsulate sensor components, nanomaterials can be functional agents in the sensor architecture. Semiconductor quantum dots (QDs) have excellent optical properties for use in sensors, such as narrow fluorescence peaks and minimal photobleaching. However, the QDs themselves do not interact with glucose, and hence have no inherent recognition ability and must be coupled to a recognition element for successful implementation. Several research groups have coupled cadmium telluride (CdTe) QDs with GOx to fabricate sensing systems similar to the one shown in Figure 3a. The luminescence of these QDs is quenched by the hydrogen peroxide generated by the enzyme in the presence of glucose. Tethering the enzyme directly to a CdTe QD [76] or using layer-by-layer assembly to form a nanofilm of CdTe QDs covered by a nanofilm of GOx [77] allows rapid optical detection of glucose. In fact, the nanofilm approach allows accurate quantification of serum samples [77]. Direct conjugation of GOx with manganese-doped zinc sulfide (ZnS) QDs also yielded a sensor capable of detecting glucose in clinical samples [78]. Similarly, the phosphorescence of TiO2/silicon dioxide (SiO2) nanocomposites is quenched by peroxide, and coupling with GOx allows serum detection of glucose comparable to current detection methods [79].
Figure 3.
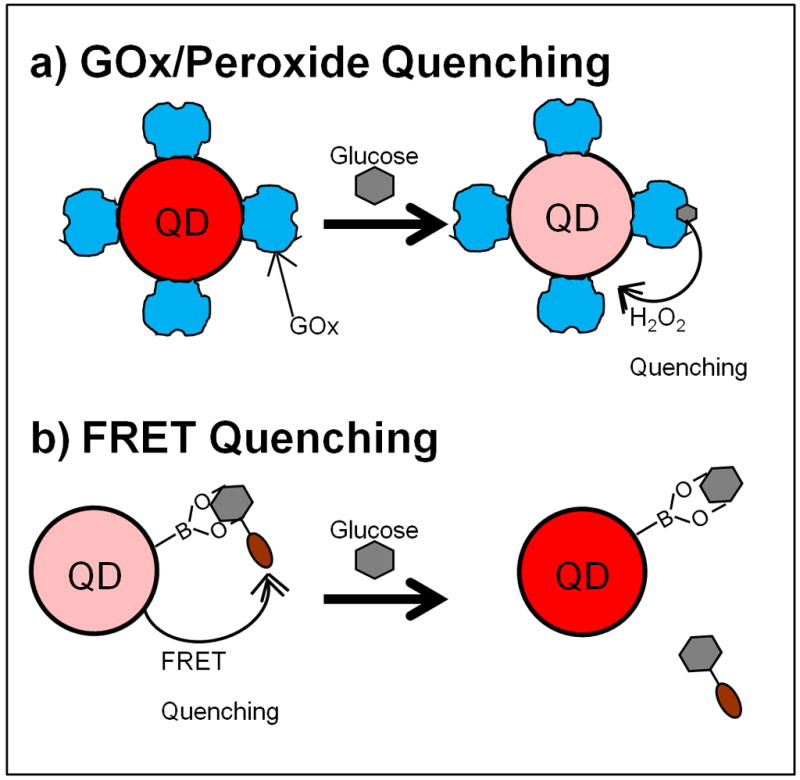
Quantum Dot-based glucose sensors. The fluorescence or phosphorescence of QDs can be quenched by hydrogen peroxide. Glucose sensors are fabricated through attachment of GOx to a QD reporter (a). In the presence of glucose, the enzyme generates hydrogen peroxide, which quenches the QD, providing an optical signal change proportional to glucose concentrations. For examples, see [76–79]. QDs can also be quenched by chromophores through FRET (b). Glucose sensors can be fabricated through attachment of a recognition element to the QD and attaching a FRET quencher to a glucose analogue. Without glucose, these molecules are bound together, quenching the QD fluorescence. Glucose displaces the quencher, increasing QD fluorescence. For examples, see [80–81].
These approaches can be applied with recognition elements other than GOx. ConA also recognizes glucose and can be tethered to QDs. However, upon glucose recognition, no peroxide is generated, so another fluorescence quenching mechanism must be used. For example, the addition of gold nanoparticles labeled with β-cyclodextrin (β-CD) quench fluorescence by FRET, as β-CD binds to ConA, bringing the nanoparticle close to the QD [80]. Glucose can displace the β-CD, which increases QD fluorescence, even in clinical samples. Boronic acids can also be attached to a QD to detect glucose; in one example, a fluorophore-labeled sugar was used as the FRET-based quencher of a cadmium selenide/ZnS QD [81] (similar to Figure 3b).
QDs can also be used as a reference signal. One demonstration of this approach uses a film of QDs as a reference, which is covered by a film of oxygen-responsive dye and finally a GOx layer [82]. The signal from the oxygen-responsive dye changes with glucose concentration as GOx activity locally depletes the oxygen, while the signal from the QDs remains constant. This allows colorimetric quantification of glucose concentrations in the hypoglycemic range. QDs can also assist amperometric glucose biosensors [83]. Upon UV irradiation, cadmium sulfide QDs can generate electron-hole pairs. Using a membrane containing platinum nanoparticles to assist with extracting the electron, these QDs increase the electrochemical signal obtained from GOx detection of glucose with the application of UV light, yielding a more sensitive current response to glucose. However, the use of UV light would preclude most application in biological samples due to the large UV absorbance of proteins. Finally, although not based on QDs, silica luminescent nanoparticles with embedded europium complexes can sense glucose through interactions between glucose and europium; direct interaction displaces water molecules, which quench fluorescence [84]. This approach yields increases in fluorescence with glucose detection and, owing in part to the optical properties of europium, allows accurate quantification in clinical samples.
As a replacement for current electrochemical sensors, fluorescent sensors based on QDs will likely be more expensive. However, these approaches could also be converted into “smart tattoo” systems through implantation into the skin, yielding the benefits described above. Of course, implantation into the body would require stringent toxicity testing because of possible problems with cadmium-containing particles. For implantation of some of the systems (such as displacement of β-CD [80] or a labeled sugar [81]), they must first be encapsulated to allow reversible signaling. QDs also have ideal optical properties for long-term implantation (i.e. they will not photobleach over time), and many of the discussed systems [77–80,82,84] have been demonstrated in clinical samples (Table 2), which bodes well for future in vivo use.
Concluding remarks
Research and development of nanosensors for the management of diabetes is an important research area and will remain so in the future. Even though progress in this field is rapid, the ultimate goal of achieving long-term, accurate, and continuous glucose monitoring in patients has not been reached. In order to help achieve this goal, future work should emphasize testing in realistic, clinical samples even for proof of concept sensor designs. These sensors should also be compared more thoroughly with commercially available sensors to better demonstrate the advantages or disadvantages of the nanosensors. These direct comparisons should help to justify the additional cost and effort to overcome manufacturing challenges associated with nanosensors compared with standard sensors. The cost and effort of large-scale manufacture of new sensor approaches must provide either extreme improvements in accuracy with minimal additional new cost or an improvement in patient quality of life. This is a large problem to overcome for the approaches that incorporate nanomaterials into electrochemical detection approaches or detect glucose through direct oxidation, as the patient must still undergo the same sampling methods (finger-stick), yielding no improvement in quality of life. In order to have an impact on diabetes, these sensors must demonstrate extremely high improvements in response, as cost is unlikely to decrease below current manufacturing approaches. In addition to cost, other questions remain about the ability for these approaches to impact clinical care (Box 3), including biocompatibility and sensor lifetime; these questions must be answered through further research in order for patients to benefit from this technology.
Box 3. Outstanding Questions.
Can nanomaterials be designed to help improve biocompatibility and sensor lifetime for implantable sensors?
Do nanomaterials improve current sensors at a level that offsets the increased cost of manufacture?
Are there additional considerations for biocompatibility of nanoscale materials?
Can nanoscale sensors minimize tissue encapsulation and protein fouling?
Can nanosensors be administered in locations to minimize glucose transport time lag?
Nanoscale sensors have the potential to drastically improve continuous glucose monitoring capabilities and improve patient quality of life. Macrosensors can be compromised during implantation through degradation of the sensor and fibrous capsule formation. Nanosensors might avoid these drawbacks, allowing more long-term monitoring and reaching the goal of a closed-loop artificial pancreas. Current diabetes treatment involves sampling blood to obtain blood-glucose concentration, calculation of the needed insulin dose and injection of the drug. The closed-loop pancreas would eliminate the need for the patient to perform these steps, as it would perform all three tasks, similar to a natural pancreas. Continuous monitoring, like that achieved by some of the systems discussed here, is a necessary part of this long-term goal. Additionally, “smart tattoo” approaches minimize the need for implantation of electrochemical sensors to attain this goal, which minimizes patient inconvenience and pain. Nanosensors have the capability to improve the lives of future patients living with diabetes.
Acknowledgments
We thank J. Matthew Dubach and Mary K. Balaconis for helpful criticisms of the manuscript. This work was supported by the National Institute of General Medicine of the National Institutes of Health under award number R01 GM084366 and by Internal Research and Development funding from The Charles Stark Draper Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. 2008. [Google Scholar]
- 2.Huang ES, et al. Projecting the Future Diabetes Population Size and Related Costs for the U.S. Diabetes Care. 2009;32:2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J. Chem Rev. Vol. 108. Washington, DC, U. S.: 2008. Electrochemical Glucose Biosensors; pp. 814–825. [DOI] [PubMed] [Google Scholar]
- 4.Burge MR, et al. Continuous Glucose Monitoring: The Future of Diabetes Management. Diabetes Spectrum. 2008;21:112–119. [Google Scholar]
- 5.Pickup JC, et al. Nanomedicine and its potential in diabetes research and practice. Diabetes/Metabolism Research and Reviews. 2008;24:604–610. doi: 10.1002/dmrr.893. [DOI] [PubMed] [Google Scholar]
- 6.Chopra N, et al. Functional One-Dimensional Nanomaterials: Applications in Nanoscale Biosensors. Anal Lett. 2007;40:2067–2096. [Google Scholar]
- 7.Vaddiraju S, et al. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosens Bioelectron. 2010;25:1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, et al. A novel approach to ultrasensitive diagnosis using supramolecular protein nanoparticles. FASEB J. 2007;21:1324–1334. doi: 10.1096/fj.06-7303com. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty S, et al. Detection of biomarker in breath: A step towards noninvasive diabetes monitoring. Current Science. 2008;94:237–242. [Google Scholar]
- 10.Zhu ZG, et al. Nano-yarn carbon nanotube fiber based enzymatic glucose biosensor. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/16/165501. [DOI] [PubMed] [Google Scholar]
- 11.Qiu JD, et al. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal Biochem. 2009;385:264–269. doi: 10.1016/j.ab.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Carbon nanotube/chitosan/gold nanoparticles-based glucose biosensor prepared by a layer-by-layer technique. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2009;29:50–54. [Google Scholar]
- 13.Lin JH, et al. One-step synthesis of silver nanoparticles/carbon nanotubes/chitosan film and its application in glucose biosensor. Sensors and Actuators B-Chemical. 2009;137:768–773. [Google Scholar]
- 14.Wen ZH, et al. Pt Nanoparticles Inserting in Carbon Nanotube Arrays: Nanocomposites for Glucose Biosensors. J Phys Chem C. 2009;113:13482–13487. [Google Scholar]
- 15.Zhang YF, et al. A Novel Glucose Biosensor Based on Glucose Oxidase Immobilized on AuPt Nanoparticle - Carbon Nanotube - Ionic Liquid Hybrid Coated Electrode. Electroanalysis. 2010;22:223–228. [Google Scholar]
- 16.Gopalan AI, et al. An electrochemical glucose biosensor exploiting a polyaniline grafted multiwalled carbon nanotube/perfluorosulfonate ionomer-silica nanocomposite. Biomaterials. 2009;30:5999–6005. doi: 10.1016/j.biomaterials.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Baby TT, Ramaprabhu S. SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta. 2010;80:2016–2022. doi: 10.1016/j.talanta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Tsai MC, Tsai YC. Adsorption of glucose oxidase at platinum-multiwalled carbon nanotube-alumina-coated silica nanocomposite for amperometric glucose biosensor. Sensors and Actuators B-Chemical. 2009;141:592–598. [Google Scholar]
- 19.Pang XY, et al. An amperometric glucose biosensor fabricated with Pt nanoparticle-decorated carbon nanotubes/TiO2 nanotube arrays composite. Sensors and Actuators B-Chemical. 2009;137:134–138. [Google Scholar]
- 20.Baby TT, et al. Metal decorated graphene nanosheets as immobilization matrix for amperometric glucose biosensor. Sensors and Actuators B-Chemical. 2010;145:71–77. [Google Scholar]
- 21.Wu XE, et al. Direct electron transfer of glucose oxidase immobilized in an ionic liquid reconstituted cellulose-carbon nanotube matrix. Bioelectrochemistry. 2009;77:64–68. doi: 10.1016/j.bioelechem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Ali SMU, et al. A fast and sensitive potentiometric glucose microsensor based on glucose oxidase coated ZnO nanowires grown on a thin silver wire. Sensors and Actuators B-Chemical. 2010;145:869–874. [Google Scholar]
- 23.Kong T, et al. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes. Sensors and Actuators B-Chemical. 2009;138:344–350. [Google Scholar]
- 24.Yang K, et al. ZnO Nanotube Arrays as Biosensors for Glucose. J Phys Chem C. 2009;113:20169–20172. [Google Scholar]
- 25.Chi BZ, et al. Synthesis of ruthenium purple nanowire array for construction of sensitive and selective biosensors for glucose detection. Sensors and Actuators B-Chemical. 2009;140:591–596. [Google Scholar]
- 26.Liu YY, et al. An Effective Amperometric Biosensor Based on Gold Nanoelectrode Arrays. Nanoscale Res Lett. 2009;4:210–215. doi: 10.1007/s11671-008-9227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Obrero G, et al. A gold nanoparticle-modified PVC/TTF-TCNQ composite amperometric biosensor for glucose determination. J Electroanal Chem. 2009;634:59–63. [Google Scholar]
- 28.Li CY, et al. Toward real-time continuous brain glucose and oxygen monitoring with a smart catheter. Biosens Bioelectron. 2009;25:173–178. doi: 10.1016/j.bios.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Santhosh P, et al. Fabrication of enzymatic glucose biosensor based on palladium nanoparticles dispersed onto poly(3,4-ethylenedioxythiophene) nanofibers. Bioelectrochemistry. 2009;75:61–66. doi: 10.1016/j.bioelechem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Yang LQ, et al. A practical glucose biosensor based on Fe3O4 nanoparticles and chitosan/nafion composite film. Biosens Bioelectron. 2009;25:889–895. doi: 10.1016/j.bios.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Luo LQ, et al. Amperometric glucose biosensor based on NiFe2O4 nanoparticles and chitosan. Sensors and Actuators B-Chemical. 2010;145:293–298. [Google Scholar]
- 32.Li JP, et al. Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sensors and Actuators B-Chemical. 2009;139:400–406. [Google Scholar]
- 33.Jimenez J, et al. Magneto-induced self-assembling of conductive nanowires for biosensor applications. J Phys Chem C. 2008;112:7337–7344. [Google Scholar]
- 34.Santhosh P, et al. Hollow spherical nanostructured polydiphenylamine for direct electrochemistry and glucose biosensor. Biosens Bioelectron. 2009;24:2008–2014. doi: 10.1016/j.bios.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZY, et al. Detection of Glucose Based on Direct Electron Transfer Reaction of Glucose Oxidase Immobilized on Highly Ordered Polyaniline Nanotubes. Anal Chem. 2009;81:1638–1645. doi: 10.1021/ac802421h. [DOI] [PubMed] [Google Scholar]
- 36.Shamsipur M, et al. Highly improved electrooxidation of glucose at a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry. 2010;77:120–124. doi: 10.1016/j.bioelechem.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang GF, et al. Enzyme-free amperometric sensing of glucose using Cu-CuO nanowire composites. Microchim Acta. 2010;168:87–92. [Google Scholar]
- 38.Cherevko S, Chung CH. The porous CuO electrode fabricated by hydrogen bubble evolution and its application to highly sensitive non-enzymatic glucose detection. Talanta. 2010;80:1371–1377. doi: 10.1016/j.talanta.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, et al. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sensors and Actuators B-Chemical. 2010;144:220–225. [Google Scholar]
- 40.Babu TGS, Ramachandran T. Development of highly sensitive non-enzymatic sensor for the selective determination of glucose and fabrication of a working model. Electrochim Acta. 2010;55:1612–1618. [Google Scholar]
- 41.Quan H, et al. Electrochemical oxidation of glucose on silver nanoparticle-modified composite electrodes. Electrochim Acta. 2010;55:2232–2237. [Google Scholar]
- 42.Feng D, et al. Electrochemical glucose sensor based on one-step construction of gold nanoparticle-chitosan composite film. Sensors and Actuators B-Chemical. 2009;138:539–544. [Google Scholar]
- 43.Wang X, et al. Non-enzymatic amperometric glucose biosensor based on nickel hexacyanoferrate nanoparticle film modified electrodes. Colloids and Surfaces B: Biointerfaces. 2010;78:363–366. doi: 10.1016/j.colsurfb.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 44.Miao FJ, et al. Amperometric glucose sensor based on 3D ordered nickel-palladium nanomaterial supported by silicon MCP array. Sensors and Actuators B-Chemical. 2009;141:338–342. [Google Scholar]
- 45.Cherevko S, Chung CH. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sensors and Actuators B-Chemical. 2009;142:216–223. [Google Scholar]
- 46.Safavi A, et al. Fabrication of a glucose sensor based on a novel nanocomposite electrode. Biosens Bioelectron. 2009;24:1655–1660. doi: 10.1016/j.bios.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 47.Luo DB, et al. Fabrication of Boron-Doped Diamond Nanorod Forest Electrodes and Their Application in Nonenzymatic Amperometric Glucose Biosensing. ACS Nano. 2009;3:2121–2128. doi: 10.1021/nn9003154. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, et al. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal Chem. 2008;80:997–1004. doi: 10.1021/ac701790z. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, et al. Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens Bioelectron. 2009;24:3329–3334. doi: 10.1016/j.bios.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 50.Rathod D, et al. Platinum nanoparticle decoration of carbon materials with applications in non-enzymatic glucose sensing. Sensors and Actuators B-Chemical. 2010;143:547–554. [Google Scholar]
- 51.Meng L, et al. Nonenzymatic Electrochemical Detection of Glucose Based on Palladium-Single-Walled Carbon Nanotube Hybrid Nanostructures. Anal Chem. 2009;81:7271–7280. doi: 10.1021/ac901005p. [DOI] [PubMed] [Google Scholar]
- 52.Jiang LC, Zhang WD. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode. Biosens Bioelectron. 2010;25:1402–1407. doi: 10.1016/j.bios.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 53.Chen XM, et al. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens Bioelectron. 2010;25:1803–1808. doi: 10.1016/j.bios.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 54.Li X, et al. Self-assembled microstructure of carbon nanotubes for enzymeless glucose sensor. Sensors and Actuators B-Chemical. 2009;136:444–450. [Google Scholar]
- 55.Ly SY, Lee JH. Human-Urine Diabetes Assay and In Vivo Rat Bladder Assay Using a Fluorine-Doped Carbon Nanotube Catheter Sensor. Ann Biomed Eng. 2009;37:2028–2033. doi: 10.1007/s10439-009-9714-1. [DOI] [PubMed] [Google Scholar]
- 56.Yang DS, et al. One-step functionalization of multi-walled carbon nanotubes by radiation-induced graft polymerization and their application as enzyme-free biosensors. Radiat Phys Chem. 2010;79:434–440. [Google Scholar]
- 57.Risveden K, et al. The region ion sensitive field effect transistor, a novel bioelectronic nanosensor. Biosens Bioelectron. 2007;22:3105–3112. doi: 10.1016/j.bios.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 58.Lee D, Cui TH. Layer-by-Layer Self-Assembled Single-Walled Carbon Nanotubes Based Ion-Sensitive Conductometric Glucose Biosensors. IEEE Sensors J. 2009;9:449–456. [Google Scholar]
- 59.Gun J, et al. Field-effect nanoparticle-based glucose sensor on a chip: Amplification effect of coimmobilized redox species. Electroanalysis. 2008;20:1748–1753. [Google Scholar]
- 60.Cella LN, et al. Single-Walled Carbon Nanotube-Based Chemiresistive Affinity Biosensors for Small Molecules: Ultrasensitive Glucose Detection. J Am Chem Soc. 2010;132:5024–5026. doi: 10.1021/ja100503b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labib M, et al. A novel competitive capacitive glucose biosensor based on concanavalin A-labeled nanogold colloids assembled on a polytyramine-modified gold electrode. Anal Chim Acta. 2010;659:194–200. doi: 10.1016/j.aca.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 62.Ngeontae W, et al. Novel potentiometric approach in glucose biosensor using silver nanoparticles as redox marker. Sensors and Actuators B-Chemical. 2009;137:320–326. [Google Scholar]
- 63.Chen XM, et al. A novel non-enzymatic ECL sensor for glucose using palladium nanoparticles supported on functional carbon nanotubes. Biosens Bioelectron. 2009;24:3475–3480. doi: 10.1016/j.bios.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 64.Mou X, et al. Long-term calibration considerations during subcutaneous microdialysis sampling in mobile rats. Biomaterials. 2010;31:4530–4539. doi: 10.1016/j.biomaterials.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zenkl G, et al. Sugar-responsive fluorescent nanospheres. Macromol Biosci. 2008;8:146–152. doi: 10.1002/mabi.200700174. [DOI] [PubMed] [Google Scholar]
- 66.Zenkl G, Klimant I. Fluorescent acrylamide nanoparticles for boronic acid based sugar sensing - from probes to sensors. Microchim Acta. 2009;166:123–131. [Google Scholar]
- 67.Billingsley K, et al. Fluorescent Nano-Optodes for Glucose Detection. Anal Chem. 2010;82:3707–3713. doi: 10.1021/ac100042e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saxl T, et al. Fluorescence lifetime spectroscopy and imaging of nano-engineered glucose sensor microcapsules based on glucose/galactose-binding protein. Biosens Bioelectron. 2009;24:3229–3234. doi: 10.1016/j.bios.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Chinnayelka S, et al. Microcapsule biosensors using competitive binding resonance energy transfer assays based on apoenzymes. Anal Chem. 2005;77:5501–5511. doi: 10.1021/ac050755u. [DOI] [PubMed] [Google Scholar]
- 70.Chinnayelka S, et al. Near-Infrared Resonance Energy Transfer Glucose Biosensors in Hybrid Microcapsule Carriers. J Sensors. 2008 doi: 10.1155/2008/346016. [DOI] [Google Scholar]
- 71.Chaudhary A, et al. Evaluation of Glucose Sensitive Affinity Binding Assay Entrapped in Fluorescent Dissolved-Core Alginate Microspheres. Biotechnol Bioeng. 2009;104:1075–1085. doi: 10.1002/bit.22500. [DOI] [PubMed] [Google Scholar]
- 72.Scodeller P, et al. Wired-enzyme core-shell Au nanoparticle biosensor. J Am Chem Soc. 2008;130:12690–12697. doi: 10.1021/ja802318f. [DOI] [PubMed] [Google Scholar]
- 73.Barone PW, et al. Modulation of single-walled carbon nanotube photoluminescence by hydrogel swelling. ACS Nano. 2009;3:3869–3877. doi: 10.1021/nn901025x. [DOI] [PubMed] [Google Scholar]
- 74.Barone PW, Strano MS. Reversible control of carbon nanotube aggregation for a glucose affinity sensor. Angew Chem Int Ed. 2006;45:8138–8141. doi: 10.1002/anie.200603138. [DOI] [PubMed] [Google Scholar]
- 75.Barone PW, Strano MS. Single walled carbon nanotubes as reporters for the optical detection of glucose. J Diabetes Sci Technol. 2009;3:242–252. doi: 10.1177/193229680900300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao LH, et al. A New Route to the Considerable Enhancement of Glucose Oxidase (GOx) Activity: The Simple Assembly of a Complex from CdTe Quantum Dots and GOx, and Its Glucose Sensing. Chemistry - A European Journal. 2008;14:9633–9640. doi: 10.1002/chem.200800681. [DOI] [PubMed] [Google Scholar]
- 77.Li XY, et al. Glucose Biosensor Based on Nanocomposite Films of CdTe Quantum Dots and Glucose Oxidase. Langmuir. 2009;25:6580–6586. doi: 10.1021/la900066z. [DOI] [PubMed] [Google Scholar]
- 78.Wu P, et al. Conjugation of Glucose Oxidase onto Mn-Doped ZnS Quantum Dots for Phosphorescent Sensing of Glucose in Biological Fluids. Anal Chem. 2010;82:1427–1433. doi: 10.1021/ac902531g. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, et al. Glucose biosensor based on the room-temperature phosphorescence of TiO2/SiO2 nanocomposite. Biosens Bioelectron. 2009;24:3706–3710. doi: 10.1016/j.bios.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 80.Tang B, et al. A new nanobiosensor for glucose with high sensitivity and selectivity in serum based on fluorescence resonance energy transfer (FRET) between CdTe quantum dots and Au nanoparticles. Chemistry - A European Journal. 2008;14:3637–3644. doi: 10.1002/chem.200701871. [DOI] [PubMed] [Google Scholar]
- 81.Freeman R, et al. Chem Commun. Cambridge, U. K.: 2009. Competitive analysis of saccharides or dopamine by boronic acid-functionalized CdSe-ZnS quantum dots; pp. 764–766. [DOI] [PubMed] [Google Scholar]
- 82.Wang XD, et al. Optical colorimetric sensor strip for direct readout glucose measurement. Biosens Bioelectron. 2009;24:3702–3705. doi: 10.1016/j.bios.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 83.Sun JJ, et al. Photoelectrochemical glucose biosensor incorporating CdS nanoparticles. Particuology. 2009;7:347–352. [Google Scholar]
- 84.Gao F, et al. A novel nonenzymatic fluorescent sensor for glucose based on silica nanoparticles doped with europium coordination compound. Talanta. 2009;80:202–206. doi: 10.1016/j.talanta.2009.06.050. [DOI] [PubMed] [Google Scholar]