Abstract
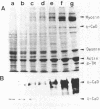
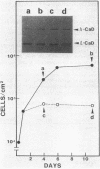
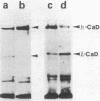
We investigated the expression of two molecular weight forms of caldesmon in a wide range of tissues and cells. The distribution of high molecular weight caldesmon (h-caldesmon, Mr 120,000-150,000) was restricted to smooth muscles where it was found in large quantity. The low molecular weight protein (l-caldesmon, Mr 70,000-80,000) was widely distributed in nonmuscle tissues and cells. Therefore, the expression of h-caldesmon might be much more specific to smooth muscles. We then examined the expressional changes of two caldesmons during phenotypic modulation of smooth muscle cells (SMCs). In developing gizzards, the expression of caldesmons switched from the l- to the h-form. Contrarily, the expression turned from h- to l-caldesmon in association with dedifferentiation of aortic SMCs in primary culture. In agreement with these observations, the levels of those mRNAs that direct the synthesis of both caldesmons were apparently in proportion to the quantities of protein, as determined by use of an in vitro translation system. In addition, h-caldesmon in smooth muscle-like BC3H1 cells increased in its amount with a concomitant reduction of l-caldesmon following serum-depleted and contact-inhibited cytodifferentiation. These results suggest that the expressional changes of two caldesmons are closely correlated with the phenotypic modulation of SMCs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Cobb J. L. Studies on the avian gizzard: the development of the gizzard and its innervation. Z Zellforsch Mikrosk Anat. 1969;98(4):599–621. doi: 10.1007/BF00347035. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Norman K. M. Identification of a secretory granule-binding protein as caldesmon. Nature. 1986 Jan 2;319(6048):68–70. doi: 10.1038/319068a0. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., McConnell J. D., Gröschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res. 1977 Feb 14;177(4):503–522. doi: 10.1007/BF00220611. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark T., Ngai P. K., Sutherland C., Gröschel-Stewart U., Walsh M. P. Vascular smooth muscle caldesmon. J Biol Chem. 1986 Jun 15;261(17):8028–8035. [PubMed] [Google Scholar]
- Dingus J., Hwo S., Bryan J. Identification by monoclonal antibodies and characterization of human platelet caldesmon. J Cell Biol. 1986 May;102(5):1748–1757. doi: 10.1083/jcb.102.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Hirabayashi T. Developmental change of protein constituents in chicken gizzards. Dev Biol. 1983 Jun;97(2):483–493. doi: 10.1016/0012-1606(83)90105-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Marston S. B. Ca2+ can control vascular smooth-muscle thin filaments without caldesmon phosphorylation. Biochem J. 1986 Jul 15;237(2):605–607. doi: 10.1042/bj2370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Caldwell K. L., Glaser L. Multiple controls for the synthesis of muscle-specific proteins in BC3H1 cells. J Cell Biol. 1982 Feb;92(2):350–356. doi: 10.1083/jcb.92.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- Nomura M., Sobue K. Caldesmon regulates the three-dimensional contraction (myosin-dependent contraction of the actin binding protein-induced actin gel). Biochem Biophys Res Commun. 1987 Apr 29;144(2):936–943. doi: 10.1016/s0006-291x(87)80054-2. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yoshikawa K., Tanaka T., Sobue K., Maruyama K. The role of tropomyosin in the interactions of F-actin with caldesmon and actin-binding protein (or filamin). Eur J Biochem. 1987 Mar 16;163(3):467–471. doi: 10.1111/j.1432-1033.1987.tb10892.x. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Sebanne R., Lindstrom J. Regulation of surface expression of acetylcholine receptors in response to serum and cell growth in the BC3H1 muscle cell line. J Biol Chem. 1983 Nov 25;258(22):13946–13953. [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Segura M., Flores M., Garcia R., Palmer E. Differential expression of gizzard actin genes during chick embryogenesis. J Biol Chem. 1979 Nov 10;254(21):11119–11125. [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainberg A., Brik H. The appearance of acetylcholine receptors triggered by fusion of myoblasts in vitro. FEBS Lett. 1978 Apr 15;88(2):327–331. doi: 10.1016/0014-5793(78)80204-x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Morimoto K., Kanda K., Maruyama K., Kakiuchi S. Reconstitution of Ca2+-sensitive gelation of actin filaments with filamin, caldesmon and calmodulin. FEBS Lett. 1982 Feb 22;138(2):289–292. doi: 10.1016/0014-5793(82)80463-8. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Takahashi K., Wakabayashi I. Caldesmon150 regulates the tropomyosin-enhanced actin-myosin interaction in gizzard smooth muscle. Biochem Biophys Res Commun. 1985 Oct 30;132(2):645–651. doi: 10.1016/0006-291x(85)91181-7. [DOI] [PubMed] [Google Scholar]
- Sobue K., Tanaka T., Kanda K., Ashino N., Kakiuchi S. Purification and characterization of caldesmon77: a calmodulin-binding protein that interacts with actin filaments from bovine adrenal medulla. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5025–5029. doi: 10.1073/pnas.82.15.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch A. R., Rubenstein P. A. Induction of vascular smooth muscle alpha-isoactin expression in BC3H1 cells. J Biol Chem. 1984 Mar 10;259(5):3152–3159. [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Tarikas H., Schubert D. Regulation of adenylate kinase and creatine kinase activities in myogenic cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2377–2381. doi: 10.1073/pnas.71.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K., Itoh K., Sobue K., Mori T., Shibata N. Purification of caldesmon and myosin light chain (MLC) kinase from arterial smooth muscle: comparisons with gizzard caldesmon and MLC kinase. J Biochem. 1987 Jan;101(1):1–9. doi: 10.1093/oxfordjournals.jbchem.a121879. [DOI] [PubMed] [Google Scholar]