Inflammatory cytokine triggered up-regulation of PD-L1 on RPE may accelerate local resolution of uveitis by inducing regulatory activity in uveitogenic T cells.
Keywords: autoimmunity, inflammation, tissue cells
Abstract
We previously reported that after exposure to inflammatory cytokines, such as IL-17 and IFN-γ, RPE cells express increased amounts of suppressor of cytokine signaling, leading to general suppression of the inflammatory response. Here, we demonstrate that RPE cells expressed increased levels of PD-L1 in response to IL-17, IFN-γ, or Poly I:C. These PD-L1hi RPE cells inhibited the pathogenic activities of IRBP-specific T cells, which usually induced uveitis when injected into naïve mice (EAU). The suppressed pathogenicity of these uveitogenic T cells after exposure to PD-L1hi RPE cells could be partially reversed by anti-PD-L1 antibodies. Nevertheless, IRBP-specific T cells pre-exposed to PD-L1hi RPE cells displayed substantial suppressor activity, which strongly inhibited the activation of fresh IRBP-Teffs in response to subsequent antigenic challenge and when transferred into naïve mice, inhibited the induction of EAU by IRBP-Teff transfer. These findings suggest that inflammatory cytokine-triggered up-regulation of PD-L1 on RPE constitutes a critical factor for inducing infiltrated uveitogenic T cells with regulatory activities, which may accelerate the natural resolution of T cell-mediated intraocular inflammation.
Introduction
Uveitis is a common inflammatory disease that affects the uvea and retina. Although its etiology is not fully understood, uveitogenic-specific Th1 cells and recently identified Th17 cells are sought to play a role in its development and progression [1–4]. However, to prevent the tissue damage and vision loss caused by this T cell-mediated inflammation, the eye as an immune-privileged organ uses an extensive array of mechanisms, by which the inflammation can be limited and the integrity of the vision function maintained. Among them, RPE cells are one of the cellular components that actively participate in immune responses in the eye [5–8]. The RPE is situated at the interface between the choroidal blood supply and the photoreceptor cell layer of the neural retina. It creates and maintains immune tolerance by the formation of the blood-eye barrier [9], the secretion of immunosuppressive factors [10, 11], expressing Fas ligand on the cell surface [12], and phagocytosis [13].
PD-1 and its ligands, PD-L1 and PD-L2, are among the most recently characterized members of the B7 family of costimulatory molecules [14]. PD-L1 and PD-L2 have overlapping functions and can act synergistically to inhibit T cell activation, proliferation, and cytokine production, and PD-L1 plays a predominant role in vivo [14, 15]. Whereas PD-L2 expression is restricted to cells of hematopoietic origin, including activated DCs and macrophages, PD-L1 is more widely expressed on hematopoietic cells, including resting and activated T cells, B cells, DCs, macrophages, and BMMCs and in nonhematopoietic organs, including the vascular endothelium, epithelium, muscle, liver, heart, pancreas, placenta, skin, and eye [16–20]. Studies using animal models of autoimmune disease have demonstrated that the PD-1 and PD-L1 interaction plays an important role in maintaining peripheral tolerance, which protects against the development of autoimmune disease, and that blockade of this interaction increases the incidence of autoimmune diseases, such as diabetes [21]; exacerbates autoimmune kidney disease [22], myocarditis [19], experimental autoimmune encephalomyelitis [23], and autoimmune hepatitis [24]; impairs fetomaternal tolerance [25]; and prevents allograft survival [26–29]. It has been reported recently that in vitro, IFN-γ-exposed RPE cells express PD-L1, which suppresses IFN-γ production by anti-CD3 mAb-activated T cells [30, 31].
In this study, we showed that RPE cells constitutively express PD-L1. Upon exposure to inflammatory cytokines, such as IFN-γ, IL-17, or the TLR3 ligand Poly I:C, they expressed increased levels of PD-L1. After exposure to PD-L1hi RPE cells, IRBP-specific T cells lost their uveitogenic activity but acquired immunosuppressive activity. Thus, the PD-1:PD-L1 interaction in the eye may play a critical role in the control of ocular inflammation and regulate the pathogenic activity of the invading autoreactive T cells.
MATERIALS AND METHODS
Animals, reagents, and cell culture
Pathogen-free female B6 mice (6–10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and were housed and maintained in the animal facilities of the University of Louisville (Kentucky, USA). All animal studies conformed to the Association for Research in Vision and Ophthalmology statement about the use of animals in ophthalmic and vision research. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed.
Murine rIL-17 and rIFN-γ were purchased from R&D Systems (Minneapolis, MN, USA). Poly I:C was obtained from Invivogen (San Diego, CA, USA; Cat. #TLRL-kit 1m). Anti mouse PD-L1 mAb was purchased from eBioscience (San Diego, CA, USA) and used in culture as a concentration of 20 μg/ml.
Isolation and culture of primary RPE cells and incubation with T cells
The method for the isolation of RPE cells has been described previously [8, 32]. The purity of the RPE cells was >95%, as assessed by staining with anti-pan keratin antibody (clone PCK-26; Sigma-Aldrich, St. Louis, MO, USA) and anti-RPE65 antibody (Novus, Littleton, CO, USA). RPE cells were used in experiments after three to five passages.
In studies involving the interaction between RPE cells and antigen-specific T cells, RPE cells were plated at 1 × 106 cells/well in a six-well plate in a 1-ml cell culture medium [8]. After overnight adherence, 100 ng/ml IL-17 was added for 24 h; then, the medium was discarded, the cells washed once with medium, and 1 × 107 T cells added to the well (RPE:T cell ratio of 1:10) for 24 h; and then, the T cells were recovered and used in experiments.
Flow cytometry analysis
Adherent RPE cells were removed by trypsinization, washed, and single-stained with fluorescein (FITC)-conjugated anti-PD-L1 mAb (eBioscience). T cells were triple-stained with combinations of FITC-, PE-, and allophycocyanin-conjugated mAb against mouse CD4/CD25/CTLA4 or CD4/CD25/Foxp3 (eBioscience). Data collection and analysis were performed using a flow cytometer (FACSCalibur, BD PharMingen, San Diego, CA, USA) and appropriate software (CellQuest, BD PharMingen).
Measurement of cytokines using an ELISA
Levels of IFN-γ, IL-17, and IL-10 were measured using commercially available ELISA kits (R&D Systems).
Actively induced and adoptively transferred experimental uveitis in B6 mice
For active induction of disease, the animals were immunized s.c. with 100 μl emulsion containing human IRBP1–20 (150 μg) and 500 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI, USA) in IFA (Sigma-Aldrich), distributed over six spots on the tail base and flank. Concurrently, 0.2 μg PTX was injected i.p.
For adoptive transfer, recipient animals were injected i.p. with 0.2 ml PBS containing 5 × 106 IRBP1–20-specific T cells, prepared as described previously [33]. The clinical course of the disease was assessed by indirect fundoscopy twice a week and graded as described previously [33]. The pathology of the disease was confirmed by histology [33].
Preparation of IRBP-specific T cells
IRBP1–20-specific T cells were prepared from IRBP1–20-immunized mice as described previously [34]. Briefly, T cells were isolated from the lymph node or spleen of B6 mice at 13 days p.i. by passage through a nylon wool column. The cells (1×107) were stimulated with 20 μg/ml IRBP1–20 in 2 ml medium in a six-well plate (Costar, Cambridge, MA, USA) in the presence of 1 × 107 irradiated, syngeneic spleen cells as APCs. After 2 days, the activated lymphoblasts were isolated by gradient centrifugation in Lymphoprep (Robbins Scientific, Mountain View, CA) and cultured in RPMI-1640 medium supplemented with 20 ng/ml IL-2.
Preparation of MOG35–55-specific T cells
B6 mice were immunized with the MOG35–55 peptide (200 μg/mouse), emulsified in IFA containing 0.6 mg M. tuberculosis (H37Ra; Difco). One day p.i., the animals received a single i.p. dose of 400 ng PTX (Sigma-Aldrich) [35], and then, 15 days later, T cells were isolated from the draining lymph nodes and spleens of immunized animals and enriched by passage through nylon wool and restimulated in vitro with immunizing peptide (20 μg/ml), presented by irradiated, syngeneic spleen cells. After 3 days, activated T cell blasts were separated on a Ficoll gradient.
Proliferation assay
T cells prepared from IRBP1–20-immunized B6 mice, with or without 24 h preincubation with RPE cells, were seeded at 4 × 105 cells/well in 96-well plates and then cultured at 37°C for 48 h in a total volume of 200 μl medium, with or without immunizing peptide in the presence of irradiated, syngeneic spleen APCs (1×105), and [3H]thymidine incorporation during the last 8 h was assessed using a microplate scintillation counter (Packard Instrument, Meriden, CT, USA). The proliferative response was expressed as the mean cpm ± sd of triplicate determinations.
Suppressor assay
Adherent RPE cells were incubated with IRBP1–20-specific T cells as described above, and then, after 24 h, the nonadherent T cells were recovered by gentle pipetting. The retrieved IRBP-T cells (3×105) were then cocultured with indicated ratios of fresh IRBP1–20 or MOG35–55 Teffs derived from peptide-immunized B6 mice on Day 13 p.i. in the presence of immunizing peptides (10 μg/ml) and APCs (irradiated, syngeneic spleen cells) in 96-well plates (Nunc, Thermo Fisher, Rochester, NY, USA) in a final volume of 200 μL medium/well. After 48 h, the proliferation of responder T cells was assessed by the [3H]thymidine incorporation assay, and cytokines released by responder fresh T cells into the culture supernatants were measured by ELISA.
Alternatively, fresh IRBP- or MOG-specific Teffs were stained with 5 μM CFSE prior to coculture. After 48 h, activated Teff blasts were separated and incubated in IL-2-containing medium for an additional 3 days. Suppressor activity was revealed by inhibition of the expansion of CFSE-labeled responder T cells [36].
Statistics
Experiments were repeated at least twice and usually, three or more times. An unpaired Student's t test for two sets of data, one-way ANOVA Dunnet for three or more means at one time, or repeated ANOVA for a clinical score of uveitis was used for statistical analysis. A P value, determined to be significantly different from those for controls, is marked with an asterisk (*P<0.05; **P<0.01) in the figures.
RESULTS
Increased expression of PD-L1 by cultured RPE cells in response to inflammation
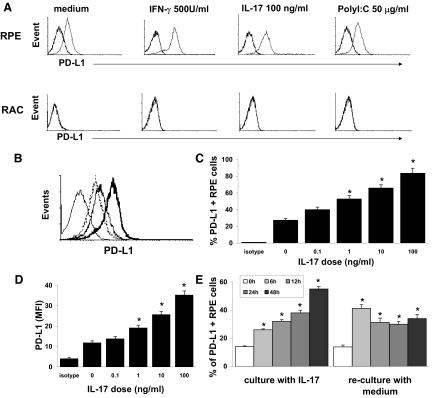
To investigate the pro- or anti-inflammatory activity of RPE cells, we examined PD-L1 expression on RPE cells, with or without prior inflammatory stimulation. As shown in Fig. 1A (upper row), RPE cells constitutively expressed a low level of PD-L1, and after exposure for 24 h to the cytokines IL-17 and IFN-γ or the TLR3 ligand Poly I:C, PD-L1 expression was markedly increased, as detected by antibody staining and FACS analysis. PD-L1 expression was not detectable on RACs before and after exposure to cytokines and Poly I:C (Fig. 1A, lower row). Using increasing doses of IL-17, PD-L1-positive RPE cells (Fig. 1B and C) and PD-L1 expression/cell, as indicated by the MFI (Fig. 1D), were found to be up-regulated in a dose-dependent manner. Thus, the RPE cells treated with 100 ng/ml IL-17 alone will hereafter be referred to as PD-L1hi RPE cells.
Figure 1. PD-L1 expression on RPE cells.
(A) PD-L1 expression is up-regulated on RPE cells but not RACs in response to inflammatory stimuli. RPE and RACs were cultured to confluence and then were incubated for 24 h with medium alone, 500 U/ml IFN-γ, 100 ng/ml IL-17, or 50 μg/ml Poly I:C, and PD-L1 expression was measured by flow cytometry (thick line: isotype control; thin line: anti-PD-L1-FITC). (B–D) The increase in PD-L1 expression on RPE cells caused by IL-17 is dose-dependent. (B) RPE cells were cultured for 24 h in medium (dotted line) or medium containing 1 ng/ml (dark, solid line) or 100 ng/ml (bold, solid line) of IL-17, and then PD-L1 expression was examined. The light, solid line is a cell stained with the isotype control antibody. (C) Analysis of PD-L1 expression after culture of RPE with increasing doses of IL-17. (D) Analysis of PD-L1 expression on a per-cell basis, quantified via MFI. (E) Kinetic analysis of PD-L1 expression by RPE cells in response to IL-17. RPE cells were cultured with 100 ng/ml IL-17, as in A, for 6–48 h, and then PD-L1 expression on RPE cells was measured by flow cytometry (left panel). After 24 h culture with IL-17, cells were washed and recultured in fresh culture medium without IL-17 for 6–48 h, and then PD-L1 expression on RPE cells was examined (right panel). The white columns are untreated cells. The results of one experiment with triplicate cultures are shown and are representative of the results for three separate. Error bars are the sd. *P < 0.05 versus medium without IL-17.
Kinetics studies showed that increased PD-L1 expression on RPE cells was seen as early as at 6 h of exposure to IL-17 and increased with time of incubation (Fig. 1E, left panel). After removal of IL-17, PD-L1 expression remained high for at least 48 h (Fig. 1E, right panel).
Exposure to PD-L1hi RPE cells significantly inhibits the uveitogenic activity of IRBP-specific T cells
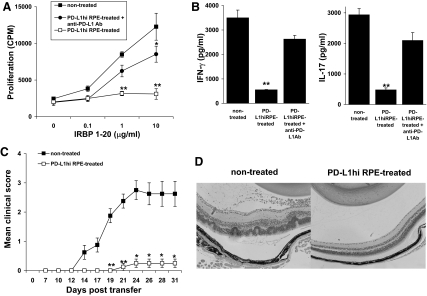
To determine whether PD-L1hi RPE cells had an inhibitory effect on IRBP-specific T cells, we first cocultured IRBP-specific T cells derived from IRBP-immunized mice for 24 h with monolayers of PD-L1hi RPE cells generated by incubation of primary RPE cells with 100 ng/ml IL-17 for 24 h. The T cells were then recovered and stimulated in vitro with irradiated, syngeneic spleen APCs and an optimal dose of IRBP1–20, and then T cell activation was assessed by proliferation and cytokine production and the pathogenic activity of the activated T cells assessed by adoptive transfer of these stimulated T cells into naïve mice. As shown in Fig. 2, preincubation with PD-L1hi RPE cells almost completely suppressed the ability of T cells to respond to subsequent antigenic challenge by proliferation (Fig. 2A) or IFN-γ and IL-17 production (Fig. 2B) and suppressed their disease-inducing ability (Fig. 2C and D), as compared with IRBP-specific T cells not incubated with PD-L1hi RPE cells. The inhibited IRBP-T cell production and cytokine production were mostly reversed when anti-PD-L1 mAb was added to the primary RPE cell cultures at the same time as the IL-17 used to generate the PD-L1hi RPE cells (Fig. 2A and B).
Figure 2. IRBP-specific T cells lose pathogenic activity after incubation with PD-L1hi RPE cells.
(A) IRBP-specific T cells (5×106) were left untreated or were preincubated with monolayers of PD-L1hi RPE cells (ratio of 10:1), with or without anti-PD-L1 mAb (20 μg/ml) for 24 h, and then, the recovered T cells were cocultured with the indicated concentration of IRBP1–20 peptide and irradiated, syngeneic, splenic APCs and their proliferation measured after 48 h. (B) Cells were treated as in A but using 10 μg/ml IRBP1–20 for stimulation, and then (B), the IFN-γ or IL-17 released by the IRBP-T cells was measured and (C) their uveitis-inducing ability after adoptive transfer (5×106/mouse; n=6 of two experiments) evaluated. (D) Pathological examination of the eye in the two groups performed on Day 31. **P < 0.01 and *P < 0.05 versus nontreated.
IRBP-specific T cells acquire a regulatory activity after exposure to PD-L1hi RPE cells
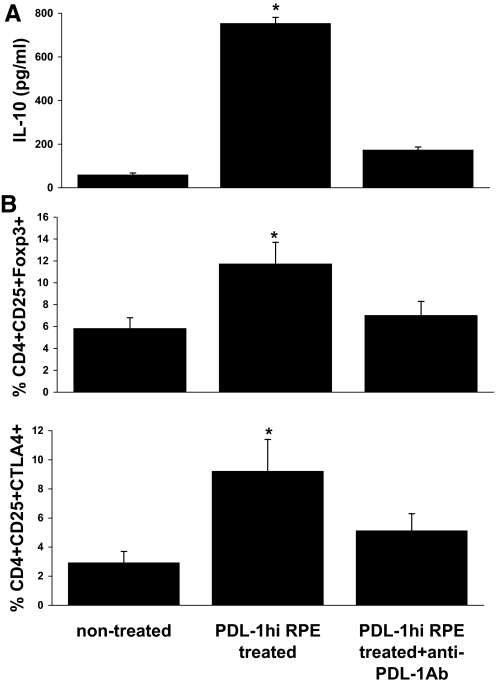
Unexpectedly, cytokine production studies revealed that in response to subsequent antigenic challenge, IRBP-specific T cells preincubated with PD-L1hi-RPE cells produced high levels of IL-10 and that this effect was blocked by addition of anti-PD-L1 antibody to the primary RPE cell cultures at the same time as the IL-17 used to generate the PD-L1hi RPE cells (Fig. 3A). Further examination of the phenotype of these PD-L1hi RPE-exposed, IRBP-specific T cells showed that they expressed increased levels of Foxp3 and CTLA4 (Fig. 3B), as compared with the same responder T cells preincubated with PD-L1-blocked RPE cells.
Figure 3. IRBP-specific T cells preincubated with PD-L1hi RPE acquire the Treg phenotype.
Cells were treated as in Fig. 2B, and then, the IL-10 produced by the IRBP-T cells was measured by ELISA (A), and CD4/CD25/Foxp3- or CTLA4-positive cells were detected by three-color antibody staining, followed by flow cytometry analysis (B). *P < 0.05 versus nontreated.
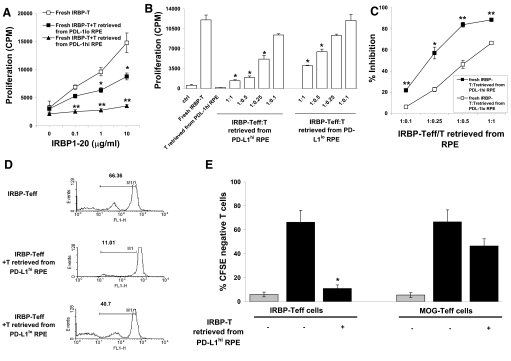
Next, we assessed the suppressive activity of IRBP-T cells preincubated with RPE cells. After 24 h coculture with monolayers of PD-L1hi or PD-L1lo RPE cells (RPE cells cultured with medium without IL-17, see Fig. 1; or with IL-17 and anti-PD-L1 mAb), the IRBP-T cells were recovered and added to freshly prepared IRBP-Teffs at a ratio of 1:1, and then, the mixture was stimulated by the immunizing antigen (IRBP1–20) in the presence of irradiated APCs; it should be noted that any proliferation is a result of the fresh, untreated cells and not the cells incubated with the PD-L1hi RPE cells, as Fig. 2 shows that such cells do not respond to antigen. IRBP-T cells pre-exposed to PD-L1hi RPE cells and to PD-L1lo RPE suppressed the proliferation (Fig. 4A) However, the extent of the suppression on proliferation was significantly potent by IRBP-T cells incubated with PD-L1hi RPE cells.
Figure 4. IRBP-T cells retrieved from the preincubation with PD-L1hi RPE potently inhibit IRBP-Teff proliferation.
IRBP-specific T cells (5×106) were preincubated with PD-L1hi or PD-L1lo RPE cells for 24 h, retrieved (named IRBP-T), and added to fresh IRBP-specific T cells (IRBP-Teff) at a ratio of 1:1 in the presence of indicated doses of IRBP1–20 and irradiated APCs (A) or at a different ratio in the presence of 10 μg/ml IRBP1–20 (B). After 48 h, the proliferation of the IRBP-Teffs in response to antigen stimulation was evaluated. (C) Percentage inhibition of the proliferation of fresh IRBP-Teffs by retrieved IRBP-T cells from the incubation with PD-L1hi or PD-L1lo RPE cells calculated from B. (D) IRBP-T cells retrieved from PD-L1hi or PD-L1lo RPE cell monolayers were tested for suppressor activity as in A. Prior to the assay, fresh T cells were stained with CFSE. The numbers indicate the percentage of CFSE-negative cells analyzed by flow cytometry. Results of one experiment, representative of three experiments, are shown. FL1-H, Fluorescence 1-height. (E) Fresh IRBP-Teffs or MOG-Teffs, prestained with CFSE, were incubated with IRBP1–20 or MOG35–55, as appropriate, and irradiated APCs in the presence (+) or absence (–) of IRBP-T cells retrieved from PD-Llhi RPE cells and then CFSE-negative cells analyzed by flow cytometry. The columns indicate the mean percentage of CFSE-negative cells after 5 days of culture. The error bars indicate the sd for the experimental results for three consecutively tested donor/responder pairs. The light-gray columns show the percent of CFSE-negative, fresh T cells without IRBP or MOG antigen stimulation Results shown are representative of those from three independent experiments. (A, B, E) **P < 0.01 and *P < 0.05 versus fresh IRBP-Teff; (C) **P < 0.01 and *P < 0.05 versus fresh IRBP-T: T from PD-L1lo RPE.
To further test whether the presence of PD-L1 on RPE could influence the efficiency of suppression by retrieved IRBP-T cells, IRBP-T cells pre-exposed to PD-L1hi or PD-L1lo RPE were recovered and cultured with fresh IRBP-Teffs in the presence of IRBP1–20 and irradiated APCs at a variety of fresh IRBP-Teff/retrieved IRBP-T ratios, as graphically depicted in Fig. 4B and C. PD-L1hi RPE enhanced pre-exposed IRBP-T cell-suppressive function at a low, fresh IRBP-Teff/retrieved IRBP-T ratio.
To evaluate whether PD-L1hi or PD-L1lo RPE-exposed IRBP-T cells affect the suppression of fresh IRBP-Teff proliferation on a per-cell basis, we performed additional suppression assays measuring CFSE dilution of fresh IRBP-Teffs by flow cytometry, in which the responder IRBP-Teffs were prelabeled with CFSE prior to coculture with retrieved IRBP-T cells, which from pre-exposed to PD-L1hi RPE, reduced fresh IRBP-Teff expansion at the single-cell level to a greater extent than IRBP-T cells retrieved from culture with PD-L1lo RPE (Fig. 4D).
To determine whether the regulatory activity induced by PD-L1hi RPE cells was antigen-specific, we examined the suppressive activity of IRBP-T cells preincubated with PD-L1hi RPE cells on fresh IRBP-Teff and MOG-specific responder T cells. Again, the proliferation of the responder T cells was assessed by the CFSE assay, in which the responder Teffs were prelabeled with CFSE. As shown in Fig. 4E, IRBP-T cells preincubated with PD-L1hi RPE cells had a much stronger inhibitory effect on the proliferative response of IRBP-Teffs to the IRBP peptide than on that of MOG-Teffs to the MOG peptide.
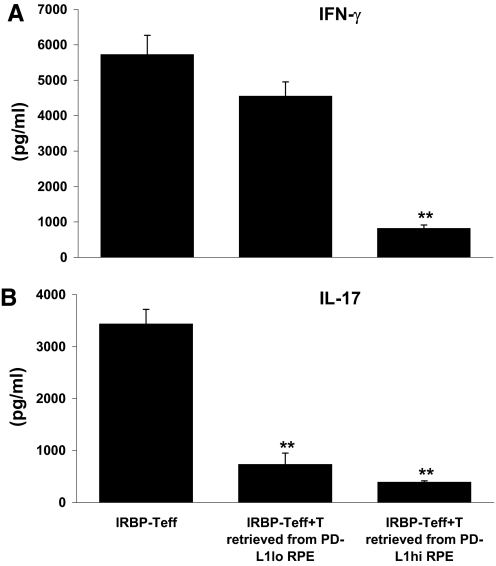
In addition, IRBP-T cells pre-exposed to PD-L1hi RPE cells suppressed IFN-γ- and IL-17-producing responses of the responder T cells, whereas IRBP-T cells pre-exposed to PD-L1lo RPE cells only inhibited IL-17-producing T cells (Fig. 5).
Figure 5. IRBP-T cells retrieved from the preincubation with PD-L1hi RPE cells reduce IFN-γ produced by IRBP-Teffs.
IRBP-specific T cells (5×106) were preincubated with PD-L1hi or PD-L1lo RPE cells for 24 h, retrieved (named IRBP-T), and added to fresh, IRBP-specific T cells (IRBP-Teff) at a ratio of 1:1 in the presence of 10 μg/ml IRBP1–20 and irradiated APCs. After 48 h, the production of Th1 and Th17 cytokines (A and B) by IRBP-Teffs, in response to antigen stimulation, was evaluated by ELISA. **P < 0.01 versus IRBP-Teff.
IRBP-T cells preincubated with PD-L1hi RPE cells suppress the induction of uveitis by fresh IRBP-Teffs
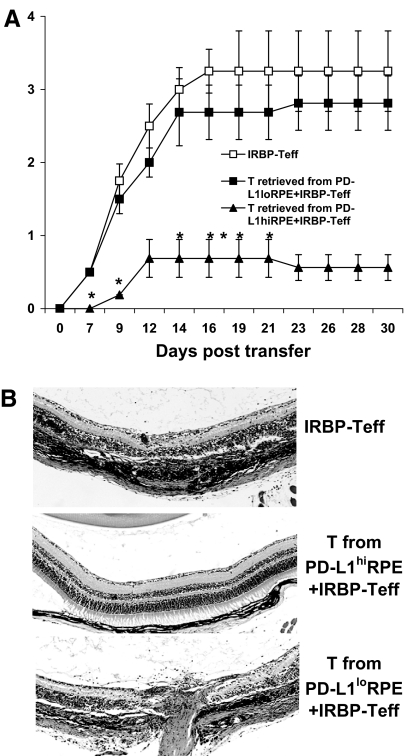
To further examine the in vivo inhibitory activity of IRBP-T cells preincubated with RPE cells, we injected naïve B6 mice with 5 × 106 fresh IRBP-Teffs, alone or together with 5 × 106 IRBP-T cells pre-exposed to PD-L1hi or PD-L1lo RPE cells. As shown in Fig. 6A and B, EAU was delayed and mild in the mice that received IRBP-T cells preincubated with PD-L1hi RPE but not those preincubated with PD-L1lo RPE cells.
Figure 6. IRBP-T cells retrieved from the preincubation with PD-L1hi RPE cells suppress EAU induced by IRBP-Teffs.
Three groups of naïve mice (n=6; two experiments) were adoptively transferred with blast IRBP-Teffs (5×106/mouse) after in vitro stimulation with IRBP1–20 and irradiated APCs for 2 days. Group 1 mice received only IRBP-Teffs (positive control). Groups 2 and 3 were injected with IRBP-T cells after incubation with PD-L1hi or PD-L1lo RPE cells, respectively (experimental groups). Disease was monitored and scored clinically (A) two times/week and histologically (B) at Day 30. *P < 0.05 versus IRBP-Teff.
DISCUSSION
The suppression of “unwanted” immune responses plays an important role in protecting the functional integrity of an organ from autoimmune attack. The parenchymal cells in the eye, including the retinal glial Müller cells [37], cells from rat ciliary bodies [38], and astrocytes [39], have been reported to have an immune regulatory effect. We [6, 8] and others [5, 7, 30, 40–43] have previously shown that RPE cells can promote or inhibit T cell activation. The effector T cells can be inhibited directly by RPE cells or indirectly by Tregs induced by RPE. The ability of RPE to induce or activate Tregs has been appreciated in mice [44, 45] and recently in humans [40]. However, the mechanisms by which these cells participate in the immune response remain largely unclear. A recent study has shown that PD-L1 expression by RPE cells is related to their direct suppressor activity on naïve T cell activation [31, 46].
In this study, we further explored the critical role of PD-L1 expressed by RPE in the maintenance of tissue tolerance and the protection of tissues from detrimental immune attack initiated by uveitogenic IRBP-specific T cells. We showed that RPE cells constitutively expressed a low level of PD-L1 and that they expressed more PD-L1 in response to the cytokines IFN-γ and IL-17. In addition, the microbial product Poly I:C, a TLR3 ligand, induced increased PD-L1 expression (Fig. 1). The expression of PD-L1 has been reported on the cells, such as RPE activated by IFN-γ [31], macrophages activated by Th1, or a combination of LPS and IFN-γ [20, 22], and myeloid-derived APCs from inflamed CNS [47]. Our study in Fig. 1 showed that exposure to IL-17 and Poly I:C, as well as IFN-γ, can up-regulate PD-L1 on RPE, indicating that multiple inflammatory molecules are able to enhance the expression of PD-L1. This response was not seen with RACs, one of few ocular tissue cells that actively participate in immune regulation during inflammation [6, 32, 48]. The IL-17-triggered up-regulation of PD-L1 on RPE became prevalent and ongoing even after the removal of the IL-17 stimulator. Importantly, compared with PD-L1lo RPE, PD-L1hi RPE cells gained increased inhibitory activity and suppressed the proliferation and cytokine production of IRBP-specific Th1 and Th17 cells, leading to the reduced ability of the IRBP-specific T cells to induce EAU (Fig. 2). The observed inhibition was not contributed by apoptosis induced by PD-L1, as we checked the cell viability of IRBP-T cells cocultured with PD-L1hi RPE prior to the experiments.
Moreover, after incubation with PD-L1hi RPE cells, responder IRBP-specific T cells gained increased regulatory activity while losing pathogenic activity. This was demonstrated by the responder T cells producing high levels of IL-10 (Fig. 3), expressing increased levels of CTLA4 and Foxp3 (Fig. 3), and most importantly, showing a potent, inhibitory effect in vitro and in vivo (Figs. 4–6).
We observed that PD-L1lo RPE cells can endow IRBP-Teffs with regulatory activity. Specifically, IL-17 production by IRPB-Teffs is inhibited, although INF-γ is not. There are at least two possible explanations for this differential effect on cytokine production. First, IL-17-producing Teffs are more susceptible to regulatory control. The second is that other cell surface molecules or soluble factors expressed by RPE cells can induce T regulatory functions. It has been shown previously by others that RPE cells secrete CTLA-2α, enabling bystander T cells to be converted into Tregs via TGF-β promotion [44, 45]. However, PD-L1hi RPE-induced regulatory activities of IRBP-T cells are far more effective than those induced by PD-L1lo RPE at limiting the proliferative capacity and Th1 cytokine production of IRBP-Teffs, consistent with the reports that PD-L1 enhances the efficiency of suppression by Tregs [20, 49]. Moreover, PD-L1hi RPE-induced regulatory activity of IRBP-T cells, when transferred in vivo, potently suppresses EAU induced by active antigen immunization or passive antigen-specific Teff transfer. Collectively, these findings support the notion that RPE cells (i.e., PD-L1lo RPE) have a constitutive inhibitory activity. However, PD-L1hi RPE cells can result in the secretion of IL-10 or the expression of CTLA-4 on IRBP-specific T cells that contribute to the regulatory effect of the RPE.
As IL-10 is one of the major inhibitory cytokines, we focused our study on IL-10. Furthermore, anti-PD-L1 antibody blocked the increase in IL-10 produced by IRBP-T cells. However, other inhibitory factors of Tregs may very well be involved in the intraocular regulation of Teffs.
It is interesting to note that IRBP-T cells incubated with PD-L1hi RPE cells showed a strong, antigen-specific, inhibitory effect, potently suppressing IRBP-Teffs but having only a marginal, suppressive effect on MOG-Teffs.
Previous studies have shown that many cytokines including IFN-γ and IL-17 are a two-edged sword, in that they can be proinflammatory or anti-inflammatory depending on which parenchymal cells are stimulated [50–54]. Parenchymal cells in the eye respond differently to stimulation with IL-17, IFN-γ, and Poly I:C [6, 32, 48]. Our study showed that these agents increased PD-L1 expression on RPE cells and enhanced their suppressive effect on pathogenic T cells via a PD-L1/PD-1 interaction, resulting in a reduction in T cell-mediated inflammation. In contrast, they had no effect on PD-L1 expression on RACs and instead, increased their proinflammatory cytokine production and antigen-presenting ability [6, 48], resulting in promotion of T cell-mediated inflammation. Thus, the severity of ocular inflammation might be balanced by the net effect of these responses in vivo.
In conclusion, PD-L1 expression by RPE cells on exposure to IFN-γ or IL-17 might constitute an important pathway by which RPE cells might promote local anti-inflammatory responses and accelerate resolution of uveitis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants EY12974 and EY14599, Vision Research Infrastructure Development grant R24 EY015636, a Research to Prevent Blindness career development award (H.S.), and the Commonwealth of Kentucky Research Challenge Trust Fund (H.J.K.). The editorial assistance of Dr. Tom Barkas is greatly appreciated.
Footnotes
- B6 mice
- C56BL/6 mice
- EAU
- experimental autoimmune uveitis
- Foxp3
- forkhead box p3
- IRBP
- interphotoreceptor retinoid-binding protein
- MFI
- mean fluorescence intensity
- MOG
- myelin oligodendrocyte glycoprotein
- PD-L1hi/lo
- programmed cell death ligand 1 high-/low-expressing
- p.i.
- postimmunization
- Poly I:C
- polyinosinic:polycytidylic
- RAC
- retinal astrocyte
- Teff
- T effector cell
- Treg
- regulatory T cell
AUTHORSHIP
Y.K. designed, performed, and analyzed the majority of the experiments of this work; G.J. contributed to some of the experiments; H.S. directed the study, planned experiments, and wrote the manuscript; and D.S. and H.J.K. helped with designing the experiments and writing the manuscript.
REFERENCES
- 1.Luger D., Silver P. B., Tang J., Cua D., Chen Z., Iwakura Y., Bowman E. P., Sgambellone N. M., Chan C. C., Caspi R. R. (2008) Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng Y., Han G., Shao H., Wang Y., Kaplan H. J., Sun D. (2007) Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 48, 4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amadi-Obi A., Yu C. R., Liu X., Mahdi R. M., Clarke G. L., Nussenblatt R. B., Gery I., Lee Y. S., Egwuagu C. E. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 13, 711–718 [DOI] [PubMed] [Google Scholar]
- 4.Cox C. A., Shi G., Yin H., Vistica B. P., Wawrousek E. F., Chan C. C., Gery I. (2008) Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J. Immunol. 180, 7414–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida K., Panjwani N., Cao Z., Streilein J. W. (2003) Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul. Immunol. Inflamm. 11, 91–105 [DOI] [PubMed] [Google Scholar]
- 6.Ke Y., Jiang G., Sun D., Kaplan H. J., Shao H. (2009) Retinal astrocytes respond to IL-17 differently than retinal pigment epithelial cells. J. Leukoc. Biol. 86, 1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugita S., Futagami Y., Smith S. B., Naggar H., Mochizuki M. (2006) Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor β. Exp. Eye Res. 83, 1459–1471 [DOI] [PubMed] [Google Scholar]
- 8.Sun D., Enzmann V., Lei S., Sun S. L., Kaplan H. J., Shao H. (2003) Retinal pigment epithelial cells activate uveitogenic T cells when they express high levels of MHC class II molecules, but inhibit T cell activation when they express restricted levels. J. Neuroimmunol. 144, 1–8 [DOI] [PubMed] [Google Scholar]
- 9.Shakib M., Zinn K. M. (1973) Fine structure and function of ocular tissues. The choroid, Bruch′s membrane, and the retinal pigment epithelium. Int. Ophthalmol. Clin. 13, 189–204 [DOI] [PubMed] [Google Scholar]
- 10.Futagami Y., Sugita S., Vega J., Ishida K., Takase H., Maruyama K., Aburatani H., Mochizuki M. (2007) Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J. Immunol. 178, 6994–7005 [DOI] [PubMed] [Google Scholar]
- 11.Sugita S. (2009) Role of ocular pigment epithelial cells in immune privilege. Arch. Immunol. Ther. Exp. (Warsz) 57, 263–268 [DOI] [PubMed] [Google Scholar]
- 12.Griffith T. S., Yu X., Herndon J. M., Green D. R., Ferguson T. A. (1996) CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 5, 7–16 [DOI] [PubMed] [Google Scholar]
- 13.Willermain F., Caspers-Velu L., Nowak B., Stordeur P., Mosselmans R., Salmon I., Velu T., Bruyns C. (2002) Retinal pigment epithelial cells phagocytosis of T lymphocytes: possible implication in the immune privilege of the eye. Br. J. Ophthalmol. 86, 1417–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H. (2008) PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki T., Honjo T. (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19, 813–824 [DOI] [PubMed] [Google Scholar]
- 16.Bolinger B., Engeler D., Krebs P., Miller S., Firner S., Hoffmann M., Palmer D. C., Restifo N. P., Tian Y., Clavien P. A., Ludewig B. (2010) IFN-γ-receptor signaling ameliorates transplant vasculopathy through attenuation of CD8+ T-cell-mediated injury of vascular endothelial cells. Eur. J. Immunol. 40, 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiegs G., Lohse A. W. (2010) Immune tolerance: what is unique about the liver. J. Autoimmun. 34, 1–6 [DOI] [PubMed] [Google Scholar]
- 18.Pinchuk I. V., Saada J. I., Beswick E. J., Boya G., Qiu S. M., Mifflin R. C., Raju G. S., Reyes V. E., Powell D. W. (2008) PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 135, 1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas J. A., Menke J., Rabacal W. A., Schoen F. J., Sharpe A. H., Kelley V. R. (2008) Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 181, 2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir M. E., Liang S. C., Guleria I., Latchman Y. E., Qipo A., Albacker L. A., Koulmanda M., Freeman G. J., Sayegh M. H., Sharpe A. H. (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203, 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fife B. T., Guleria I., Gubbels B. M., Eagar T. N., Tang Q., Bour-Jordan H., Yagita H., Azuma M., Sayegh M. H., Bluestone J. A. (2006) Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 203, 2737–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menke J., Lucas J. A., Zeller G. C., Keir M. E., Huang X. R., Tsuboi N., Mayadas T. N., Lan H. Y., Sharpe A. H., Kelley V. R. (2007) Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J. Immunol. 179, 7466–7477 [DOI] [PubMed] [Google Scholar]
- 23.Salama A. D., Chitnis T., Imitola J., Ansari M. J., Akiba H., Tushima F., Azuma M., Yagita H., Sayegh M. H., Khoury S. J. (2003) Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mataki N., Kikuchi K., Kawai T., Higashiyama M., Okada Y., Kurihara C., Hokari R., Kawaguchi A., Nagao S., Kondo T., Itoh K., Miyakawa H., Miura S. (2007) Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am. J. Gastroenterol. 102, 302–312 [DOI] [PubMed] [Google Scholar]
- 25.Taglauer E. S., Yankee T. M., Petroff M. G. (2009) Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 80, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori J., Wang M., Miyashita M., Tanemoto K., Takahashi H., Takemori T., Okumura K., Yagita H., Azuma M. (2006) B7–H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 177, 5928–5935 [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Han R., Hancock W. W. (2007) Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur. J. Immunol. 37, 2983–2990 [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Popoola J., Khandwala S., Vadivel N., Vanguri V., Yuan X., Dada S., Guleria I., Tian C., Ansari M. J., Shin T., Yagita H., Azuma M., Sayegh M. H., Chandraker A. (2008) Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation 117, 660–669 [DOI] [PubMed] [Google Scholar]
- 29.Morita M., Fujino M., Jiang G., Kitazawa Y., Xie L., Azuma M., Yagita H., Nagao S., Sugioka A., Kurosawa Y., Takahara S., Fung J., Qian S., Lu L., Li X. K. (2010) PD-1/B7–H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am. J. Transplant. 10, 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattori T., Kezuka T., Usui Y., Okunuki Y., Takeuchi M., Maruyama K., Haneda M., Shirato S., Goto H. (2009) Human iris pigment epithelial cells suppress T-cell activation via direct cell contact. Exp. Eye Res. 89, 358–364 [DOI] [PubMed] [Google Scholar]
- 31.Sugita S., Usui Y., Horie S., Futagami Y., Aburatani H., Okazaki T., Honjo T., Takeuchi M., Mochizuki M. (2009) T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest. Ophthalmol. Vis. Sci. 50, 2862–2870 [DOI] [PubMed] [Google Scholar]
- 32.Jiang G., Ke Y., Sun D., Han G., Kaplan H. J., Shao H. (2008) Reactivation of uveitogenic T cells by retinal astrocytes derived from experimental autoimmune uveitis-prone B10RIII mice. Invest. Ophthalmol. Vis. Sci. 49, 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao H., Liao T., Ke Y., Shi H., Kaplan H. J., Sun D. (2006) Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp. Eye Res. 82, 323–331 [DOI] [PubMed] [Google Scholar]
- 34.Shao H., Fu Y., Song L., Sun S., Kaplan H. J., Sun D. (2003) Lymphotoxin β receptor-Ig fusion protein treatment blocks actively induced, but not adoptively transferred, uveitis in Lewis rats. Eur. J. Immunol. 33, 1736–1743 [DOI] [PubMed] [Google Scholar]
- 35.Shao H., Huang Z., Sun S. L., Kaplan H. J., Sun D. (2004) Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest. Ophthalmol. Vis. Sci. 45, 4060–4065 [DOI] [PubMed] [Google Scholar]
- 36.Shao H., Sun S. L., Kaplan H. J., Sun D. (2004) Characterization of rat CD8+ uveitogenic T cells specific for interphotoreceptor retinal-binding protein 1177–1191. J. Immunol. 173, 2849–2854 [DOI] [PubMed] [Google Scholar]
- 37.Caspi R. R., Roberge F. G., Nussenblatt R. B. (1987) Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science 237, 1029–1032 [DOI] [PubMed] [Google Scholar]
- 38.Helbig H., Gurley R. C., Palestine A. G., Nussenblatt R. B., Caspi R. R. (1990) Dual effect of ciliary body cells on T lymphocyte proliferation. Eur. J. Immunol. 20, 2457–2463 [DOI] [PubMed] [Google Scholar]
- 39.Sun D., Coleclough C., Whitaker J. N. (1997) Nonactivated astrocytes downregulate T cell receptor expression and reduce antigen-specific proliferation and cytokine production of myelin basic protein (MBP)-reactive T cells. J. Neuroimmunol. 78, 69–78 [DOI] [PubMed] [Google Scholar]
- 40.Horie S., Sugita S., Futagami Y., Yamada Y., Mochizuki M. (2010) Human retinal pigment epithelium-induced CD4(+)CD25(+) regulatory T cells suppress activation of intraocular effector T cells. Clin. Immunol. 136, 83–95 [DOI] [PubMed] [Google Scholar]
- 41.Kaestel C. G., Jorgensen A., Nielsen M., Eriksen K. W., Odum N., Holst N. M., Ropke C. (2002) Human retinal pigment epithelial cells inhibit proliferation and IL2R expression of activated T cells. Exp. Eye Res. 74, 627–637 [DOI] [PubMed] [Google Scholar]
- 42.Sugita S., Streilein J. W. (2003) Iris pigment epithelium expressing CD86 (B7–2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 198, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamiri P., Zhang Q., Streilein J. W. (2004) Vulnerability of allogeneic retinal pigment epithelium to immune T-cell-mediated damage in vivo and in vitro. Invest. Ophthalmol. Vis. Sci. 45, 177–184 [DOI] [PubMed] [Google Scholar]
- 44.Sugita S., Horie S., Nakamura O., Maruyama K., Takase H., Usui Y., Takeuchi M., Ishidoh K., Koike M., Uchiyama Y., Peters C., Yamamoto Y., Mochizuki M. (2009) Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2α during inflammatory conditions. J. Immunol. 183, 5013–5022 [DOI] [PubMed] [Google Scholar]
- 45.Sugita S., Horie S., Nakamura O., Futagami Y., Takase H., Keino H., Aburatani H., Katunuma N., Ishidoh K., Yamamoto Y., Mochizuki M. (2008) Retinal pigment epithelium-derived CTLA-2α induces TGFβ-producing T regulatory cells. J. Immunol. 181, 7525–7536 [DOI] [PubMed] [Google Scholar]
- 46.Sugita S., Usui Y., Horie S., Futagami Y., Yamada Y., Ma J., Kezuka T., Hamada H., Usui T., Mochizuki M., Yamagami S. (2009) Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Invest. Ophthalmol. Vis. Sci. 50, 263–272 [DOI] [PubMed] [Google Scholar]
- 47.Schreiner B., Bailey S. L., Shin T., Chen L., Miller S. D. (2008) PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38, 2706–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang G., Ke Y., Sun D., Wang Y., Kaplan H. J., Shao H. (2009) Regulatory role of TLR ligands on the activation of autoreactive T cells by retinal astrocytes. Invest. Ophthalmol. Vis. Sci. 50, 4769–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francisco L. M., Salinas V. H., Brown K. E., Vanguri V. K., Freeman G. J., Kuchroo V. K., Sharpe A. H. (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ke Y., Liu K., Huang G. Q., Cui Y., Kaplan H. J., Shao H., Sun D. (2009) Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J. Immunol. 182, 3183–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing Z., Gauldie J., Cox G., Baumann H., Jordana M., Lei X. F., Achong M. K. (1998) IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 101, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flaishon L., Topilski I., Shoseyov D., Hershkoviz R., Fireman E., Levo Y., Marmor S., Shachar I. (2002) Cutting edge: anti-inflammatory properties of low levels of IFN-γ. J. Immunol. 168, 3707–3711 [DOI] [PubMed] [Google Scholar]
- 53.Barrett S. P., Gleeson P. A., de Silva H., Toh B. H., van,Driel I. (1996) Interferon-γ is required during the initiation of an organ-specific autoimmune disease. Eur. J. Immunol. 26, 1652–1655 [DOI] [PubMed] [Google Scholar]
- 54.Christen U., Wolfe T., Mohrle U., Hughes A. C., Rodrigo E., Green E. A., Flavell R. A., von Herrath M. G. (2001) A dual role for TNF-α in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 166, 7023–7032 [DOI] [PubMed] [Google Scholar]