Review discusses studies analyzing how the immune system generates Treg cells that can recognize self-peptides and prevent harmful autoimmune responses.
Keywords: Foxp3, thymocyte, T cell receptor, selection, immune regulation
Abstract
The cataclysmic disease that develops in mice and humans lacking CD4+ T cells expressing the transcription factor Foxp3 has provided abundant evidence that Foxp3+CD4+ Tregs are required to suppress a latent autoreactivity of the immune system. There is also evidence for the existence of tissue-specific Tregs that can act to suppress regional autoimmune responses, suggesting that Tregs exert their effects, in part, through responding to self-peptides. However, how the immune system generates a repertoire of Tregs that is designed to recognize and direct regulatory function to self-peptides is incompletely understood. This review describes studies aimed at determining how T cell recognition of self-peptide(s) directs Treg formation in the thymus, including discussion of a modified “avidity” model of thymocyte development. Studies aimed at determining how TCR specificity contributes to the ability of Tregs to suppress autoimmune diseases are also discussed.
Introduction
The immune system responds to invading microorganisms with the expansion and differentiation of T and B cells that act together to eliminate the infectious agent [1]. Superimposed on these processes is the requirement that T and B cells that can react with self-antigens be prevented from mediating autoimmune damage to the host′s own cells and tissues [2]. A major mechanism for establishing T and B cell tolerance to self-antigens is the clonal elimination of self-reactive lymphocytes [3]. In some settings, induction of functional inactivation also appears to limit the potential self-reactivity of lymphocytes that evade deletion during their development [4]. In recent years, however, clear evidence has emerged for the existence of Tregs that play a crucial and active role in preventing autoreactive lymphocytes that evade negative selection from mediating autoimmune pathology [5, 6]. The best-characterized Treg population was first identified as CD4+CD25+ T cells, which comprise 5–10% of the peripheral CD4+ T cell repertoire in mice and humans and can exert potent, suppressive activity in in vitro culture systems [7]. Subsequent studies identified Foxp3 as a transcription factor that is expressed selectively in CD4+CD25+ Tregs, and Foxp3 is now used routinely to identify this Treg population [8].
Mice and humans lacking cells expressing Foxp3 spontaneously develop a wasting disease that is characterized by lymphocyte infiltration into multiple sites and tissues and can be prevented by transfer of Tregs from normal hosts [7]. This seems to imply that Tregs might mediate their protective effects by recognizing self-antigens in peripheral organs and tissues and controlling a latent autoreactivity that exists in the normal immune repertoire. However, how the immune system generates T cells that can recognize and direct regulatory activity toward self-peptides remains poorly understood. That the peripheral T cell repertoire is shaped by the specificity and reactivity of the thymocyte TCR for self-peptides is broadly accepted and embodied in the “avidity” model of thymocyte development [9]. Thus, studies using fetal thymic organ culture provided evidence that weak signals transmitted by peptide-loaded MHC molecules can rescue thymocytes from programmed cell death (“positive selection”) based on the ability of the TCR to react with host MHC molecules, which are mostly occupied by self-peptides [10–12]. This ensures that only those thymocytes whose TCRs have a capacity to recognize the host's MHC molecules when they are displaying foreign peptides will be exported to the periphery. By contrast, stronger signals through the TCR could cause thymocytes to undergo deletion and represented a mechanism by which those TCRs that are overtly reactive with self-peptide:MHC complexes can be prevented from being exported to the periphery, where they could mediate autoimmune damage. How TCR signaling by self-peptide:MHC complexes contributes to CD4+CD25+Foxp3+ Treg formation and fits within this general scheme of CD4+ T cell development is currently not understood. Similarly, how signaling by self-peptides directs the effector activity of Tregs in vivo remains to be defined. This review will describe studies that have been carried out to determine how TCR specificity for self-peptides guides the development and activity of Tregs, including recent studies that attempt to fit this process within the avidity model of thymocyte development.
ROLE OF THE THYMUS IN TREG FORMATION
The first evidence that Tregs are generated in the thymus came from the finding that subjecting mice that are less than 3 days old to thymectomy led to the development of a multi-organ autoimmune disease [13, 14]. Additional evidence pointing to the thymus as a source of cells that can exert dominant tolerance came from elegant studies by LeDourain and colleagues [15], in which engraftment of thymic epithelium could induce transplantation tolerance in quails and mice. These and additional studies in rats led to a proposal that the formation of Tregs is “the third function of the thymus” (along with positive selection and deletion) [16]. However, the identity of these putative Tregs was unclear until the seminal finding that autoimmune disease could be prevented in Day 3-thymectomized mice by the adoptive transfer of CD4+CD25+ T cells from unmanipulated adult mice [17]. Interestingly, CD4+CD25+ Tregs were found to be present in diseased, Day 3-thymectomized mice but at reduced frequencies relative to unmanipulated controls, and whether the failure of these cells to prevent autoimmunity is because they are present in insufficient numbers or because they lack certain TCR specificities that are required to prevent disease development has not been established [18]. Additional support for the thymic production of Tregs came with the demonstration that CD4+CD8–CD25+ thymocytes can exert regulatory function and could be isolated at early time-points before CD4+CD25+ T cells had populated the periphery [19]. These conclusions were confirmed and extended when the key role of Foxp3 in CD4+CD25+ Treg development was discovered [20, 21], and it was shown that CD4+CD8– [CD4 SP (CD4SP)] thymocytes begin to express Foxp3 at Day 2 after birth in neonatal mice, following which, CD4+Foxp3+ T cells began to accumulate in the periphery and after 3 weeks, reached levels similar to those found in adult mice [22]. Thus, by the year 2000, the idea that the thymus produces Tregs that are required to prevent the spontaneous development of cataclysmic autoimmune disease was beginning to become accepted, but little was known about the mechanisms underlying the formation of such cells. In particular, these studies were unable to determine whether Tregs were selected based on interactions with self-peptides or if they arose in a stochastic manner independent of the TCR.
TREG FORMATION IN TCR TRANSGENIC MICE
Investigations of the role that TCR specificity plays in thymocyte development are problematic in unmanipulated mice as a result of the enormous diversity of the TCRs that can be generated through gene rearrangement, and of the peptides that can interact with these TCRs during thymic selection. Indeed, the key breakthrough that led to the avidity model of thymocyte selection outlined above was the use of TCR transgenic mice (which greatly increased the representation of thymocytes expressing a defined TCR), along with additional knockout mice that allowed the peptides that are presented to thymocytes to be experimentally manipulated [10–12]. Efforts to understand the role of TCR specificity in Treg selection have similarly been facilitated by the use of TCR transgenic mice to analyze the ability of particular TCRs to undergo selection into the Treg pathway.
Initial observations of Treg development in mice expressing transgenic TCRs provided additional, early clues that Tregs were important in preventing autoimmunity. Transgenic mice expressing a MBP-specific TCR that had been derived from an encephalitogenic CD4+ T cell clone were found not to develop disease unless they were mated into a RAG−/− background, in which case, the mice developed autoimmune encephalitis [23, 24]. As the disease could be prevented by the adoptive transfer of CD4+ T cells from normal mice, it was concluded that encephalitis development was at least partly a result of a failure of immune regulation [23, 24]. Subsequent studies showed that the development of CD4+CD25+ T cells in these mice depends on the coexpression of allelically included TCR α-chains, along with the transgenic TCR, and that these cells were absent in the RAG−/− background (which forced exclusive expression of the transgenic TCR and thereby, prevented CD4+CD25+ Treg formation) [25]. A similar reliance on expression of endogenous TCR chains for Treg formation has been noted in other TCR transgenic mice with specificities for OVA or influenza virus HA, where 5–10% of the CD4+ T cells in RAG-sufficient mice have been found to express Treg markers (such as CD25 and Foxp3) and are restricted to cells expressing low levels of the clonotypic TCR (and therefore, coexpress endogenous TCR chains) [19, 26–28]. By contrast, in RAG−/− backgrounds, <1% of the CD4+ T cells were Tregs, resembling the findings made with the MBP-specific CD4+ TCR transgenic mice.
These findings provided initial evidence that TCR specificity can be an important determinant in directing Treg development and appeared to suggest that some TCRs might be incapable of recognizing a ligand(s) that is required for Treg formation in the thymus. As is described in more detail in the next section, additional evidence emerged that the ability of the TCR to react with self-peptides is a critical requirement for Treg development and that TCRs that could not undergo Treg selection in a RAG−/− background did not have an appropriate specificity for a selecting peptide. Recently, however, another mechanism that regulates Treg formation has been uncovered, which can also impact the formation of Tregs in RAG−/− backgrounds. Two groups of workers took the approach of isolating TCRs from purified Tregs and reintroducing these TCRs into mice by transgenesis or by retroviral transduction in bone marrow chimeras [29, 30]. In each set of studies, the expectation was that thymocytes would be “instructed” to enter the Treg pathway, as the TCR presumably conferred specificity for whatever selecting ligand induced the formation of the original Treg. Notably, however, efficient formation of Tregs only occurred under conditions of low clonal frequency and was impaired when a high percentage of thymocytes expressed the Treg-derived TCR (as is the case in RAG−/− backgrounds). These studies provided evidence that there exists a “niche” that limits the number of Tregs that can be generated intrathymically, and in one of the studies, it was suggested that this might explain why Treg formation was not observed in RAG−/− backgrounds [29]. However, the other study showed that although the number of Tregs that could be formed was limited by niche size, greater numbers were found in RAG−/− mice that expressed TCRs derived from Tregs versus conventional CD4+ T cells [30]. This latter finding argues that the Treg-derived TCR is instructed to undergo selection to become a Treg, as it interacts with some ligand that is not recognized by the TCR expressed by conventional CD4+ T cells, although it does not reveal the nature of the ligand that mediates this selection. A similar experimental approach had provided evidence previously that Tregs recognize self-ligands when a Treg-derived TCR was found to undergo deletion in transgenic mice. However, why this TCR did not promote Treg formation and instead, caused thymocyte deletion was not established [31].
TREG FORMATION IN RESPONSE TO SELF-PEPTIDES
A key observation regarding the ligands that can induce Treg formation came from studies in which mice expressing a HA-specific TCR transgene were mated with mice that expressed HA as a neo-self-antigen [26, 32]. TS1 mice express a transgenic TCR that is specific for the major I-Ed-restricted T cell determinant from the influenza PR8 HA (termed S1) and can be detected by the clonotypic mAb 6.5 [33]. As noted above, CD4+ T cells expressing high levels of the clonotypic 6.5 TCR undergo little or no Treg formation in TS1 mice [32]. However, when TS1 mice were mated with HA28 transgenic mice that express the HA as a neo-self-antigen (to produce TS1×HA28 mice), the proportion of 6.5+CD4+ T cells in the periphery that expressed CD25 increased to ∼50%, and these cells could exert regulatory activity in vitro [32]. Additional studies showed that these 6.5+CD4+CD25+ Tregs were formed based on interactions between thymocytes expressing the HA-specific 6.5 TCR and the HA self-peptide in the thymus [26]. The 6.5+CD4+CD25+ Tregs could also be found when TS1 mice that had been mated into RAG−/− backgrounds were used to generate bone marrow chimeras with HA28 mice or in intact TS1×HA28.Cα−/− mice (in which endogenous TCR α-chain gene rearrangement is selectively suppressed) [26, 28]. These studies provided the first evidence that TCR specificity for self-peptides can be a prerequisite for the formation of Tregs.
These initial studies were quickly followed by additional studies showing the formation of HA-specific Tregs when TS1 mice were mated with additional lineages expressing HA under the control of other promoters [34–36] and in DO11.10 mice expressing the OVA-specific KJ-126 TCR that had been mated with mice expressing OVA as a self-peptide [27, 37]. However, similar studies in another system yielded a different model of Treg formation; transgenic mice expressing the MCC-specific AND TCR were mated with mice expressing the cognate MCC-derived peptide under the control of an inducible promoter that allowed the levels of expression of the self-peptide to be regulated [38]. Increased percentages of Tregs were found in the mice that expressed the self-peptide, but this appeared to be a result of deletion of clonotypic non-Tregs rather than an induced formation of increased numbers of Tregs in response to the self-peptide. Based on these observations, a mechanism of Treg formation was proposed that involves some stochastic process that leads a subset of cells to become committed to a Treg pathway, which then protects these cells from subsequent deletion by the self-antigen [38]. Key to this model was the finding that no net increases in the number of Tregs were found in mice that coexpressed the TCR and the self-peptide versus those expressing the TCR alone, as would be predicted for a model in which specificity for self-peptides is required to actively induce Treg formation. At the time that model was proposed, it was further argued that no such net increases in Treg formation in response to self-peptides had been observed in any experimental system. However, there is now clear evidence that such self-peptide-induced increases in Treg formation can occur and that these increases cannot be reconciled with the model that was proposed based on the studies in AND mice. Why these studies did not reveal self-peptide-induced Treg formation is not known, but it is worth noting that the AND transgenic mouse is one in which the TCR transgene is expressed by virtually all CD4+ thymocytes, and it is possible that the process of intraclonal competition outlined above limited the formation of Tregs in these studies. In any event, the studies in the HA and OVA systems have firmly established that self-peptides can be critical ligands for promoting the development of Tregs expressing defined TCRs.
THYMOCYTE DELETION AND TREG FORMATION AS INTERTWINED PROCESSES
One of the prominent features of the studies in the HA and OVA systems was that the self-peptide that induced Treg formation was recognized by the TCR as an agonist. It is well established that TCRs can possess a wide range of reactivities with peptide:MHC complexes, and agonist peptides are typically defined as peptides with which the TCR is strongly reactive [39]. Indeed, in most cases, including the HA and OVA models described here, the agonist peptide corresponds to the antigen that was used to immunize mice and generate the original T cell clones from which the TCR was derived to make the transgenic mouse, and is capable of strongly stimulating CD4+ T cells expressing this TCR. However, the avidity model of thymocyte selection holds that thymocytes that are strongly reactive with self-peptides undergo deletion to prevent cells that could potentially react with self-peptides from being exported to the periphery [10–12]. How then to place the notion of agonist-induced Treg formation into this scheme has been uncertain; indeed, what parameters would dictate whether a thymocyte undergoes deletion or Treg formation, and whether these are distinct versus inter-related events, has not been clear.
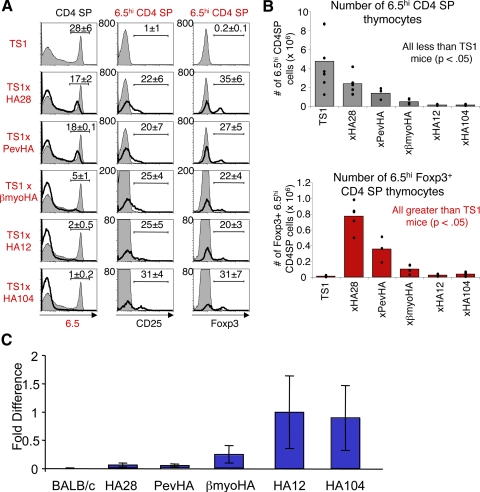
We addressed this issue recently by expanding our analysis of 6.5+CD4+CD25+Foxp3+ Treg formation that occurs in TS1 × HA transgenic mice when HA expression is driven by several different promoters and enhancers [40]. We found that thymocytes expressing the 6.5 TCR are subjected to varying degrees of deletion by the S1 peptide in the different TS1 × HA lineages. However, among the 6.5hiCD4SP thymocytes that evade deletion in each lineage, the proportion that acquires CD25 or Foxp3 expression is quite similar (Fig. 1A). As a result of these two processes, the numbers of 6.5hiFoxp3+CD4SP thymocytes varied substantially in the different TS1 × HA lineages. In all cases, however, significantly higher numbers of 6.5hiFoxp3+CD4SP thymocytes developed in TS1 × HA mice than in TS1 mice, which do not coexpress the S1 peptide (Fig. 1B). Notably, the amount of HA transgene mRNA in the different lineages correlated well with the degree of deletion of 6.5hiCD4SP thymocytes; those lineages that induced only modest deletion of 6.5hiCD4SP thymocytes accompanied by abundant 6.5hi Treg formation contained low levels of HA mRNA, and higher levels were found in lineages that induced more efficient deletion (Fig. 1C). To determine how expression in different thymic cell types might impact 6.5hiCD4SP thymocyte development, we generated bone marrow chimeras and found that deletion and Treg formation among 6.5hiCD4SP thymocytes could be induced in mice in which HA expression is targeted to radio-resistant (i.e., epithelial) or bone marrow-derived cells [40]. Collectively, these analyses provided evidence that deletion and CD4+Foxp3+ thymocyte formation are intertwined rather than distinct processes. Moreover, they argue against the possibility that discrete thymic cell types play distinct roles in inducing deletion versus Treg formation. Instead, the overall amount of a self-peptide appears to be an important factor that governs the extent to which autoreactive CD4SP thymocytes are subject to deletion, and of the cells that evade deletion, similar proportions acquire Foxp3 expression (Fig. 2).
Figure 1. The S1 self-peptide induces thymocytes expressing the 6.5 TCR to undergo deletion and Treg formation in TS1 × HA mice.
(A) Histograms show 6.5 expression by CD4SP thymocytes (left panels) and CD25 expression (middle panels) or Foxp3 expression (right panels) by 6.5hiCD4SP thymocytes from the indicated TS1 × HA (black lines) mice, overlaying TS1 (filled) mice, with mean percentages indicated. (B) Graphs show numbers of 6.5hiCD4SP thymocytes (upper graph) and of 6.5hiCD4SPFoxp3+ thymocytes (lower graph) from indicated TS1 × HA mice. Bars denote means, and individual values are indicated (●). (C) Graph shows relative amounts of HA transgene mRNA in total thymic mRNA preparations from indicated lineages of HA transgenic mice as determined by real-time PCR. Bars indicates means ± sem.
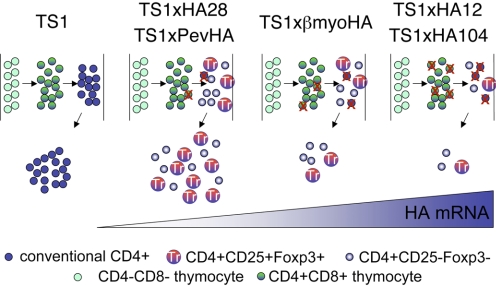
Figure 2. Thymocyte deletion and Treg formation are intertwined processes in TS1 × HA transgenic mice.
Schematic drawing depicts selection processes shaping the development of 6.5hi thymocytes in mice that do not express HA or that express increasing amounts of HA mRNA in the thymus. In TS1 mice that do not express any HA mRNA, thymocytes expressing the 6.5 TCR undergo progressive development from the CD4–CD8– (“double-negative”) stage, through the CD4+CD8+ (“double-positive”) stage, to the mature CD4+CD8– (“SP”) stage, following which, they are exported to the periphery as CD4+ T cells. In the different TS1 × HA lineages, the presence of increasing amounts of HA mRNA causes increasing degrees of thymocyte deletion (indicated as a red “X”), and there is a progressive decrease in the number of thymocytes expressing the 6.5 TCR that mature to the CD4SP developmental stage. Among the thymocytes that evade deletion, similar proportions become Foxp3+ at the CD4SP stage in the different lineages and are exported to the periphery to populate the peripheral Treg repertoire. One effect of this process is that a greater number of Tregs are produced when 6.5hi thymocytes encounter the HA as a low-abundance antigen than are produced when the HA is more highly expressed. Another is that HA-specific Tregs are exported to the periphery along with 6.5hiCD4SPFoxp3– T cells that could potentially contribute to the latent autoreactivity of the peripheral CD4+ T cell repertoire.
A MODIFIED AVIDITY MODEL OF THYMOCTYE DEVELOPMENT
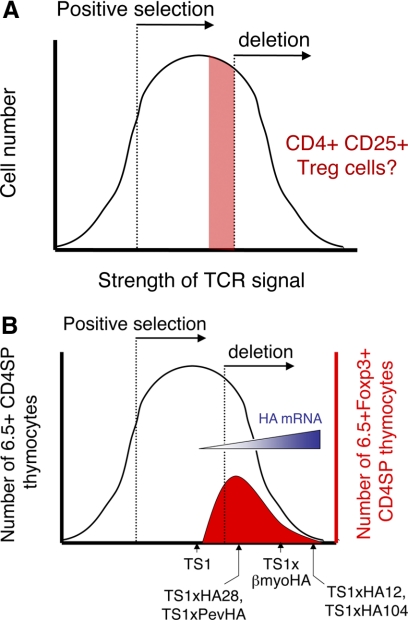
As introduced above, the discovery that agonist self-peptides can induce Treg formation has been difficult to reconcile with the avidity model of thymocyte selection, in which the strength of the TCR signal determines whether a developing thymocyte undergoes death by neglect, positive selection, or deletion. Based primarily on experiments in which peptides were introduced into fetal thymic organ culture, these studies are typically presented as a bell-shaped curve, in which the recovery of thymocytes is plotted against the amount of peptide [or by extension, the avidity of the interaction with self-peptide(s)] that is encountered by the thymocyte. One idea has been that Treg formation might be promoted by TCR signaling that is below a threshold for overt deletion [6] (Fig. 3A). However, our findings in TS1 × HA mice suggest an alternative model, in which the TCR signaling intensity that induces TCR deletion and Treg formation overlaps and that Tregs are formed as a relatively constant percentage of the thymocytes that evade deletion by the agonist self-peptide (Fig. 3B). There are several notable features of this model: First, the overall number of Tregs that is formed is actually determined by the extent of thymocyte deletion, and as noted above, this in turn, is proportional to the amount of self-peptide that is encountered in the thymus. Moreover, the extent of deletion appears to be limited by the amount of self-peptide that is present; more self-peptide causes more deletion under conditions where the frequency of thymocyte precursors is fixed by the use of the TS1 TCR transgene, and interestingly, this implies that the size of the niche that is available for deletion is proportional to the amount of the self-peptide that is presented in the thymus. This idea that niche size may affect the extent of Treg formation by determining the extent of thymocyte deletion extends recent studies showing that under conditions where the amount of self-peptide that is presented remains constant, the extent of Treg formation can be influenced by the frequency of thymocyte precursors [29, 30].
Figure 3. Treg formation in a modified avidity model of thymocyte development.
The avidity model of thymocyte development holds that the strength of the TCR signal determines whether a developing thymocyte undergoes death by neglect, positive selection, or deletion. The outcome of these different TCR signals is typically presented as a bell-shaped curve, in which the recovery of thymocytes is plotted against the amount of peptide [or, by extension, the avidity of the interaction with self-peptide(s)], which is encountered by the thymocyte. One idea has been that Treg formation might be promoted by TCR signaling that is below a threshold for overt deletion (A). However, our data are more consistent with a model in which the strength of the TCR signal necessary to induce deletion and Treg formation overlaps (B). As represented in the figure, this model is based on the finding that 6.5hi thymocytes undergo increasing deletion in response to higher amounts of HA mRNA in the different TS1 × HA lineages, and among the cells that evade deletion, similar proportions become Tregs.
Second, the net result of these processes is that the Treg repertoire that is ultimately produced, is biased toward low-abundance self-peptides. Thus, the formation of Tregs, as represented in Figure 2, is heavily biased toward those mice that express low amounts of HA mRNA, and assuming that the results we have obtained with different HA transgenes are paralleling differences in how bona fide self-antigens are expressed (e.g., some in high amounts, some in low amounts), then this model would suggest that the Treg repertoire that is generated in unmanipulated mice (and humans) may be biased toward low-abundance self-antigens that induce inefficient deletion. There is now clear evidence that the transcription factor AIRE plays a specialized role in directing thymic expression of otherwise tissue-specific self-peptides and may participate in this process, although any conclusion that AIRE participates directly in the formation of Tregs remains controversial [41]. A focusing of the Treg repertoire toward low-abundance self-peptides may also explain why a study failed to detect Treg activity against self-peptides, as the target self-peptides recognized by Tregs might be too rare to induce detectable activation of Treg hybridomas in in vitro culture systems [42]. It also suggests that the Treg repertoires that are expressed by outbred individuals may be quite idiosyncratic, as their composition would be imposed by low abundance peptides that may show greater variation between individuals than is the case for highly abundant self-peptides.
A third feature of this model is that the formation of CD4+Foxp3+ Tregs is accompanied by the formation of a population of CD4+ T cells with the same reactivity for the selecting self-peptide but are not Foxp3+. The fact that mice and humans that lack Foxp3+ T cells develop autoimmune disease spontaneously is dramatic evidence that the normal immune repertoire contains CD4+ T cells that are intrinsically reactive with self-peptides, but the precise specificities of such CD4+ T cells are unknown. The findings in TS1 × HA mice provide evidence that among a cohort of thymocytes expressing the same TCR, some can undergo selection to enter the Treg lineage, and others do not. The conclusion that only a subset of the thymocytes that evade deletion acquires Foxp3 expression resembles studies in other systems showing Foxp3 expression in only a subset of susceptible thymocytes and may reflect heterogeneity in the expression of additional molecules and/or epigenetic modifications of target genes that are required for Foxp3 expression [43]. Moreover, this model is supported by sequence analyses that have revealed some overlap between the TCR genes that are used in Foxp3+CD4+ and Foxp3–CD4+ T cells [44]. It will be important to test this model further using additional transgenic and nontransgenic systems to determine the extent to which the Treg and effector cell repertoires that are produced by the thymus represent overlapping versus distinct sets of specificities.
ROLE OF TCR SPECIFICITY IN DIRECTING TREG ACTIVITY
Although there is accumulating evidence that TCR recognition of self-peptides plays an important role in Treg formation, how TCR specificity guides Treg effector function is less well understood. Studies carried out using mixtures of purified CD4+CD25+ T cells and effector cells obtained from TCR transgenic mice in in vitro assays have provided evidence that Tregs must be stimulated via their own TCR to become activated, but once activated, they can mediate bystander suppression [45, 46]. These targets of suppression include CD4+ T cells with a different TCR specificity, CD4+ T cells recognizing peptides presented by a different APC molecule, or even CD8+ T cells that are activated by non-APC ligands (e.g., tetramer complexes of MHC molecules bearing the appropriate peptide) [45, 46]. Notably, we demonstrated further that 6.5+CD4+CD25+ Tregs from TS1 × HA mice could mediate bystander suppression in vitro in response to analogs of the S1 peptide, indicating that Tregs that have been generated in response to an agonist peptide can exert suppressor function in response to peptides with which the TCR is only weakly cross-reactive [46]. This raises the possibility that Tregs might have different specificity requirements for formation versus activation, which could allow Tregs to become activated by a broader range of peptides than mediate their formation. It is important to note, however, that these conclusions are based on studies in in vitro proliferation assays, and how they may translate to the more complex environment that is encountered in vivo is not clear.
As introduced above, elegant studies in animal models have provided evidence that Tregs can exhibit organ and tissue specificity in their ability to prevent autoimmune disease, although the precise specificity of the Tregs for self-peptides was not determined. Thus, CD4+CD25+ cells from athyroid rats were able to adoptively suppress autoimmune diabetes, but not autoimmune thyroiditis, in thymectomized hosts treated with split-dose radiation [47]. In these experiments, CD4+CD25+ cells from intact animals were able to suppress disease in both organs, as were CD4+CD25+ cells from the thymus of athyroid animals, indicating that ablation of the thyroid resulted in a loss of organ-specific Treg activity in the periphery rather than a loss of thyroid-specific Treg formation in the thymus. Subsequent studies using neonatal thymectomy as a model of organ-specific autoimmune disease supported this conclusion [48, 49]. In these studies, BAF mice that were subjected to thymectomy prior to Day 3 of life developed dachryoadenitis, autoimmune ovarian disease in females and EAP in males, and CD4+CD25+ cells isolated from intact (prostate antigen+) male BAF mice were significantly more efficient at adoptively suppressing EAP than CD4+CD25+ cells isolated from (prostate antigen–) female donors. Furthermore, CD4+CD25+ Tregs that were capable of suppressing EAP efficiently were absent in male donors that lacked a prostate and were present in female donors engrafted with a prostate and given testosterone to induce prostate-antigen expression. Although the specificity of the protective Tregs was not assessed directly in these experiments, a subsequent report by the same group suggested that presentation of ovarian antigens in the draining LN is responsible for maintaining a population of organ-specific Tregs, as Tregs from the draining LN were 15–50 times more efficient than those of the nondraining LN at suppressing autoimmune diseases of ovary, prostate, and lacrimal glands [50]. Moreover, this difference disappeared upon autoantigen ablation and returned upon autoantigen re-expression [50]. Collectively, these data imply that TCR recognition of self-antigens in the periphery plays a dominant role in maintaining the peripheral Treg repertoire and in activating Treg suppressor function.
ROLE OF TREG SPECIFICITY IN TRANSGENIC MODELS OF AUTOIMMUNE DISEASE
Just as the use of TCR transgenic mice to limit the diversity of the T cell repertoire has been helpful in elucidating the mechanisms of thymocyte and Treg development, they have also been used to examine the impact of Treg specificity in mouse models of autoimmune disease. As introduced above, in early studies using a MBP-specific TCR transgenic mouse, the development of disease was inhibited by the development of Tregs bearing allelically included TCR α-chains [23]. Interestingly, whereas the presence of endogenous TCR α-chains was required to mediate the formation of Tregs in these mice, they were not sufficient to promote regulatory activity, as CD4+CD25+ cells that had been depleted of clonotype+ cells were unable to prevent encephalitis in MBP.RAG−/− mice [25]. Thus, the endogenous CD4+CD25+ cell repertoire in MBP mice mediates Treg selection but lacks the requisite TCRs to prevent autoimmunity against CNS antigens, and coexpression of the MBP-specific transgenic TCR confers specificity that allows these Tregs to prevent encephalomyelitis, presumably by recognizing MBP as a target autoantigen.
Studies using Tregs obtained from TCR transgenic mice have provided additional evidence that TCR specificity for an autoantigen can direct Treg function in vivo. We generated a lineage of HA transgenic mice (designated HACII mice), which was engineered to express the HA as a target self-antigen driven by a MHC class II promoter to direct expression of the HA transgene to APCs (including B cells and dendritic cells) [51]. We used these mice to develop a system in which the HA is a surrogate autoantigen that is expressed by anti-dsDNA B cells, and showed that provision of HA-specific CD4+ T cell help to these B cells in a nonautoimmune-prone background would overcome their anergic state and result in the production of anti-dsDNA antibodies. Notably, coadministration of HA-specific CD4+CD25+ Tregs from TS1 × HA28 mice prevented this activation, providing evidence that Tregs can prevent autoimmune responses by recognizing the same target autoantigen as is recognized by effector CD4+ T cells [52].
Similar evidence supporting a role for TCR specificity in directing Treg function in vivo has been obtained in studies involving diabetes-prone NOD mice and transgenic mice expressing the islet cell-specific BDC2.5 TCR. NOD mice carrying the BDC2.5 transgene are protected from diabetes by a population of CD4+CD25+ Tregs that coexpresses the BDC2.5 TCR and endogenous TCR chains [53]. To establish that TCR specificity can direct Treg function in vivo, CD4+CD25+ cells from BDC2.5 mice were expanded in vitro by dendritic cells pulsed with a mimetope peptide to activate the BDC2.5 TCR and were shown to suppress diabetes development in adoptive transfer studies [54]. CD4+CD25+ T cells from BDC2.5 mice that were expanded by incubation with anti-CD3 and IL-2 were shown to be similarly able to suppress diabetes development, and in this case, the expanded cells were shown to be enriched in specificity for an islet autoantigen, as they proliferated in pancreatic LNs but not in other LNs following transfer into NOD mice [55]. However, no such protection was observed when CD4+CD25+ T cells obtained from GAD286 TCR transgenic mice were similarly expanded and used in adoptive transfer studies, although this TCR is directed to the known islet autoantigen GAD. The authors concluded that the key difference between the CD4+CD25+ T cells from BDC2.5 and GAD286 mice is the extent of deletion of thymocytes expressing the clonotypic TCRs; thus, thymocytes expressing the BDC2.5 TCR undergo inefficient deletion, and the GAD286 thymocytes are deleted more extensively. As this is presumably a result of differences in the presentation of the respective autoantigens in the thymus, these conclusions are notable with respect to the modified “avidity” model of Treg formation proposed here, which predicts that the Treg repertoire is biased toward low-abundance antigens that induce inefficient thymocyte deletion. By this model, the efficient deletion of GAD-specific thymocytes in GAD286 mice also has the effect of purging the Treg repertoire of GAD-specific Tregs. As was observed in TS1 × HA transgenic mice that express high levels of the HA mRNA, the efficient deletion of GAD-specific thymocytes would also limit the number of GAD-specific Tregs that can be produced, as Tregs arise from thymocytes that have evaded deletion. By contrast, the less-efficient deletion of thymocytes in BDC2.5 mice could allow a greater number of autoantigen-specific Tregs to be generated from the cohort of thymocytes that evaded deletion. These Tregs could then be expanded and exert their suppressive effect by recognizing the islet autoantigen.
These studies suggest that the absence of Tregs that can recognize disease-associated autoantigens may be an important predisposing factor in autoimmunity, but other studies suggest that alternative factors can also make important contributions to these processes. A mouse lineage termed TXA23 expresses a transgenic TCR that is specific for gastric H+/K+ ATPase and develops spontaneous gastritis despite the presence of CD4+CD25+ Tregs [56]. Notably, gastritis can be induced in recipient mice by transfer of small numbers of naïve CD4+ thymocytes from TXA23 mice into immunodeficient mice, but this disease is suppressed by cotransfer of polyclonal CD4+CD25+ T cells from BALB/c mice. Thus, polyclonal Tregs with TCRs directed to diverse self-peptides can suppress disease caused by a population of autoantigen-specific CD4+ T cells, at least in an adoptive transfer setting where lymphopenia-induced proliferation might contribute to the development of gastritogenic CD4+ T cells [57]. The fact that disease develops in intact TXA23 mice, which contain CD4+CD25+ Tregs, may therefore be a result of the high frequency of transgenic TCR-bearing CD4+ T cells, which overwhelms this regulation. These observations in turn raise the possibility that the development of autoimmune disease might in some cases reflect an imbalance between the frequency of Tregs directed to diverse self-peptides and effector cells directed to disease-associated autoantigens. Within this framework, it is also possible that autoreactive CD4+ T cells that have differentiated into different effector cell types might have differing susceptibilities to Treg-mediated suppression, as it was shown further that CD4+ T cells from TXA23 mice, which have been differentiated in vitro into Th1, Th2, or Th17 cells, can have differing sensitivities to regulation by polyclonal Tregs [58].
We are beginning to analyze how TCR specificity can affect Treg function in autoimmune states by analyzing TS1 × HACII mice (generated by mating TS1 mice with HACII mice). TS1 × HACII mice develop inflammatory arthritis spontaneously, which affects the majority of mice and begins to manifest itself at 8–10 weeks of age [51]. An interesting feature of this disease model is that arthritis develops in the context of massive thymocyte deletion and despite the presence of HA-specific CD4+CD25+Foxp3+ T cells that are capable of mediating suppression in vitro. Why disease develops despite the presence of these putative antigen-specific Tregs is uncertain. One possibility is that they are at a numerical disadvantage initially in these mice and that although they are present in diseased mice, their numbers are insufficient at some critical, early period to prevent disease from developing. Another possibility is that the severe deletion of thymocytes that occurs in TS1 × HACII mice limits the range of specificities that can be produced among cells expressing endogenous TCR chains, and the overall TCR repertoire lacks the appropriate specificities to prevent disease development. In this regard, it is noteworthy that cells bearing many of the hallmarks of Tregs have been found in human rheumatoid arthritis patients, and why these cells fail to prevent disease development is similarly obscure [59]. One of the challenges in understanding arthritis in humans is that the targets of autoaggressive CD4+ T cells are not known, and how Treg specificity may shape disease outcome is even more obscure. Our ongoing studies in TS1 × HACII mice, in which the identity of the self-peptide that is recognized by effector CD4+ T cells and Tregs is known and can be manipulated, should allow the role that TCR specificity plays in directing Treg function in vivo to be more fully defined and aid in the development of effective therapeutic strategies.
CONCLUSION
Treg formation is required to prevent the body from succumbing to a latent pathological autoreactivity and may also play a role in preventing the development of regional autoimmune responses. Studies carried out using transgenic mouse models have revealed mechanisms by which the specificity of the TCR for self-peptides can guide the development of a self-reactive Treg repertoire and have led to the development of a modified “avidity” model of thymocyte development that encompasses Treg formation. Additional studies have suggested a role for TCR specificity in guiding Treg activity in autoimmune disease models. A more thorough understanding of these processes will be important for the development of effective Treg-based therapies.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI59166 and AI24541), from Sibley Memorial Hospital, and by the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health. D. M. S. is supported by The Wistar Institute Cancer Training grant (T32 CA09171). We thank Alissa Basehoar, Christina Mergenthaler, Abigail Liebow, Lori Mroz, Nardine Zakhary, and Victoria Garcia for their invaluable technical help and for maintaining the transgenic mouse lineages.
Footnotes
- AIRE
- autoimmune regulator
- BAF
- C57BL/6 × A/J F1
- dsDNA
- double-stranded DNA
- EAP
- experimental autoimmune prostatitis
- Foxp3
- forkhead box p3
- GAD
- glutamic acid decarboxylase
- HA
- hemagglutinin
- LN
- lymph node
- MBP
- myelin basic protein
- MCC
- moth cytochrome C
- RAG–/–
- recombinase-activating gene-deficient
- S1
- site 1 peptide
- SP
- single-positive
- Treg
- regulatory T cell
AUTHORSHIP
Donald M. Simons and Andrew J. Caton contributed to the conception, design, analysis, and interpretation of data and writing of this manuscript. Cristina Cozzo Picca, Soyoung Oh, Olivia A. Perng, Malinda Aitken, and Jan Erikson contributed to the conception, design, analysis, interpretation of data, and editing of this manuscript.
REFERENCES
- 1.Marrack P., Kappler J. (1994) Subversion of the immune system by pathogens. Cell 76, 323–332 [DOI] [PubMed] [Google Scholar]
- 2.Nossal G. J. (1994) Negative selection of lymphocytes. Cell 76, 229–239 [DOI] [PubMed] [Google Scholar]
- 3.Sprent J., Kishimoto H. (2002) The thymus and negative selection. Immunol. Rev. 185, 126–135 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz R. H. (1997) T cell clonal anergy. Curr. Opin. Immunol. 9, 351–357 [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S. (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101, 455–458 [DOI] [PubMed] [Google Scholar]
- 6.Shevach E. M. (2000) Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18, 423–449 [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. (2004) Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y., Rudensky A. Y. (2007) Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8, 457–462 [DOI] [PubMed] [Google Scholar]
- 9.Starr T. K., Jameson S. C., Hogquist K. A. (2003) Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 [DOI] [PubMed] [Google Scholar]
- 10.Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. (1994) T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 [DOI] [PubMed] [Google Scholar]
- 11.Jameson S. C., Hogquist K. A., Bevan M. J. (1994) Specificity and flexibility in thymic selection. Nature 369, 750–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashton-Rickardt P. G., Bandeira A., Delaney J. R., Van Kaer L., Pircher H. P., Zinkernagel R. M., Tonegawa S. (1994) Evidence for a differential avidity model of T cell selection in the thymus. Cell 76, 651–663 [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S., Takahashi T., Nishizuka Y. (1982) Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J. Exp. Med. 156, 1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S., Takahashi T., Nishizuka Y. (1982) Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J. Exp. Med. 156, 1577–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Douarin N., Corbel C., Bandeira A., Thomas-Vaslin V., Modigliani Y., Coutinho A., Salaün J. (1996) Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol. Rev. 149, 35–53 [DOI] [PubMed] [Google Scholar]
- 16.Seddon B., Mason D. (2000) The third function of the thymus. Immunol. Today 21, 95–99 [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 [PubMed] [Google Scholar]
- 18.Asano M., Toda M., Sakaguchi N., Sakaguchi S. (1996) Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184, 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. (1999) Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162, 5317–5326 [PubMed] [Google Scholar]
- 20.Hori S., Nomura T., Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 21.Fontenot J. D., Gavin M. A., Rudensky A. Y. (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 [DOI] [PubMed] [Google Scholar]
- 22.Fontenot J. D., Dooley J. L., Farr A. G., Rudensky A. Y. (2005) Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202, 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares-Villagomez D., Wang Y., Lafaille J. J. (1998) Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188, 1883–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Keere F., Tonegawa S. (1998) CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 188, 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hori S., Haury M., Coutinho A., Demengeot J. (2002) Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 99, 8213–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan M. S., Boesteanu A., Reed A. J., Petrone A. L., Holenbeck A. E., Lerman M. A., Naji A., Caton A. J. (2001) Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 [DOI] [PubMed] [Google Scholar]
- 27.Kawahata K., Misaki Y., Yamauchi M., Tsunekawa S., Setoguchi K., Miyazaki J., Yamamoto K. (2002) Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 168, 4399–4405 [DOI] [PubMed] [Google Scholar]
- 28.Larkin J., III, Rankin A. L., Picca C. C., Riley M. P., Jenks S. A., Sant A. J., Caton A. J. (2008) CD4+CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J. Immunol. 180, 2149–2157 [DOI] [PubMed] [Google Scholar]
- 29.Bautista J. L., Lio C-W. J., Lathrop S. K., Forbush K., Liang Y., Luo J., Rudensky A. Y., Hsieh C-S. (2009) Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10, 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung M. W., Shen S., Lafaille J. J. (2009) TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J. Exp. Med. 206, 2121–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiPaolo R. J., Shevach E. M. (2009) CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur. J. Immunol. 39, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan M. S., Riley M. P., von Boehmer H., Caton A. J. (2000) Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur. J. Immunol. 30, 136–144 [DOI] [PubMed] [Google Scholar]
- 33.Kirberg J., Baron A., Jakob S., Rolink A., Karjalainen K., von Boehmer H. (1994) Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apostolou I., Sarukhan A., Klein L., von Boehmer H. (2002) Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3, 756–763 [DOI] [PubMed] [Google Scholar]
- 35.Lerman M. A., Larkin J., III, Cozzo C., Jordan M. S., Caton A. J. (2004) CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J. Immunol. 173, 236–244 [DOI] [PubMed] [Google Scholar]
- 36.Aschenbrenner K., D′Cruz L. M., Vollmann E. H., Hinterberger M., Emmerich J., Swee L. K., Rolink A., Klein L. (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 [DOI] [PubMed] [Google Scholar]
- 37.Walker L. S., Chodos A., Eggena M., Dooms H., Abbas A. K. (2003) Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198, 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Santen H. M., Benoist C., Mathis D. (2004) Number of Treg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J. Exp. Med. 200, 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kersh G. J., Allen P. M. (1996) Essential flexibility in the T-cell recognition of antigen. Nature 380, 495–498 [DOI] [PubMed] [Google Scholar]
- 40.Picca C. C., Oh S., Panarey L., Aitken M., Basehoar A., Caton A. J. (2009) Thymocyte deletion can bias Treg formation toward low-abundance self-peptide. Eur. J. Immunol. 39, 3301–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathis D., Benoist C. (2007) A decade of AIRE. Nat. Rev. Immunol. 7, 645–650 [DOI] [PubMed] [Google Scholar]
- 42.Pacholczyk R., Kern J., Singh N., Iwashima M., Kraj P., Ignatowicz L. (2007) Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity 27, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lio C-W. J., Hsieh C-S. (2008) A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsieh C. S., Zheng Y., Liang Y., Fontenot J. D., Rudensky A. Y. (2006) An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7, 401–410 [DOI] [PubMed] [Google Scholar]
- 45.Thornton A. M., Shevach E. M. (2000) Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164, 183–190 [DOI] [PubMed] [Google Scholar]
- 46.Larkin J., III, Picca C. C., Caton A. J. (2007) Activation of CD4+ CD25+ regulatory T cell suppressor function by analogs of the selecting peptide. Eur. J. Immunol. 37, 139–146 [DOI] [PubMed] [Google Scholar]
- 47.Seddon B., Mason D. (1999) Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 189, 877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setiady Y. Y., Ohno K., Samy E. T., Bagavant H., Qiao H., Sharp C., She J. X., Tung K. S. (2006) Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood 107, 1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samy E. T., Setiady Y. Y., Ohno K., Pramoonjago P., Sharp C., Tung K. S. (2006) The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol. Rev. 212, 170–184 [DOI] [PubMed] [Google Scholar]
- 50.Wheeler K. M., Samy E. T., Tung K. S. K. (2009) Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J. Immunol. 183, 7635–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rankin A. L., Reed A. J., Oh S., Cozzo Picca C., Guay H. M., Larkin J., III, Panarey L., Aitken M. K., Koeberlein B., Lipsky P. E., Tomaszewski J. E., Naji A., Caton A. J. (2008) CD4+ T cells recognizing a single self-peptide expressed by APCs induce spontaneous autoimmune arthritis. J. Immunol. 180, 833–841 [DOI] [PubMed] [Google Scholar]
- 52.Seo S. J., Fields M. L., Buckler J. L., Reed A. J., Mandik-Nayak L., Nish S. A., Noelle R. J., Turka L. A., Finkelman F. D., Caton A. J., Erikson J. (2002) The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity 16, 535–546 [DOI] [PubMed] [Google Scholar]
- 53.Kanagawa O., Militech A., Vaupel B. A. (2002) Regulation of diabetes development by regulatory T cells in pancreatic islet antigen-specific TCR transgenic nonobese diabetic mice. J. Immunol. 168, 6159–6164 [DOI] [PubMed] [Google Scholar]
- 54.Tarbell K. V., Yamazaki S., Olson K., Toy P., Steinman R. M. (2004) CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199, 1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Q., Henriksen K. J., Bi M., Finger E. B., Szot G., Ye J., Masteller E. L., McDevitt H., Bonyhadi M., Bluestone J. A. (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McHugh R. S., Shevach E. M., Margulies D. H., Natarajan K. (2001) A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur. J. Immunol. 31, 2094–2103 [DOI] [PubMed] [Google Scholar]
- 57.Datta S., Sarvetnick N. (2009) Lymphocyte proliferation in immune-mediated diseases. Trends Immunol. 30, 430–438 [DOI] [PubMed] [Google Scholar]
- 58.Stummvoll G. H., DiPaolo R. J., Huter E. N., Davidson T. S., Glass D., Ward J. M., Shevach E. M. (2008) Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J. Immunol. 181, 1908–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh S., Rankin A. L., Caton A. J. (2010) CD4+CD25+ regulatory T cells in autoimmune arthritis. Immunol. Rev. 233, 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]