Hypertonic stress activates T cells by inducing the controlled release of ATP through pannexin-1 hemichannels and activation of ATP-gated ion channels P2X1, P2X4, and P2X7.
Keywords: trauma, ATP, purinergic signaling, MAPK, IL-2
Abstract
Hypertonic saline (HS) resuscitation increases T cell function and inhibits posttraumatic T cell anergy, which can reduce immunosuppression and sepsis in trauma patients. We have previously shown that HS induces the release of cellular ATP and enhances T cell function. However, the mechanism by which HS induces ATP release and the subsequent regulation of T cell function by ATP remain poorly understood. In the present study, we show that inhibition of the gap junction hemichannel pannexin-1 (Panx1) blocks ATP release in response to HS, and HS exposure triggers significant changes in the expression of all P2X-type ATP receptors in Jurkat T cells. Blocking or silencing of Panx1 or of P2X1, P2X4, or P2X7 receptors blunts HS-induced p38 MAPK activation and the stimulatory effects of HS on TCR/CD28-induced IL-2 gene transcription. Moreover, treatment with HS or agonists of P2X receptors overcomes T cell suppression induced by the anti-inflammatory cytokine IL-10. These findings indicate that Panx1 hemichannels facilitate ATP release in response to hypertonic stress and that P2X1, P2X4, and P2X7 receptor activation enhances T cell function. We conclude that HS and P2 receptor agonists promote T cell function and thus, could be used to improve T cell function in trauma patients.
Introduction
T cell suppression following trauma impairs cellular immune defenses, which can lead to posttraumatic infectious complications and sepsis, the leading causes of death in trauma patients [1–3]. To reduce the risk of such complications, different approaches that prevent or overcome posttraumatic T cell suppression have been explored [4]. Among the different strategies that have been considered is the restoration of T cell function with hypertonic fluids that modulate immune responses. Hypertonic saline (HS) and similar hyperosmotic fluids have been used successfully to resuscitate trauma patients [5, 6]. These fluids have a tonicity that is nearly 10 times higher than that of plasma. Infusion of such resuscitation fluids raises plasma osmolarity, causing an osmotic shift of extravascular fluid into the circulatory system, which rapidly restores blood volume and tissue perfusion after trauma [7, 8].
HS treatment enhances cell-mediated immune responses in vivo, and we found that HS resuscitation elicits immunomodulatory effects that decrease the risk of posttraumatic sepsis [9–11]. However, the exact mechanisms by which HS treatment increases T cell function remain unclear. In vitro studies have shown that HS treatment at physiologically relevant levels induces robust ATP release from Jurkat T cells and from primary human T cells [12], resulting in increased intracellular Ca2+ signaling, p38 MAPK activation, IL-2 production, and T cell proliferation [5, 13, 14]. In animal sepsis models, treatment with ATP has been shown to reduce organ damage, restore immune competence, and increase survival [15]. Under physiological conditions, cellular ATP can be released by T cells and other cell types during apoptosis or necrosis or be released from intact cells in a more controlled manner by vesicular release or through specific transporter and membrane channel proteins [16, 17]. In T cells, the gap junction hemichannel pannexin-1 (Panx1) has been proposed to mediate ATP release [18]. However, the role of Panx1 in HS-induced ATP release is not known.
Upon its release into the extracellular space, ATP can influence T cell activation processes by stimulating P2X receptors that are expressed on the cell surface [19]. The P2X receptor family consists of seven distinct members (P2X1–P2X7) that function as ATP-gated ion channels [18–20]. Jurkat T cells and primary human T cells express several of these P2X receptor subtypes [21]. With the exception of P2X5 [22], activation of P2X receptors induces Ca2+ influx and subsequent downstream cell signaling pathways [23]. In the present study, we investigated the mechanism by which HS treatment induces ATP release from T cells and P2X receptor subtypes that are involved in the up-regulation of T cell function by HS.
MATERIALS AND METHODS
Materials
ATP and ATPγS, NF023, GdCl3, TNP-ATP, and apyrase were purchased from Sigma-Aldrich (St. Louis, MO, USA). BzATP, A-438079, and 10Panx-1 were from Tocris Bioscience (Ellisville, MO, USA), and IL-10 was purchased from Calbiochem (San Diego, CA, USA).
Cells and cell stimulation
Jurkat cells (clone E6-1) were purchased from ATCC (Manassas, VA, USA) and maintained in RPMI 1640 (ATCC) supplemented with 10% heat-inactivated FCS (Atlanta Biologicals, Lawrenceville, GA, USA) and 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). For ATP release experiments, cells were resuspended in HBSS (Thermo Scientific, Agawam, MA, USA) at a concentration of 5 × 106/ml. Cell suspensions (250 μl) were incubated with the different inhibitors described below or with HBSS as a negative control. Cells were subjected to hypertonic stimulation by adding appropriate volumes of 1 M NaCl in HBSS to increase the tonicity of cell suspensions to a hypertonicity level of 40 mM beyond isotonicity (40 mM HS). All experiments were carried out at 37°C on a vibration isolation table to minimize mechanical stimulation. Cell viability after the different treatments was ≥97% as determined by trypan blue staining.
Quantification of ATP release by HPLC
ATP release from Jurkat T cells was assessed by HPLC. After cell stimulation with 40 mM HS for 1 min, samples were placed on ice and gently centrifuged (400 × g for 2 min), and supernatants were subjected to perchloric acid (Sigma-Aldrich) treatment to precipitate protein. Adenine compounds in the supernatants were subjected to the following procedure to generate fluorescent products: 150 μl culture supernatants or nucleotide standard solution was incubated at 72°C for 30 min with 25 mM Na2HPO4 and 1 M chloroacetaldehyde (Sigma-Aldrich) in a final reaction volume of 200 μl to induce the formation of 1,N6-etheno derivatives, as published previously [24]. Samples were placed on ice, alkalinized with 50 μl 0.5 M NH4HCO3, and analyzed using a Waters HPLC system (Waters, Milford, MA, USA) and a Waters 474 fluorescence detector as described previously [24, 25].
Assessment of gene expression
To determine IL-2 gene expression, cells were brought to a concentration of 5 × 105 cells/ml and pretreated with various antagonists or HBSS for 20 min. Cells were then stimulated with 40 mM HS and Dynabeads (Dynal Inc., Lake Success, NY, USA), coated with anti-CD3 and anti-CD28 antibodies (BD PharMingen, San Jose, CA, USA) as described previously [13], at a ratio of one bead/cell. Cells were harvested 4 h after stimulation, and RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). cDNA (20 μl) was synthesized using SuperScript® III RT (Invitrogen), according to the manufacturer′s protocol. For negative controls, first-strand cDNA synthesis was carried out in the absence of RT. IL-2 and β-actin gene expression were assessed by real-time PCR, as described previously [19, 25]. Primers used to quantify Panx1 mRNA expression were purchased from Qiagen. To determine changes in P2X receptor expression, cells were stimulated with 40 mM HS for 1, 4, and 20 h, and RNA was extracted and cDNA synthesized as described above. For positive controls, human brain cDNA was used as the template for real-time PCR (BioChain, Hayward, CA, USA). The primer sets and conditions used for real-time PCR have been published previously [25].
MAPK expression and activation
To determine MAPK activation, 2.5 × 105 cells in a volume of 500 μl were pretreated with various antagonists or HBSS for 20 min at 37°C on an isolation table. Cells were then stimulated with 40 mM HS and/or Dynabeads (Dynal Inc.), coated with anti-CD3 and anti-CD28 antibodies (BD PharMingen) as described previously [13], at a ratio of one bead/cell for 5 min. Samples were put on ice, centrifuged, lysed in 100 μl mammalian protein extraction reagent (Thermo Scientific) containing phosphatase inhibitor (Thermo Scientific) and protease inhibitor cocktail (Sigma-Aldrich), centrifuged, resuspended in 100 μl SDS buffer containing 50 μM DTT, and boiled for 5 min. Proteins were separated by SDS-PAGE using 12% Tris-glycine polyacrylamide gels (Invitrogen) and transferred to PVDF membranes (Millipore, Bedford, MA, USA). Immunoblotting was performed using antibodies that recognize the phosphorylated forms of p38 (Thr180/Tyr182) and ERK1/2 (Thr202/Tyr204) MAPK and antibodies recognizing active and inactive p38 and ERK (Cell Signaling Technology, Beverly, MA, USA), according to standard procedures. For densitometry analyses of Western blot bands, NIH Image J 1.34 s software was used.
Panx1 and P2X receptor silencing by siRNA
siRNA constructs targeting the gap junction hemichannel Panx1 (sense: 5′GCAUCAAAUCAGGGAUCCUUU3′; antisense: 5′AGGAUCCCUGAUUUGAUGCUU3′) or the ATP-gated ion channels P2X1 (sense: 5′GGCCGAGAACUUCACUCUUtt3′; antisense: 5′AAGAGUGAAGUUCUCGGCCtc3′), P2X4 (sense: 5′GUACUACAGAGACCUGGCUtt3′; antisense: 5′ AGCCAGGUCUCUGUAGUACtt3′), and P2X7 (sense: 5′GGUGAAAGAGGAGAUCGUGtt3′; antisense: 5′CACGAUCUCCUCUUUCACCtc3′) were purchased from Ambion (Austin, TX, USA), and a nonsense siRNA control was purchased from Qiagen. Jurkat cells were transfected with a NEON electroporation system (Invitrogen) using 100 μl cells at a concentration of 5 × 106 cells/ml in electroporation buffer and 400 nM Panx1 siRNA or 500 nM siRNA targeting P2X1, P2X4, or P2X7 mRNA. Electroporation was carried out using the following settings: 100 μl tip volume, 1700 V pulse voltage, 20 ms pulse width, one pulse. Electroporated cells were transferred to antibiotic-free medium containing 10% heat-inactivated FCS, and experiments were carried out 24 h or 48 h after transfection with Panx1 siRNA or P2X receptor siRNA, respectively. Successful siRNA knockdown was confirmed by real-time RT-PCR as described above. The silencing efficiency was tested using Alexa488-conjugated nonsense siRNA and found to be ≥93%.
Statistical analyses
Unless otherwise indicated, data are presented as mean ± sd. Sets of data were compared using the two-tailed unpaired Student's t tests, and P < 0.05 was considered significant.
RESULTS
HS induces ATP release through Panx1 hemichannels
We have shown previously that treatment of T cells and other leukocytes with HS induces rapid release of cellular ATP [13, 14, 26]. Using a microscopy technique established in our laboratory, we were able to visualize ATP release from living T cells [12]. We found that treatment of Jurkat T cells with 40 mM HS induces the release of ATP, resulting in extracellular ATP concentrations that reach up to 80 μM near the cell surface [12]. This release is accompanied by rapid T cell shrinkage within seconds after HS exposure (Fig. 1A and Supplemental Movie 1). We found that the released ATP diffuses away from the cell surface, thus allowing extracellular ATP to act not only in an autocrine manner on the cells that release ATP but also in a paracrine manner, influencing surrounding cells.
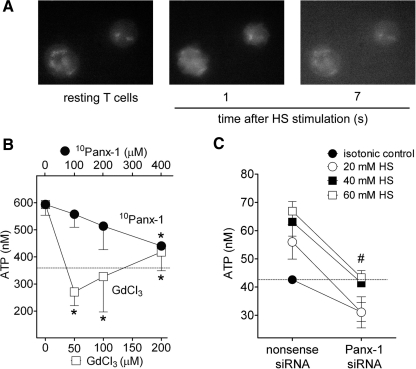
Figure 1. HS treatment induces ATP release from T cells mainly through Panx1.
(A; see Supplemental Movie 1) Jurkat T cells were exposed to HS (40 mM), and ATP release was assessed using a fluorescence microscope method. (B) Jurkat T cells were treated with increasing concentration of GdCl3 or the 10Panx-1 for 20 min and exposed to HS (40 mM), and ATP release was assessed by HPLC analysis of cell supernatants 1 min after HS exposure. *P < 0.05 compared with vehicle control. (C) ATP concentrations of Jurkat T cells treated with siRNA for Panx1 or with a nonsense control siRNA and exposed to different concentrations of HS. #P < 0.05 for all HS concentrations compared with nonsense siRNA controls. The dotted lines indicate ATP concentrations in the supernatant of control cells stimulated under isotonic conditions.
To determine the mechanism of ATP release in response to hypertonic stress, we pretreated Jurkat cells with different concentrations of the nonspecific, stretch-activated channel inhibitor GdCl3 or with the hemichannel-specific 10Panx-1. The cells were then stimulated with 40 mM HS for 1 min, and ATP release was assessed by measuring ATP concentrations in the cell supernatants using HPLC. The ATP concentrations in the cell supernatants of unstimulated cells were ∼350 nM, and HS-treated cells generated ATP concentrations in the bulk cell supernatant that were approximately twice as high, reaching an average of 600 nM (Fig. 1B). Pretreatment of cells with GdCl3 completely blocked HS-induced ATP release, and inhibition of Panx1 hemichannels with 10Panx-1 reduced ATP release in a concentration-dependent manner (Fig. 1B). To further examine the role of Panx1 in hypertonic stress-induced ATP release, we silenced Panx1 gene expression in Jurkat T cells. The cells were then exposed to 20, 40, or 60 mM HS or to isotonic control fluid. Silencing of Panx1 reduced ATP release significantly in response to stimulation with HS at all concentrations (Fig. 1C). These findings indicate that the Panx1 hemichannel is a key molecule that contributes to cellular ATP release in response to HS-induced mechanical perturbations of the cell membrane.
HS-induced p38 MAPK activation requires ATP release through Panx1
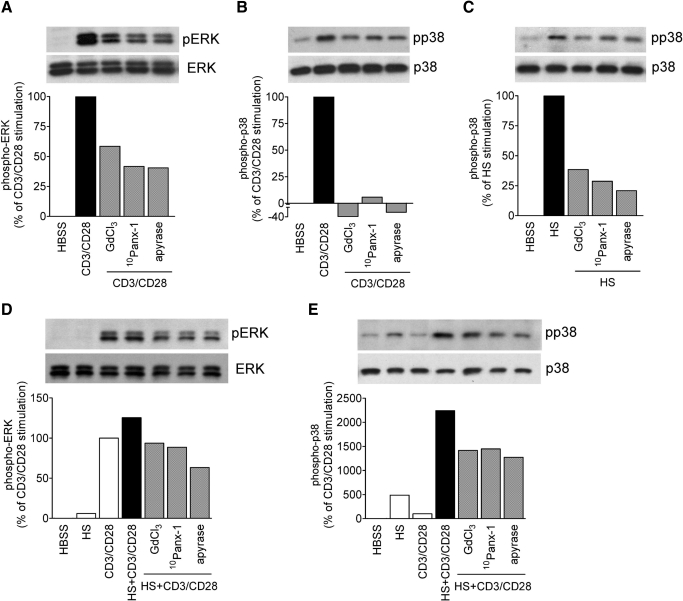
T cell activation involves tyrosine phosphorylation of MAPKs that leads to MAPK activation, IL-2 expression, T cell proliferation, and the initiation of the adaptive immune response [27]. Previously, we reported that HS treatment or addition of extracellular ATP induces p38 MAPK activation and IL-2 production in T cells [5, 13]. To assess the involvement of Panx1 and purinergic signaling in these processes, we examined how GdCl3, 10Panx-1, and apyrase, a scavenger of released ATP, affect MAPK activation and IL-2 gene transcription. CD3/CD28 stimulation of Jurkat T cells induces strong phosphorylation of the ERK-1 and ERK-2 MAPK members (Fig. 2A) but only modest phosphorylation of p38 MAPK (Fig. 2B). Conversely, treatment with HS (40 mM) induces strong phosphorylation of p38 MAPK but not of ERK1/2 (Fig. 2D and E). We found that CD3/CD28-induced stimulation of ERK1/2 and p38 MAPK can be reduced by inhibition of ATP release with GdCl3 or 10Panx-1 or by ATP hydrolysis with apyrase (Fig. 2A and B). Similarly, HS-induced stimulation of p38 MAPK was blunted in cells pretreated with GdCl3, 10Panx-1, or apyrase (Fig. 2C). CD3/CD28 stimulation, in addition to HS treatment, increased ERK activation only moderately compared with CD3/CD28 stimulation under isotonic conditions in the absence of HS (Fig. 2D). However, HS treatment markedly up-regulated phosphorylation of p38 MAPK of cells that were stimulated via CD3/CD28 (Fig. 2E). Pretreatment with GdCl3 or 10Panx-1 or removal of extracellular ATP with apyrase reduced p38 MAPK activation in HS and CD3/CD28-costimulated cells, suggesting that ATP release through Panx1 hemichannels is critical for the stimulatory effect of HS on p38 MAPK signaling.
Figure 2. ATP release and extracellular ATP facilitate HS-induced MAPK activation.
Jurkat T cells were stimulated for 5 min in the presence or absence of HS (40 mM) and with or without microbeads carrying anti-human CD3 and CD28 antibodies. ERK1/2 and p38 MAPK phosphorylation (p) was assessed in the absence or presence of the maxi-anion channel inhibitor GdCl3 (100 μM), 10Panx-1 (200 μM), or the ATP hydrolyzing enzyme apyrase (20 U/ml). Control cells were treated with isotonic culture medium.
Enhancement of IL-2 gene transcription by HS requires ATP release by Panx1
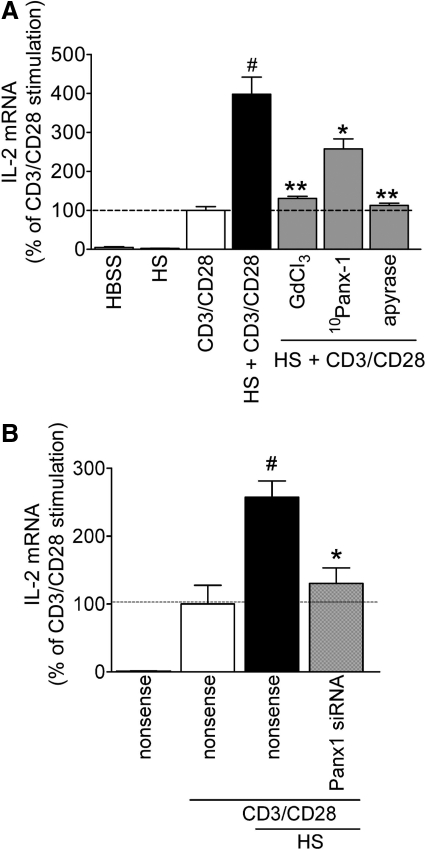
HS treatment of T cells increases IL-2 protein production by ATP release and p38 MAPK activation [5, 13]. Here, we found that stimulation of T cells via CD3/CD28 in the presence of 40 mM HS results in a four-fold increase in IL-2 mRNA expression (Fig. 3A). These increases were evident within 4 h of cell stimulation, which prompted us to assess IL-2 mRNA levels rather than IL-2 protein expression as a readout of early cell activation events. Pretreatment with GdCl3, 10Panx-1, or apyrase significantly reduces this enhancing effect of HS on IL-2 mRNA expression. To further assess the role of Panx1 in the stimulatory effect of HS on T cell activation, we silenced Panx1 gene expression in Jurkat cells. Knockdown of Panx1 expression completely abolishes the enhancement of IL-2 mRNA expression by HS, and transfection with nonsense siRNA had no discernible effect (Fig. 3B). Taken together, these findings demonstrate that Panx1 is required for HS-induced ATP release, subsequent stimulation of P2 receptors, and enhancement of T cell activation.
Figure 3. ATP release and extracellular ATP facilitate HS-induced IL-2 gene transcription.
(A) Jurkat T cells were pretreated with pharmacological inhibitors of ATP release or with the ATP scavenger apyrase, or (B) were transfected with 400 nM siRNA targeting pannexin-1 gene transcription or with nonsense siRNA. IL-2 mRNA levels were assessed 4 h after stimulation with anti-human CD3 and CD28 antibody-coated beads and 40 mM HS (#P<0.05 compared to CD3/CD28 stimulation; *P<0.05, **P<0.01 compared to HS + CD3/CD28; two-tailed unpaired Student's t tests; n=3).
HS treatment alters P2X receptor expression of T cells
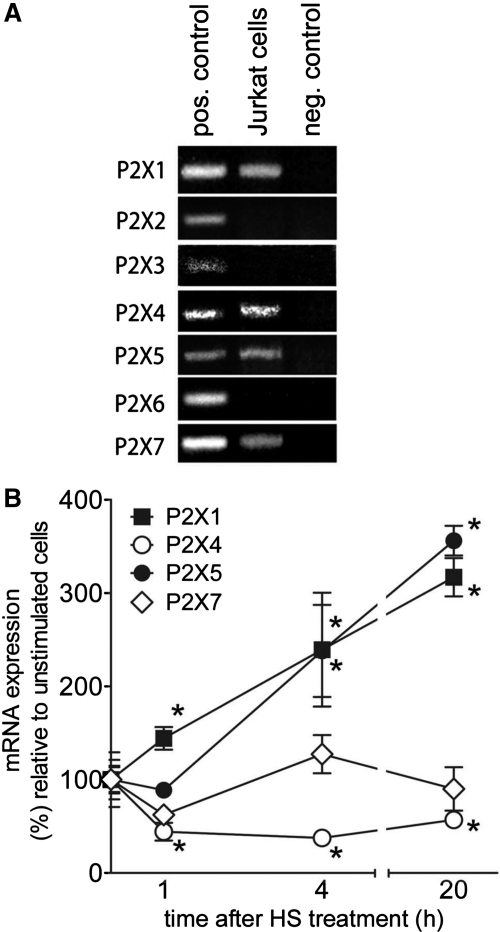
Previous work suggests that P2X7 receptors play a central role in T cell activation and in the enhancement of T cell function by HS [13, 18, 19]. However, the potential involvement of other members of the P2X receptor family in the immunomodulatory effect of HS on T cell function is unknown. Using RT-PCR analysis, we found that Jurkat cells, like primary CD4+ T cells [28], express P2X1, P2X4, and P2X5 receptors, in addition to P2X7 receptors (Fig. 4A). Treatment of Jurkat cells with 40 mM HS results in time-dependent changes in the expression of these receptors. HS significantly up-regulated gene transcription of P2X1 and P2X5 receptors and suppressed P2X4 mRNA levels by 50% within 1 h of HS treatment. Although P2X7 mRNA expression returned to baseline levels after an initial decline, P2X4 mRNA expression remained below baseline levels for at least 20 h after HS treatment (Fig. 4B). These changes imply a potential role of P2X1, P2X5, P2X7, and P2X4 in the response of T cells to hypertonic stress.
Figure 4. P2XR expression in T cells.
(A) P2X receptor expression in Jurkat cells was evaluated by RT-PCR analysis. Human brain cDNA was used as a template for positive controls, and cDNA synthesis in the absence of RT was used as a negative control. (B) Using real-time RT-PCR analysis, changes in P2X1, P2X4, P2X5, and P2X7 receptor expression were determined after stimulation of Jurkat cells with 40 mM HS for 0, 1, 4, and 20 h (*P<0.05 compared with unstimulated controls; two-tailed unpaired Student's t tests; n=3).
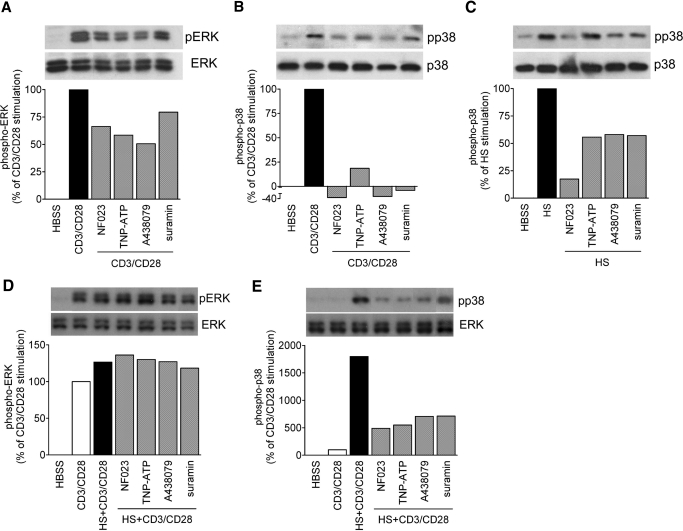
P2X1, P2X4, and P2X7 contribute to T cell activation by HS
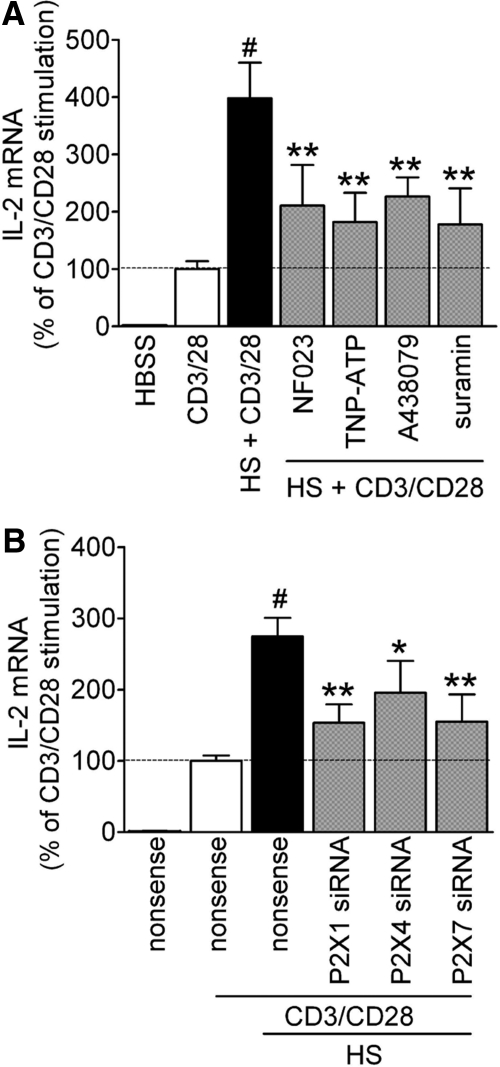
Our findings have shown that the release of ATP into the extracellular space and autocrine feedback via P2 receptors are crucial steps for the enhancement of T cell activation by HS. These events may trigger P2X receptor-mediated Ca2+ influx [13]. Therefore, we studied the role of the different P2X receptors in the response of T cell to HS. As P2X5 has weak Ca2+ channel activity compared with other P2X receptor family members [22], we focused the following studies on P2X1, P2X4, and P2X7 receptors. To examine how these different P2X receptor subtypes contribute to HS-induced enhancement of T cell activation, we used inhibitors of P2X1 (NF023), P2X1 and P2X4 (TNP-ATP), P2X7 (A-438079), and all P2 receptors combined (suramin). These antagonists inhibited ERK1/2 and p38 phosphorylation in response to CD3/CD28 stimulation (Fig. 5A and B). HS-induced p38 phosphorylation was also inhibited by P2X receptor antagonists (Fig. 5C); however, HS did not induce ERK1/2 phosphorylation (data not shown). Interestingly, none of the P2 receptor antagonists that we studied had any discernible effect on ERK activation in response to combined stimulation via CD3/CD28 and HS (Fig. 5D). However, the same antagonists strongly inhibited the enhancing effects of HS on p38 MAPK activation (Fig. 5E) as well as on IL-2 mRNA expression (Fig. 6A). To further examine which of these P2X receptor subtypes is required for the enhancing effects of HS, we silenced P2X1, P2X4, and P2X7 receptors using siRNA and found significant decreases in IL-2 mRNA transcription compared with controls treated with nonsense siRNA (Fig. 6B). Taken together, these findings show that P2X1 and P2X4, as well as P2X7 receptors, are necessary for HS-induced up-regulation of p38 MAPK activation and IL-2 transcription in T cells.
Figure 5. P2X receptors and p38 MAPK activation contribute to enhanced T cell activation by HS.
Jurkat T cells were stimulated for 5 min in the presence or absence of HS (40 mM) and with or without microbeads carrying anti-human CD3 and CD28 antibodies. ERK1/2 and p38 MAPK activation was assessed in the presence or absence of pharmacological inhibition of P2X1 (NF023; 10 μM), P2X1 and P2X4 (TNP-ATP; 30 μM), P2X7 (A-438079; 10 μM), or the general P2 receptor inhibitor suramin (200 μM). Control cells were incubated with isotonic culture medium.
Figure 6. P2X receptors contribute to enhanced IL-2 gene transcription in response to HS.
(A) IL-2 mRNA expression was assessed in the presence or absence of pharmacological inhibition of P2X1 (NF023; 10 μM), P2X1 and P2X4 (TNP-ATP; 30 μM), P2X7 (A-438079; 10 μM), or the general P2 receptor inhibitor suramin (200 μM). Control cells were incubated with isotonic culture medium. (B) Jurkat cells were treated with siRNA targeting P2X1, P2X4, or P2X7 receptors or with nonsense siRNA (500 nM siRNA), and IL-2 mRNA expression was determined (#P<0.05 compared with CD3/CD28 stimulation; *P<0.05 and **P<0.01 compared with HS+CD3/CD28 stimulation; two-tailed unpaired Student's t tests; n=3).
P2X receptor stimulation counteracts IL-10-mediated T cell suppression
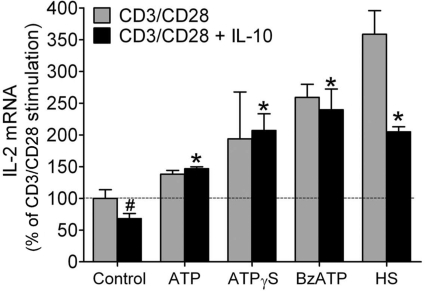
Anti-inflammatory cytokines such as IL-10 are involved in the suppression of T cell function in trauma patients, which leads to immunosuppression and sepsis [29, 30]. IL-10 levels are elevated in trauma patients [30, 31], and HS treatment can be used to overcome T cell suppression in response to IL-10 and other immunosuppressive mediators [5]. Our findings presented above indicate that autocrine stimulation of P2X receptors mediates the enhancing effects of HS. Thus, P2X receptor antagonists should elicit similar effects and may be used instead of HS to overcome T cell suppression. To test this hypothesis, we pretreated T cells with IL-10 at a concentration of 50 pg/ml and found that this causes a 25% suppression of IL-2 mRNA synthesis. Addition of exogenous ATP, the nonhydrolyzable ATP analog ATPγS, or the P2X1/P2X7-selective agonist BzATP significantly increased IL-2 mRNA expression. ATP and ATPγS were used at a concentration of 100 μM, which approximates ATP concentrations found near the cell surface of T cells in response to HS treatment [12]. BzATP was used at a final concentration of 10 μM, as this agonist is more potent than ATP in stimulating P2 receptors [32, 33]. Treatment with these agonists or with 40 mM HS significantly increased IL-2 mRNA transcription well beyond IL-2 mRNA transcription of control cells stimulated in the absence of IL-10 (Fig. 7). These findings indicate that P2 receptor agonists may be equally effective as HS in overcoming T cell suppression. Thus, P2 receptor agonists may be able to modulate T cell function, and these agents may be particularly beneficial to improve the function of T cells in immunosuppressed trauma patients.
Figure 7. P2 receptor agonists restore T cell function in the presence of IL-10.
Jurkat T cells were pretreated with IL-10 (50 pg/ml) for 1 h and then stimulated with microbeads carrying anti-human CD3/CD28 antibodies in the absence or presence of ATP (100 μM), the nonhydrolyzable ATP analog ATPγS (100 μM), the P2X1/P2X7 agonist BzATP (10 μM), or HS (40 mM), and IL-2 mRNA transcription was determined (#P<0.05 compared with CD3/CD28 stimulation; *P<0.05 compared with CD3/CD28 stimulation after IL-10 treatment; two-tailed unpaired Student's t tests; n=3).
DISCUSSION
Various animal models have shown that treatment with ATP can reduce organ damage, restore immune competence, and increase survival after hemorrhage, shock, and trauma [10]. It is thought that these beneficial effects are not only a result of the restoration of cellular ATP levels but that ATP additionally stimulates purinergic receptors that regulate immune cells and the functions of other cell types that are involved in disease progression. Similar to ATP infusion, protective effects are also seen upon HS treatment in numerous animal models of shock and trauma [6]. Hypertonic resuscitation fluids were found to reduce activation of caspase-3 and to prevent apoptosis of inflammatory cells, which could be an additional mechanism by which HS reverses immunosuppression [34, 35]. Aside from its beneficial effect in trauma patients, osmotic stimulation of T cells may be a more general physiological process that could control T cell function in specific organs such as those in the gastric and urinary tracts, where external osmolarity can reach levels of up to 1400 mmol/kg [36]. Moreover, immune organs such as the thymus and lymph nodes may provide specific osmotic environments that help regulate T cell responses [37]. We have shown that HS treatment induces the release of ATP from T cells and that this response increases T cell function [12, 13]. In this study, we show that Panx1 hemichannels are responsible for this HS-induced ATP release and that inhibition of Panx1 hemichannels or P2X receptors blunts the stimulatory effect of HS on T cell responses.
Cells can release ATP through multiple mechanisms that include physical cell damage, vesicular release, and ATP release through stretch-activated pores and specialized channels [16, 17]. Recently, Panx1 hemichannels have been implicated in the release of ATP from T cells [18, 38]. We found that inhibition of Panx1 hemichannels blocks ATP release, p38 MAPK activation, and IL-2 gene transcription in response to HS, implicating Panx1 hemichannels as a key player in the release of ATP by hypertonic stress. However, not all of the enhancing effects of HS were fully abrogated by blocking Panx1 hemichannels, which suggests that HS may also induce ATP release through additional mechanisms.
Extracellular ATP can activate P2X receptors that function as ATP-gated ion channels and promote T cell activation by facilitating Ca2+ influx [18–20]. We found that Jurkat T cells express P2X1, P2X4, P2X5, and P2X7 receptors. Although P2X1, P2X4, and P2X7 allow for substantial influx of Ca2+ from the extracellular space, P2X5 possesses only limited Ca2+ channel activity [20, 22]. In a previous study, we proposed that P2X7 receptors may be responsible for HS-induced enhancements of T cell activation [13]. However, here, we found that P2X1 and P2X4 receptors are also involved in the response of T cells to HS. These findings suggest that these three P2X receptor subtypes that recognize ATP with different affinities [20, 21] synergize in facilitating T cell responses to HS. The specific roles of these receptors may depend on extracellular ATP concentrations and the location of these receptors relative to Panx1 hemichannels, where ATP is released. Another possible level of synergistic interaction of the different receptor subtypes is through the formation of heteromeric receptor structures that are comprised of different P2X receptor subunits. Such P2X receptor heteromers have been observed in other cell types and may exhibit distinct receptor properties compared with their homomeric P2X receptor counterparts [20, 39, 40]. In addition, activation of P2YR subtypes and Ca2+ release from intracellular stores may contribute to T cell activation through ATP and its breakdown products.
The finding that P2X receptors play a central role in HS-induced T cell activation suggests that direct P2XR stimulation could be used as a tool to augment T cell function and to restore the function of suppressed T cells, for example, in immunosuppressed patients. IL-10 is an anti-inflammatory cytokine that promotes immunosuppression; however, the mechanisms of its inhibitory effect on T cell function are still not fully understood. IL-10 has been shown to inhibit T cell growth and IL-2 secretion [41], apparently by interfering with CD28-induced activation processes [42]. We found that IL-10 suppresses IL-2 gene transcription of Jurkat T cells in response to CD3/CD28 costimulation and that treatment with HS or with P2 receptor agonists restores IL-2 gene transcription in the presence of IL-10. Moreover, we found that these P2 receptor agonists are able to increase IL-2 transcription beyond the baseline level of control cells. Treatment with ATPγS, BzATP, or HS resulted in a stronger increase of IL-2 transcription compared with treatment with ATP, implying that hydrolysis of ATP by ectonucleoside triphosphate diphosphohydrolases, such as CD39 or related ectonucleotidases, may generate products that counteract the stimulatory effects of P2X receptors. A potential candidate is the hydrolytic ATP product adenosine, which stimulates A2 adenosine receptors and suppresses leukocyte and T cell function [14, 26].
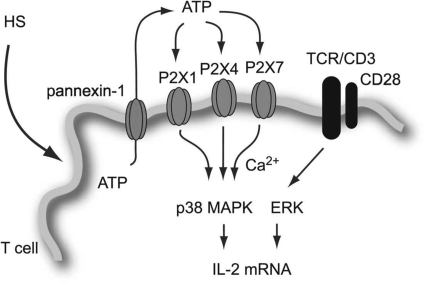
In summary, this study shows that the immunostimulatory effects of HS treatment are mediated by the controlled cellular release of ATP through Panx1 hemichannels and stimulation of P2X1, P2X4, and P2X7 receptors, which promote p38 MAPK activation and IL-2 gene transcription (Fig. 8). We conclude that direct targeting of these P2X receptors with agonists may provide a novel approach to overcome immunosuppression, for example, in trauma patients.
Figure 8. Proposed mechanism of T cell activation by hypertonic stress.
Hyperosmotic stress induces membrane deformation and ATP release through the gap junction hemichannel Panx1. The released ATP activates P2X1, P2X4, and P2X7 receptors, which facilitate an influx of extracellular Ca2+, phosphorylation of p38 MAPK, and activation of IL-2 production to promote T cell function.
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health grants R01 GM-51477, GM-60475, AI-072287, and R01 AI-080582 (W.G.J.) and DOD/CDMRP grant PR043034 (W.G.J.).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- A-438079
- 3-{[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl}pyridine hydrochloride
- ATCC
- American Type Culture Collection
- ATPγS
- adenosine 5′-O-(3-thio)triphosphate
- BzATP
- 3′-O-(4-benzoyl)benzoyl ATP
- Ca2+
- calcium ion
- GdCl3
- gadolinium trichloride
- HS
- hypertonic saline
- NF023
- 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulfonic acid
- Panx1
- pannexin-1
- 10Panx-1
- pannexin-1 mimetic inhibitory protein
- siRNA
- small interfering RNA
AUTHORSHIP
T.W. performed functional assays. L.Y. was responsible for P2X receptor expression assays. M.M., Y.S., Y.Y., and Y.C. performed functional assays and provided advice. W.G.J. provided overall direction and supervised project planning.
DISCLOSURE
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Lederer J. A., Rodrick M. L., Mannick J. A. (1999) The effects of injury on the adaptive immune response. Shock 11, 153–159 [DOI] [PubMed] [Google Scholar]
- 2.Oberholzer A., Oberholzer C., Moldawer L. L. (2001) Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16, 83–96 [DOI] [PubMed] [Google Scholar]
- 3.Ertel W., Kremer J. P., Kenney J., Steckholzer U., Jarrar D., Trentz O., Schildberg F. W. (1995) Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85, 1341–1347 [PubMed] [Google Scholar]
- 4.Miller A. C., Rashid R. M., Elamin E. M. (2007) The ″T″ in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J. Trauma 63, 1407–1417 [DOI] [PubMed] [Google Scholar]
- 5.Loomis W. H., Namiki S., Hoyt D. B., Junger W. G. (2001) Hypertonicity rescues T cells from suppression by trauma-induced anti-inflammatory mediators. Am. J. Physiol. Cell Physiol. 281, C840–C848 [DOI] [PubMed] [Google Scholar]
- 6.Shukla A., Hashiguchi N., Chen Y., Coimbra R., Hoyt D. B., Junger W. G. (2004) Osmotic regulation of cell function and possible clinical applications. Shock 21, 391–400 [DOI] [PubMed] [Google Scholar]
- 7.Brod V. I., Krausz M. M., Hirsh M., Adir Y., Bitterman H. (2006) Hemodynamic effects of combined treatment with oxygen and hypertonic saline in hemorrhagic shock. Crit. Care Med. 34, 2784–2791 [DOI] [PubMed] [Google Scholar]
- 8.Zakaria el R., Tsakadze N. L., Garrison R. N. (2006) Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery 140, 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junger W. G., Liu F. C., Loomis W. H., Hoyt D. B. (1994) Hypertonic saline enhances cellular immune function. Circ. Shock 42, 190–196 [PubMed] [Google Scholar]
- 10.Coimbra R., Hoyt D. B., Junger W. G., Angle N., Wolf P., Loomis W., Evers M. F. (1997) Hypertonic saline resuscitation decreases susceptibility to sepsis after hemorrhagic shock. J. Trauma 42, 602–606, discussion 606–607 [DOI] [PubMed] [Google Scholar]
- 11.Coimbra R., Junger W. G., Liu F. C., Loomis W. H., Hoyt D. B. (1995) Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)-induced T-cell suppression. Shock 4, 45–49 [DOI] [PubMed] [Google Scholar]
- 12.Corriden R., Insel P. A., Junger W. G. (2007) A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am. J. Physiol. Cell Physiol. 293, C1420–C1425 [DOI] [PubMed] [Google Scholar]
- 13.Loomis W. H., Namiki S., Ostrom R. S., Insel P. A., Junger W. G. (2003) Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J. Biol. Chem. 278, 4590–4596 [DOI] [PubMed] [Google Scholar]
- 14.Yip L., Cheung C. W., Corriden R., Chen Y., Insel P. A., Junger W. G. (2007) Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock 27, 242–250 [DOI] [PubMed] [Google Scholar]
- 15.Nalos M., Asfar P., Ichai C., Radermacher P., Leverve X. M., Froba G. (2003) Adenosine triphosphate-magnesium chloride: relevance for intensive care. Intensive Care Med. 29, 10–18 [DOI] [PubMed] [Google Scholar]
- 16.Praetorius H. A., Leipziger J. (2009) ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodin P., Burnstock G. (2001) Purinergic signaling: ATP release. Neurochem. Res. 26, 959–969 [DOI] [PubMed] [Google Scholar]
- 18.Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1, ra6. [DOI] [PubMed] [Google Scholar]
- 19.Yip L., Woehrle T., Corriden R., Hirsh M., Chen Y., Inoue Y., Ferrari V., Insel P. A., Junger W. G. (2009) Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 23, 1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 21.Bours M. J., Swennen E. L., Di Virgilio F., Cronstein B. N., Dagnelie P. C. (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 [DOI] [PubMed] [Google Scholar]
- 22.Bo X., Jiang L. H., Wilson H. L., Kim M., Burnstock G., Surprenant A., North R. A. (2003) Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 63, 1407–1416 [DOI] [PubMed] [Google Scholar]
- 23.Burnstock G. (2007) Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Shukla A., Namiki S., Insel P. A., Junger W. G. (2004) A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 76, 245–253 [DOI] [PubMed] [Google Scholar]
- 27.Crispin J. C., Tsokos G. C. (2009) Transcriptional regulation of IL-2 in health and autoimmunity. Autoimmun. Rev. 8, 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T cell activation at the immune synapse. Blood, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scumpia P. O., Moldawer L. L. (2005) Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit. Care Med. 33, S468–S471 [DOI] [PubMed] [Google Scholar]
- 30.Woehrle T., Du W., Goetz A., Hsu H. Y., Joos T. O., Weiss M., Bauer U., Brueckner U. B., Marion Schneider E. (2008) Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine 41, 322–329 [DOI] [PubMed] [Google Scholar]
- 31.Heizmann O., Koeller M., Muhr G., Oertli D., Schinkel C. (2008) Th1- and Th2-type cytokines in plasma after major trauma. J. Trauma 65, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 32.Michel A. D., Xing M., Humphrey P. P. (2001) Serum constituents can affect 2′-& 3′-O-(4-benzoylbenzoyl)-ATP potency at P2X(7) receptors. Br. J. Pharmacol. 132, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738 [DOI] [PubMed] [Google Scholar]
- 34.Chen L. W., Su M. T., Chen P. H., Liu W. C., Hsu C. M. (2010) Hypertonic saline enhances host defense and reduces apoptosis in burn mice by increasing Toll-like receptors. Shock, Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 35.Sharma P., Mongan P. D. (2010) Hypertonic sodium pyruvate solution is more effective than ringers ethyl pyruvate in the treatment of hemorrhagic shock. Shock 33, 532–540 [DOI] [PubMed] [Google Scholar]
- 36.Lentner C., Lentner C., Wink A. (1981) Geigy Scientific Tables, Vol. 1 Ciba-Geigy Corporation, West Caldwell, New Jersey, p. 53 [Google Scholar]
- 37.Ho S. N. (2006) Intracellular water homeostasis and the mammalian cellular osmotic stress response. J. Cell. Physiol. 206, 9–15 [DOI] [PubMed] [Google Scholar]
- 38.Pelegrin P., Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubyak G. R. (2007) Go it alone no more—P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol. Pharmacol. 72, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 40.Nicke A., Kerschensteiner D., Soto F. (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J. Neurochem. 92, 925–933 [DOI] [PubMed] [Google Scholar]
- 41.Taga K., Mostowski H., Tosato G. (1993) Human interleukin-10 can directly inhibit T-cell growth. Blood 81, 2964–2971 [PubMed] [Google Scholar]
- 42.Akdis C. A., Joss A., Akdis M., Faith A., Blaser K. (2000) A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 14, 1666–1668 [DOI] [PubMed] [Google Scholar]