Abstract
A discussion on paper by Park and Scott revealing the signaling pathways leading to the synthesis of a potent immunoregulatory oxysterol by macrophage and dendritic cells.
Keywords: oxysterols, bioactive lipid, phagocyte
25-Hydroxycholesterol is a metabolite of cholesterol that is produced and secreted by macrophages in response to TLR activation. This oxysterol has potent and wide-ranging effects in the immune system that include suppressing the production of IgA by B cells, directing the migration of activated B cells in the germinal follicle, and controlling the differentiation of monocytes into macrophages. In this issue, Park and Scott [1] report that DCs also respond to TLR ligands by increasing the expression of the cholesterol 25-hydroxylase gene, which encodes the enzyme that converts cholesterol to 25-hydroxycholesterol. They show that induction is mediated by type I IFNs through the IFNR and the JAK/STAT1 pathway (Fig. 1A) [1]. Their findings extend the 25-hydroxylase response to the DC and demonstrate that TLR ligands and cytokines induce this gene in phagocytes through well-characterized signaling pathways.
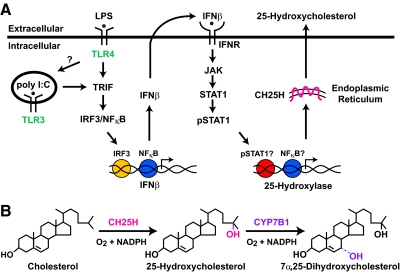
Figure 1. Signaling pathways in macrophages and DCs leading to the induction of cholesterol 25-hydroxylase and the reactions catalyzed by this enzyme.
(A) TLR ligands activate cell surface and intracellular receptors that signal through several adapters leading to the expression of IFN-β. The binding of this cytokine to the IFNR activates a transcription factor cascade, which in turn, induces the cholesterol 25-hydroxylase gene. The product of this gene is an enzyme of the ER that produces 25-hydroxycholesterol by the reaction shown in B. 25-Hydroxycholesterol is metabolized further to a 7α-hydroxylated sterol by the CYP7B1 oxysterol 7α-hydroxylase. Question marks denote steps in the signaling pathways that have not yet been confirmed by direct experimental evidence; these steps include the role of TLR4 in signaling from an intracellular compartment and the direct involvements of phosphorylated (p)STAT1 and NF-κB in expression of the cholesterol 25-hydroxylase gene.
The search for the physiological role of 25-hydroxycholesterol began in the mid-1970s when the oxysterol was shown to suppress cholesterol synthesis in cultured cells. Later, it was found that 25-hydrocholesterol interferes with the activation of transcription factors that regulate the expression of genes involved in fatty acid and cholesterol synthesis [2]. Based on these findings, the prediction was that loss of the oxysterol would alter lipid metabolism profoundly.
The synthesis of 25-hydroxycholesterol is catalyzed by the enzyme cholesterol 25-hydroxylase, which uses cholesterol and molecular oxygen as substrates and NADPH as a cofactor (Fig. 1B). The gene encoding cholesterol 25-hydroxylase (CH25H in humans; Ch25h in mice) is present in a majority of vertebrate species but absent from lower organisms such as yeast and flies [3]. Ch25h is expressed at low levels in the mouse lung, heart, and kidney but not in tissues active in fatty acid and cholesterol synthesis, such as the liver. These results suggest that the enzyme functions outside of lipid metabolism, and in support of this idea, 25-hydroxylase knockout mice regulate fatty acid and cholesterol metabolism normally (unpublished results).
A flurry of recent findings, including those of Park and Scott [1], reveals an unexpected role for the enzyme and its oxysterol product in the immune system. Initial studies with cultured macrophages showed that 25-hydroxylase gene expression is quiescent in resting cells but is rapidly induced hundreds-of-fold when cells are activated with various TLR ligands [4, 5]. Enhanced transcription of the gene is accompanied by large increases in 25-hydroxylase and secretion of 25-hydroxycholesterol into the medium. Treatment of mice with a selective TLR4 agonist activates Ch25h in tissues that are rich in macrophages (e.g., liver, lung, and brain), leading to marked increases in serum and tissue 25-hydroxycholesterol [5]. Similarly, injection of a TLR4 ligand (LPS) into human subjects produces a transient increase in serum 25-hydroxycholesterol [4].
Park and Scott [1] now report that Ch25h is also induced by TLR activation in cultured DCs. Ligands for the intracellular TLR3 (poly I:C) and the cell surface TLR4 (LPS) have the largest effect, and activation requires the presence of TRIF, a protein that mediates intracellular signaling by TLR3 and TLR4. Induction of 25-hydroxylase is impaired but not absent in cells lacking another adaptor in the TLR pathway, MyD88. The observation that TRIF is more active than MyD88 suggests that signaling by TLR4 from an intracellular compartment, as opposed to from the cell surface, is more important for activation of 25-hydroxylase in phagocytic cells [6].
Park and Scott [1] show further that the IFN-β gene is induced together with 25-hydroxylase by TLR3 and TLR4 ligands, which is consistent with TRIF being the dominant adaptor leading to the activation of the IFN-β gene [7]. Furthermore, experiments in knockout mice and with pharmacological inhibitors reveal that stimulation of 25-hydroxylase requires IFNR, JAK, and STAT1. These results, together with others [5], suggest that the signaling pathway leading to 25-hydroxylase gene activation in macrophages and DCs is minimally composed of TLR3/4 → TRIF → IRF3/NF-κB → IFN-β → IFNR → JAK/STAT1 → 25-hydroxylase (Fig. 1A).
We can conclude from the findings of Park and Scott [1] that induction of 25-hydroxylase by TLR ligands is a general response of phagocytes to infection. The immunological consequences of gene induction and secretion of 25-hydroxycholesterol are now being explored. Bauman et al. [5] show that 25-hydroxycholesterol is a potent (EC50≈65 nM) and selective suppressor of IgA production by B cells. In vitro experiments indicate that the oxysterol acts by at least two mechanisms: reducing proliferation of B cells in response to some cytokines (e.g., IL-2) but not others (e.g., TGF-β1 and IL-5) and suppressing expression of the activation-induced cytidine deaminase gene, the product of which is necessary for rearrangement of the Ig heavy chain gene to the IgA type. Observations in mice confirm that 25-hydroxycholesterol has similar effects in vivo [5]: 25-hydroxylase knockout mice, which do not synthesize the oxysterol in response to TLR activation, have supraphysiological levels of IgA in their sera, mucosa, and lungs. Conversely, mice that are deficient in the enzyme that metabolizes 25-hydroxycholesterol (the CYP7B1 oxy-sterol 7α-hydroxylase; Fig. 1B), which have abnormally high levels of the oxysterol in their sera, have low levels of IgA in these compartments.
What other effects does 25-hydroxycholesterol have in the adaptive immune system? In a recently awarded patent (WO 2010/066689 A2), Baumgarten et al. claim that the 7α-hydroxylated metabolite of the oxysterol (Fig. 1B) is a high-affinity ligand for the EBI2, a G protein-coupled receptor that is induced in B cells upon viral infection. In nonvirally infected cells, EBI2 directs the migration of antigen-stimulated B cells to different locations within the germinal follicles of the spleen and LN [8]. This movement is an integral part of the adaptive immune response to infection and when disrupted, leads to reductions in early antibody responses to T cell-dependent antigens. Taken together, these findings predict that mice and humans deficient in the enzyme that produces the 7α-hydroxylated metabolite of 25-hydroxycholesterol [9] will phenocopy EBI2-deficient mice.
Thus, 25-hydroxycholesterol and its metabolites have far-ranging effects in the adaptive immune system, but what about the innate immune system? Ecker et al. [10] show that 25-hydroxycholesterol suppresses the differentiation of monocytes into macrophages. This activity may represent a negative-feedback mechanism that would ultimately reduce the number of phagocytes capable of synthesizing 25-hydroxycholesterol.
To wrap up, the work of Park and Scott [1] contributes to a rapidly growing body of evidence that identifies 25-hydroxycholesterol as a potent bioactive lipid in the innate and adaptive immune systems. In this regard, the oxysterol and its metabolites join eicosanoids and sphingosine-1-phosphate as important modulators of the immune response to infection. The commercial availability of 25-hydroxycholesterol and of an antagonist of the actions of the oxysterol [11] should facilitate further studies that expand the role of these cholesterol derivatives in immunology.
ACKNOWLEDGMENTS
Research in the authors' laboratories is supported by grants from the National Institutes of Health (HL20948, GM69338, and DK81182) and by the Robert A. Welch Foundation (I-0971). We thank Helen Hobbs and Jay Horton for their review of this commentary.
SEE CORRESPONDING ARTICLE ON PAGE 1081
- EBI2
- EBV-induced gene 2
- IRF3
- IFN regulatory factor 3
- poly I:C
- polyinosinic:polycytidylic acid
- TRIF
- Toll/IL-1R domain-containing adaptor-inducing IFN-β
REFERENCES
- 1. Park K., Scott A. L. (2010) Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 88, XXX–XXX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown M. S., Goldstein J. L. (2009) Cholesterol feedback: from Schoenheimer′s bottle to Scap's MELADL. J. Lipid Res. 50, S15–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lund E. G., Kerr T. A., Sakai J., Li W-P., Russell D. W. (1998) cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273, 34316–34348 [DOI] [PubMed] [Google Scholar]
- 4. Diczfalusy U., Olofsson K. E., Carlsson A. M., Gong M., Golenbock D. T., Rooyackers O., Fläring U., Björkbacka H. (2009) Marked up-regulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50, 2258–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauman D. R., Bitmansour A. D., McDonald J. G., Thompson B. M., Russell D. W. (2009) 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA 106, 16764–16769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasius A. L., Beutler B. (2010) Intracellular Toll-like receptors. Immunity 32, 305–315 [DOI] [PubMed] [Google Scholar]
- 7. Honda K., Taniguchi T. (2006) IRFs: master regulators of signaling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 [DOI] [PubMed] [Google Scholar]
- 8. Pereira J. P., Kelly L. M., Cyster J. G. (2010) Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol. 22, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stiles A. R., McDonald J. G., Bauman D. R., Russell D. W. (2009) CYP7B1: one cytochrome P450, two human genetic diseases, and multiple physiological functions. J. Biol. Chem. 284, 28485–28489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ecker J., Liebisch G., Englmaier M., Grandl M., Robenek H., Schmitz G. (2010) Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 107, 7817–7822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janowski B. A., Shan B., Russell D. W. (2001) The hypercholesterolemic agent LY295427 reverses suppression of sterol regulatory element-binding protein processing mediated by oxysterols. J. Biol. Chem. 276, 45408–45416 [DOI] [PubMed] [Google Scholar]