Mechanism behind transient peripheral tolerance induced after T cell deletion is identified as dependent on the cytotoxic activity of TRAIL-expressing CD8+ Treg.
Keywords: apoptosis, immunosuppression, TCR-tg
Abstract
Peripheral tolerance controls the action of self-reactive T cells that escape thymic deletion. We showed previously that deletion of Ag-specific CD4+ T cells induced a CD8+ Treg population that maintained tolerance by deleting T cells with the same Ag specificity. The present study explored the mechanism of action of these CD8+ Treg. Following OT-II T cell deletion by soluble OVA323–339, B6 mice were unresponsive to challenge after CFA/OVA immunization, and Trail−/− or Dr5−/− mice were immune, although all strains displayed similar OT-II peripheral deletion. Interestingly, B6 mice remained tolerant to OVA even after a second infusion of OT-II T cells. Tolerance could be transferred to naïve recipients using CD8+ T cells from B6 or Dr5−/− mice that experienced peptide-induced peripheral OT-II deletion but not from Trail−/− mice. Subsequent investigation found that the mechanism of action of the CD8+ Treg was TRAIL-mediated OT-II T cell deletion in a TCR-specific manner. Furthermore, the tolerance was transient, as it was established by 14 days after peptide injection but lost by Day 56. Together, these data provide evidence to suggest that the mechanism behind transient peripheral tolerance induced following T cell deletion is the cytotoxic activity of TRAIL-expressing CD8+ Treg.
Introduction
The concept of immunological tolerance was first described almost 100 years ago [1], but it was the work of Gershon and Kondo [2] in the 1970s that showed that tolerance was mediated by T cells and could be transferred from tolerant individuals to naïve recipients (i.e., it is “infectious”). There has been a resurgence in the investigation of T cell-mediated immune regulation in recent years, and it is now clear that Treg play a critical role in the maintenance of immunological homeostasis [3]. Although a number of mechanisms have been identified by which Treg control the ability of the immune system to respond to challenge, it is also well-established that lymphocyte apoptosis is an important component in the induction and maintenance of tolerance [4].
For many years, apoptotic cells were believed to be a passive influence on the immune system, as they were rapidly cleared from the body by phagocytic cells. The realization that apoptotic cells are themselves tolerogenic and actually play a more “active” role in suppressing immunity has been key to our investigation of immune tolerance [5, 6]. Apoptosis of cells in organs such as the eye, pancreas, and small intestine is related directly to and required for suppressed immunity [6–8], and it has been proposed that this tolerogenic response is a mechanism to prevent autoimmunity [9]. Similarly, apoptosis of tumor cells can induce immune tolerance, preventing the generation of important antitumor immune responses [10]. Experimentally, the injection of soluble peptide Ag into mice containing TCR-tg T cells has provided evidence to suggest that apoptotic deletion is a mechanism to control specific populations of T cells [11–13]. Previous studies by our group have identified the generation of CD8+ Treg following soluble peptide-induced peripheral T cell deletion that is required for tolerance induction [13], and the induction of tolerance and generation of the CD8+ Treg were also found to be dependent on Fas/FasL-mediated apoptosis of the soluble, peptide-stimulated cells. Moreover, the CD8+ Treg inhibited the subsequent response of T cells sharing the same Ag specificity, and we proposed that this mechanism was in place to regulate immune responses to self that might be generated following deletion of a large number of cells containing potential autoantigens. The precise mechanism behind the functional activity of the CD8+ Treg cells, though, went undefined.
The induction of immune tolerance by apoptotic cells has been attributed to a number of mechanisms, including the production of immunosuppressive cytokines from phagocytic cells [14], production of inhibitors from the dead cell itself [15, 16], effects on the maturation of the DC [17, 18] deletion of reactive T cell clones, T cell anergy (clonal inactivation), induction of immune deviation (generation of Th2 T cells over Th1), and active regulation via Treg [6, 14, 19, 20]. In recent years, the pivotal role that CD4+ T cells play in the induction of CD8+ T cell responses has been highlighted [21–23], where most CD8+ T cell-mediated responses depend on concomitant CD4+ T cell priming to be effective. In addition, CD8+ T cell priming in the absence of CD4+ T cell help leads to their deletion from the periphery, an effect that can be overcome by supplying help during the initial priming phase [24]. The priming of CD8+ T cells in the absence of CD4+ T cell help also alters CD8+ T cell programming, which was only revealed after restimulation. Specifically, CD8+ T cells activated without CD4+ T cell help express TRAIL and undergo activation-induced cell death upon secondary Ag stimulation [23]. In the present study, we extended our previous line of investigation to test the hypothesis that soluble peptide-induced peripheral deletion of a large number of Ag-specific T cells generates a TRAIL-dependent regulatory mechanism that is required for subsequent immunological unresponsiveness. Our studies revealed peripheral deletion of adoptively transferred OT-II T cells after systemic delivery of soluble OVA323–339 induced a state of tolerance that was indeed mediated by TRAIL-expressing CD8+ Treg, which specifically deleted any potential Ag-reactive T cell clones in a TCR-dependent manner. Further, soluble peptide-induced peripheral deletion of large numbers of Ag-specific T cells only transiently regulated systemic immunity, perhaps to initially prevent any potential autoimmune responses, while allowing the system to eventually recover to deal with consequent pathogen challenges.
MATERIALS AND METHODS
Animals and reagents
B6 mice were purchased from the National Cancer Institute (Bethesda, MD, USA). Trail−/− [25] and Dr5−/− [26] B6 mice were obtained from Amgen (Seattle, WA, USA) and Dr. Wafik El-Deiry (University of Pennsylvania, Philadelphia, PA, USA), respectively. OT-I, OT-II, and SMARTA mice were obtained from Drs. Robert Cook, Yi Luo, and Steven Varga (University of Iowa, Iowa City, IA, USA), respectively. The Trail−/− and Dr5−/− mice were also bred with OT-II mice to create Trail−/− OT-II and Dr5−/− OT-II mice. All tg and knockout mice are on the H-2b background, permitting the adoptive transfer of cells among the different mice. Groups usually consisted of at least four mice, and experiments were repeated at least twice before reporting. All animal procedures were performed according to National Institutes of Health guidelines and approved by the University of Iowa Institutional Animal Care and Use Committee. Whole OVA, HEL, and CFA were purchased from Sigma-Aldrich (St. Louis, MO, USA). OVA323–339 was purchased from Biosynthesis Inc. (Lewisville, TX, USA).
Purification of T cells
OT-II T cells were isolated by negative selection using a CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA), per the manufacturer's instruction. Using this method, the purity of the CD4+ T cells was consistently >90%. For adoptive transfer experiments, spleens were isolated from OVA323–339-treated mice, and CD8+ or CD4+ T cells were purified by negative selection using a CD8+ or CD4+ T cell isolation kit (Miltenyi Biotec), per the manufacturer's instruction. Enriched CD8+ T cells, CD4+ T cells, or OT-II T cells were adoptively transferred i.v. via the retro-orbital plexus.
Peptide-induced peripheral deletion of OT-II T cells
One million (106) OT-II T cells were seeded into naive B6, Trail−/−, or Dr5−/− mice 24 h before injecting OVA323–339 (300 μg in PBS) i.p. To measure the expansion and contraction of the OT-II T cells, three mice were killed at various times after peptide injection, and the percentage of CD4+ Vα2+ Vβ5.1, 5.2+ OT-II T cells in the spleen was determined by flow cytometry.
Immune response to protein Ag
To generate an immune response to OVA or HEL, mice were immunized s.c. with 0.2 ml 100 μg OVA or HEL, emulsified 1:1 in CFA. After 7 days, mice were challenged in the right footpad with 100 μg heat-aggregated OVA or HEL in 0.033 ml PBS and in the left footpad with 0.033 ml PBS. Measurements were taken 24 h later using an engineer's micrometer. Values are expressed in micrometers (±se) and represent the difference between the right (Ag challenge) and the left (PBS challenge) footpad.
In vivo cytotoxicity assay
Purified OT-II T cells or normal CD4+ T cells were suspended at 5 × 106 cells/ml in PBS. CFSE (1 μl/ml of a 5-mM stock) was added to the OT-II T cells for the CFSEhigh population (test population), and a tenfold dilution was used for the CFSElow population (reference population). Cells were incubated for 10 min at room temperature, and the reaction was stopped by the addition of an equal volume of FBS. Cells were washed with PBS, counted, and resuspended to the appropriate volume. Five million target cells (CFSEhigh) and 5 million reference cells (CFSElow) were injected i.v. into naïve or tolerant recipients that had been immunized with CFA/OVA 7 days earlier. Spleens were harvested 18 h later and analyzed by flow cytometry. A total of 5000 events in the reference population was collected, and the number of target cells recovered was enumerated. Unimmunized B6 mice were used as controls. The percent reduction in the number of recovered CFSEhigh cells in the unimmunized versus the tolerant mice was considered the percent killing. Individual mice were analyzed, and the percent killing in each group of mice was determined.
Statistical analysis
Significant differences between groups were evaluated using a two-tailed Student's t test.
RESULTS
Peripheral deletion of CD4+ T cell induces tolerance in B6 mice but not Trail−/− or Dr5−/− mice
TCR-tg T cell-containing mice injected with soluble peptide Ag produce substantial T cell apoptosis that results in Ag-specific immune suppression [11–13]. As our previous studies—examining the impact of ex vivo-induced apoptotic cells (by γ-irradiation) administered i.v.—revealed that the subsequently induced immunosuppression was mediated by TRAIL-expressing CD8+ Treg [19], the purpose of the present set of experiments was to examine the extent to which tolerance—generated after soluble peptide-induced peripheral T cell deletion—was also dependent on a functioning TRAIL/DR5 pathway. Thus, B6, Trail−/−, and Dr5−/− mice were seeded with 106 WT, Trail−/−, or Dr5−/− OT-II T cells, respectively, and OVA323–339 was administered i.p. 24 h later. OVA323–339 injection led to the proliferation of >95% of the OT-II T cells within 3 days after injection (Supplemental Fig. 1). Mice were then immunized s.c. with whole OVA protein in CFA 28 days after receiving OVA323–339 and challenged with OVA 7 days later. The DTH response was measured after 24 h, and these data show that B6 mice were unable to respond to OVA challenge after OVA/CFA immunization, and Trail−/− (Fig. 1A) and Dr5−/− mice (Fig. 1B) displayed immunity.
Figure 1. OVA323–339-induced OT-II T cell peripheral deletion results in OVA-specific tolerance that is dependent on TRAIL, even when a second infusion of naïve OT-II T cells occurs.
(A and B) WT, Trail−/−, or Dr5−/− OT-II T cells (106) were adoptively transferred into B6, Trail−/−, or Dr5−/− recipients, respectively, and OVA323–339 (300 μg) was injected i.p. 24 h later. After 28 days, mice were immunized with CFA/OVA and then challenged with 33 μl 3 mg/ml OVA in the right footpad and PBS in the left footpad 7 days later. (C) B6 were seeded with 106 WT OT-II T cells and injected with OVA323–339, as in A. After 28 days, the B6 mice received a second infusion of 106 freshly isolated, naive WT or Dr5−/− OT-II T cells. On the same day as the second infusion, the mice were immunized s.c. with CFA/OVA. The mice were footpad-challenged with OVA 7 days later. (D) B6 mice were seeded with 106 WT OT-II T cells and injected with OVA323–339, as in A. After 28 days, the mice were immunized with CFA/OVA or CFA/HEL. Challenge with OVA or HEL in the footpad occurred 7 days later. X, CFA/OVA or CFA/HEL. (A–D) Measurements were taken 24 h after footpad challenge using an engineer's micrometer, and the difference between the right (Ag challenge) and left (PBS challenge) footpad showed the intensity of the DTH response. *P < 0.05 versus immune control group. All groups consisted of at least four mice, and data presented are representative of at least two independent experiments.
One possible explanation for the data in Fig. 1A and B was that the injection of soluble OVA323–339 led to the elimination of Ag-reactive cells in B6 mice but not in Trail−/− or Dr5−/− mice. We showed previously that the deletion of TCR-tg T cells stimulated in vivo with soluble peptide Ag was dependent on Fas/FasL, and the Fas/FasL-mediated death of the peptide-stimulated T cells was required for the subsequent regulation of systemic immunity [13]. Thus, when we examined the number of OT-II T cells in OVA323–339-injected B6, Trail−/−, and Dr5−/− mice, all three strains had similar OT-II expansion and contraction (Supplemental Fig. 2). These results suggest that TRAIL/DR5 interactions are not required for the deletion of the soluble peptide-stimulated T cells, but TRAIL/DR5 is needed at a different (efferent?) stage in the regulation of the systemic immunity in this model system. Based on these data, we then examined whether adding a second population of naïve OT-II T cells to the B6 mice that experienced OVA323–339-induced OT-II peripheral deletion would restore immunity. In this experiment, B6 mice that previously had OT-II T cells undergo OVA323–339-induced peripheral deletion were given a second infusion of naïve OT-II T cells on the same day as CFA/OVA immunization. These recipients were challenged after 7 days and the DTH response measured 24 h later. Surprisingly, these mice were still unable to mount a response to OVA, even after this second infusion of naïve OT-II cells (Fig. 1C). In contrast, B6 mice that received Dr5−/− OT-II T cells on the day of CFA/OVA immunization now displayed immunity to OVA. The tolerance observed in the soluble OVA323–339-treated B6 mice was also specific for the anti-OVA response, as immunization of tolerant B6 mice with CFA/HEL resulted in immunity after challenge (Fig. 1D). Thus, the data in Fig. 1 indicate that peptide-induced peripheral deletion of CD4+ T cells in vivo results in a state of tolerance that is TRAIL-dependent and directed toward the Ag to which the deleted T cells respond.
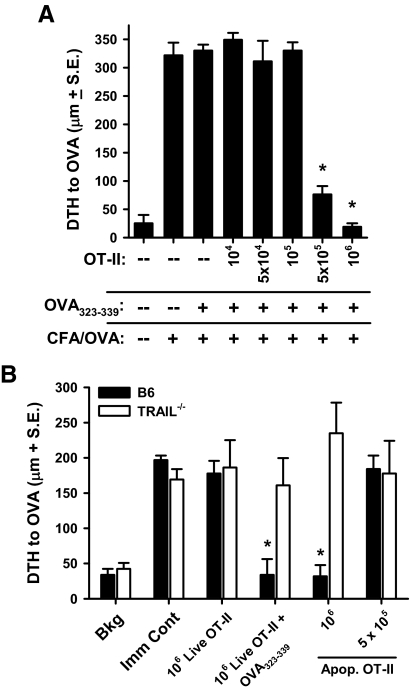
Immune tolerance after peptide-induced peripheral deletion is dependent on a threshold number of dead OT-II T cells
Deletion of Ag-specific cells occurs during the resolution of an immune response and is dependent on death receptor-induced apoptosis [27, 28] and Bcl-2 interacting mediator of cell death (Bim) expression [29, 30]. The endogenous cells undergoing apoptosis in these settings do not typically induce tolerance, as the immune system is not overwhelmed with a large number of apoptotic cells, and the activated apoptotic cells may be expressing CD154 [31]. This suggested to us that there may be a threshold to the number of apoptotic cells needed to trigger tolerance. Thus, we examined the minimum starting number of OT-II T cells required to induce tolerance by seeding B6 mice with varying numbers of OT-II T cells (ranging from 104 to 106) prior to OVA323–339 injection. This experiment revealed that the tolerance was indeed dependent on the initial number of OT-II T cells seeded into the recipient mice, as the transfer of 105 or fewer OT-II T cells failed to induce tolerance (Fig. 2A). Furthermore, the tolerance observed in the B6 mice was dependent on the presence of apoptotic OT-II T cells and not Ag-mediated (i.e., OVA323–339) activation, as direct infusion of 106 γ-irradiated (apoptotic) OT-II T cells induced tolerance (Fig. 2B). Similarly treated Trail−/− mice, in contrast, were not rendered tolerant to OVA after the injection of the apoptotic OT-II T cells. As in Fig. 2B, the tolerogenic effect of directly injecting apoptotic OT-II T cells i.v. was also dependent on the number of cells transferred, as the transfer of half as many apoptotic OT-II T cells was now unable to induce tolerance. Together, the data in Fig. 2 suggest that there needs to be a threshold amount of antigenic debris generated from the apoptotic OT-II T cells to induce TRAIL-dependent tolerance.
Figure 2. The number of OT-II T cells and apoptosis influences the induction of tolerance.
(A) Increasing numbers of WT OT-II T cells (none, 104, 5×104, 105, 5×105, and 106) were adoptively transferred into B6 mice 24 h before OVA323–339 injection. Mice were immunized with CFA/OVA 28 days later and challenged with 33 μl 3 mg/ml OVA in the right footpad and PBS in the left footpad 7 days later. (B) B6 or Trail−/− mice received the indicated number of live or irradiated (3000R) WT OT-II T cells i.v. In addition, some of the live WT OT-II T cell recipient mice were then injected with OVA323–339, as in A. After 28 days, all groups were immunized with CFA/OVA and then challenged in the footpad with OVA 7 days later. Bkg, background; Apop., apoptosis. (A and B) Measurements were taken after 24 h using an engineer's micrometer, and the difference between the right (Ag challenge) and left (PBS challenge) footpad showed the intensity of the DTH response. *P < 0.05 versus immune control (Imm Cont) group. All groups consisted of at least four mice, and data presented are representative of at least two independent experiments.
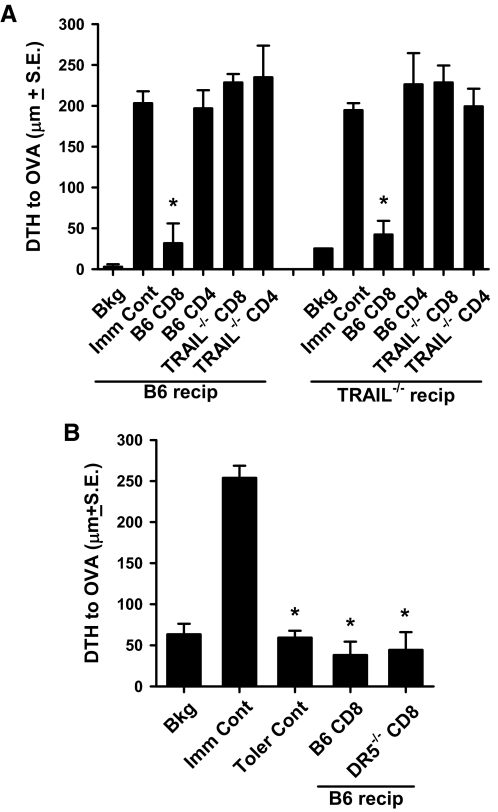
OVA323–339-induced OT-II T cell deletion generates TRAIL-expressing CD8+ T cells that transfer tolerance
As the tolerance in the present system is dependent on the apoptotic death of the OVA323–339-stimulated TCR-tg T cells [13], we tested the hypothesis that peptide-induced peripheral T cell deletion generates a TRAIL-expressing CD8+ Treg that mediates tolerance. Splenic CD4+ and CD8+ T cells were purified from B6 and Trail−/− mice that had previously endured OVA323–339-induced OT-II T cell peripheral deletion, and we then transferred these cells individually into naïve B6 or Trail−/− mice. The recipient mice were immunized immediately with CFA/OVA and challenged 7 days later. Only the CD8+ T cells from B6 mice could transfer tolerance (Fig. 3A), and the tolerance could be transferred to B6 and Trail−/− mice. Interestingly, Dr5−/− mice, which were themselves not tolerized by peptide-induced Dr5−/− OT-II T cell deletion (see Fig. 1B), did possess CD8+ Treg, whose functional importance was only revealed after transfer into DR5-expressing recipient mice (Fig. 3B). Together, these results suggest the key role of TRAIL-expressing CD8+ Treg in this experimental model of immune tolerance, but the DR5-expressing target remained undefined.
Figure 3. TRAIL-expressing CD8+ T cells transfer tolerance.
WT, Trail−/−, or Dr5−/− OT-II T cells (106) were adoptively transferred into B6 (A and B), Trail−/− (A), or Dr5−/− (B) recipients, respectively, and OVA323–339 (300 μg) was injected i.p. 24 h later. After 28 days, splenic CD8+ or CD4+ T cells were isolated and transferred to naïve B6 or Trail−/− recipients (recip), which were immunized immediately s.c. with CFA/OVA (100 μg). The mice were challenged with 33 μl 3 mg/ml OVA in the right footpad and PBS in the left footpad 7 days later. Measurements were taken after 24 h using an engineer's micrometer, and the difference between the right (Ag challenge) and left (PBS challenge) footpad showed the intensity of the DTH response. *P < 0.05 versus immune control group. All groups consisted of at least four mice, and data presented are representative of at least two independent experiments. Toler Cont, Tolerance control.
Tolerance is dependent on TCR-specific, TRAIL-mediated cellular cytotoxicity
TRAIL is a proapoptotic molecule that can induce apoptotic death in cells bearing a death-inducing TRAIL receptor [32] (DR5 in mice [33]). As tolerance in the present system is mediated by TRAIL (Fig. 1A), and tolerance was maintained even after the infusion of a second population of DR5-expressing OT-II T cells at the time of CFA/OVA immunization (Fig. 1C), we tested the hypothesis that the TRAIL-dependent tolerance was related to the cytotoxic elimination of OVA323–339-specific CD4+ T cells. B6, Trail−/−, or Dr5−/− mice were seeded with WT, Trail−/−, or Dr5−/− OT-II T cells, respectively, and then given OVA323–339. CFA/OVA immunization occurred on Day 28, and 7 days later, the mice were infused with a 1:1 ratio of syngeneic B6 CD4+ T cells (CFSElow reference cells) and Dr5+/+ or Dr5−/− OT-II T cells {CFSEhigh targets; note: naïve B6 [34] and OT-II cells express DR5 (Supplemental Fig. 3)}. After 18 h, the number of remaining OT-II T cells was quantitated with respect to the reference cells. Tolerant B6 mice showed significant deletion of Dr5+/+ OT-II T cell targets, and Trail−/− mice did not (Supplemental Fig. 3 and Fig. 4A). Interestingly, there was also significant lysis of Dr5+/+ OT-II target cells in Dr5−/− mice, which is consistent with the ability of Dr5−/− CD8+ T cells to transfer tolerance (see Fig. 3B). Depletion of CD8+ T cells from OT-II/OVA323–339-tolerized B6 mice prior to target cell injection confirmed that the cytotoxicity was mediated by CD8+ T cells (Fig. 4B). In addition, cytotoxicity in this system was specific for the OT-II TCR, as other CD4+ TCR-tg T cells (SMARTA) or OVA257-264-specific CD8+ (OT-I) T cells were not deleted in tolerant animals. Based on these data, we then predicted that there would be limited, if any, secondary OT-II T cell expansion in B6 mice after immunization with CFA/OVA following OVA323–339-induced OT-II T cell peripheral deletion. This was indeed the case (Fig. 4C). Conversely, CFA/OVA-induced secondary expansion of Trail−/− OT-II T cells did occur in Trail−/− mice previously injected with soluble OVA323–339. Thus, these data suggest that tolerance in this system is mediated by a CD8+ T cell that displays TRAIL-mediated cytotoxicity directed toward a specific TCR-bearing T cell.
Figure 4. Deletion of naïve OT-II T cells in “tolerized” B6 mice is dependent on TRAIL-mediated cytotoxicity.
(A) WT, Trail−/−, or Dr5−/− OT-II T cells (106) were adoptively transferred into B6, Trail−/−, or Dr5−/− recipients, and OVA323–339 (300 μg) was injected i.p. 24 h later. After 28 days, the mice were immunized s.c. with CFA/OVA (100 μg). After another 7 days, recipient mice received 5 × 106 B6 CD4+ T cells (reference cells: CFSElow) and 5 × 106 OT-II or Dr5−/− OT-II T cells (targets: CFSEhigh). Spleens were harvested 18 h later, and the relative number of labeled OT-II and CD4+ T cells was determined by flow cytometry. Cumulative data (from three independent experiments), normalized to the values obtained from naïve mice, are presented. (B) B6 mice were seeded with 106 WT OT-II T cells, injected with OVA323–339, and immunized with CFA/OVA as in A. Mice then received 5 × 106 B6 CD4+ T cells (reference cells: CFSElow) and 5 × 106 OT-II T cells, SMARTA CD4+ T cells, or OT-I T cells (targets: CFSEhigh) 7 days after immunization. In addition, some of the mice were depleted of CD8+ cells with three daily 100 μg doses of anti-CD8 mAb (2.43) starting on the day of CFA/OVA immunization. Cumulative data (from two independent experiments), normalized to the values obtained from naïve mice, are presented. (C) Secondary expansion of Trail−/− OT-II cells occurs in Trail−/− mice, but OT-II cells do not undergo secondary expansion in WT B6 mice. WT or Trail−/− OT-II T cells (106) were adoptively transferred into B6 or Trail−/− recipients, and OVA323–339 (300 μg) was injected i.p. 24 h later. After 28 days, the mice were immunized with OVA/CFA (0.2 ml 100 μg OVA emulsified 1:1 in CFA) s.c. At various times (3, 7, and 28 days) after OVA323–339 injection or OVA/CFA immunization (2, 5, and 7 days), mice (four mice/time-point) were killed and the percentage of OT-II cells determined in the spleen by flow cytometry. Data are representative of two independent experiments.
Tolerance induced by peripheral T cell deletion is transient
In all of the experiments described to this point, the experimental protocol called for CFA/OVA immunization 28 days after OVA323–339 injection. To examine the minimal amount of time needed between the induction of peptide-induced peripheral OT-II T cell deletion and immunization to achieve tolerance, as well as seeing how long the tolerance lasted, we immunized groups of mice with CFA/OVA as early as 7 days or as late as 56 days after OVA323–339 administration. The data show that mice immunized with CFA/OVA 7 days after OVA323–339 administration displayed strong immunity to OVA challenge, but there was a significant suppression of immunity (compared with the immune control group) in mice immunized 14–49 days after OVA323–339 administration, tolerant to OVA challenge (Fig. 5A). These results suggest that sufficient time must elapse after OVA323–339-induced OT-II T cell expansion and contraction for tolerance to be established. Furthermore, the data indicate that the tolerance is transient and wanes with time, as immunity (as measured by the intensity of the DTH response) had returned to the same magnitude as the immune control group in those mice immunized 56 days after OVA323–339 administration. We then compared the in vivo deletion of OT-II T cells when CFA/OVA immunization occurred 28 and 60 days after OVA323–339 injection. In concordance with the DTH data in Fig. 5A, we detected a significant decrease in the lysis of OT-II target cells in mice immunized at least 60 days post-OVA323–339 injection compared with the mice immunized 28 days after OVA323–339 administration (Fig. 5B). Thus, when considered in sum, the data provide evidence to suggest that transient peripheral tolerance induced after extensive in vivo peripheral T cell deletion induced by the systemic administration of soluble Ag is mediated by the cytotoxic activity of TRAIL-expressing CD8+ Treg.
Figure 5. OVA323–339-induced tolerance is transient.
(A) WT OT-II T cells (106) were adoptively transferred into B6 recipients, and OVA323–339 (300 μg) was injected i.p. 24 h later. The mice were then immunized with CFA/OVA on the indicated days after OVA323–339 injection. The mice were challenged with 33 μl 3 mg/ml OVA in the right footpad and PBS in the left footpad 7 days later. Measurements were taken after 24 h using an engineer's micrometer, and the difference between the right (Ag challenge) and left (PBS challenge) footpad showed the intensity of the DTH response. *P < 0.05 versus the immune control group. All groups consisted of at least four mice, and data presented are representative of at least two independent experiments. (B) B6 mice were seeded with 106 WT OT-II T cells and injected with OVA323–339, as in A. After 28 days or at least 60 days, the mice were immunized s.c. with CFA/OVA (100 μg). Recipient mice received 5 × 106 B6 CD4+ T cells (reference cells: CFSElow) and 5 × 106 OT-II T cells (targets: CFSEhigh) 7 days after immunization. Spleens were harvested 18 h later, and the relative numbers of OT-II and CD4+ T cells were determined by flow cytometry. Cumulative data (from three independent experiments), normalized to the values obtained from naïve mice, are presented.
DISCUSSION
The goal of thymic selection is to rid the body of thymocytes that express a TCR with high specificity for self-peptide:MHC complexes. Although central tolerance is efficient in shaping the eventual, mature T cell repertoire, some self-reactive T cells still make it through thymic selection. Numerous peripheral tolerance mechanisms are in place (such as anergy, deletion, and active suppression by Treg [11, 35]) to inhibit the activation of any circulating autoreactive T cells, as the immune system is continuously exposed to the remains of dead cells generated during normal cellular turnover. Recently published work from our laboratory using an i.v. tolerance model, where apoptotic splenocytes are administered directly i.v., described the importance of TRAIL-expressing CD8+ Treg in the maintenance of tolerance [19]. As relatively large numbers of i.v.-administered, ex vivo-induced apoptotic cells were required to induce tolerance in this system, we wondered whether the induction of substantial T cell apoptosis in vivo following Ag-induced expansion and contraction would also produce an active tolerogenic state mediated by TRAIL-expressing CD8+ Treg. It has been known for nearly 50 years that adjuvant-free Ag injected systemically (i.v. or i.p.) in a soluble form induces T cell unresponsiveness (or tolerance) instead of immunity [36–39]. In recent years, the use of TCR-tg T cells and their corresponding peptide Ag has provided some mechanistic insight into this phenomenon, largely as a result of the fact that it was possible to track the response of an Ag-specific monoclonal population of T cells. Depending on the study being done, the characterization of the TCR-tg T cells frequently required large numbers of these cells to be seeded into syngeneic mice. In addition, to induce tolerance in this system it was necessary to seed recipient mice with high numbers of these TCR-tg T cells. Thus, the experiments in this report were designed to investigate the mechanism of tolerance generated by apoptotic lymphocytes using a well-established model of soluble peptide-induced peripheral deletion of TCR-tg CD4+ T cells [11–13]. The data generated show that CD8+ T cells from B6 mice that experienced peptide-induced peripheral deletion mediate tolerance by deleting CD4+ T cells with the same Ag specificity in a TRAIL-dependent, TCR-specific manner. In addition, the tolerance established after injection of soluble peptide Ag was transient, with normal immunity returning by 8 weeks after peptide injection.
The role of CD8+ T cells in immune suppression was described more than 40 years ago by Battisto and Bloom [40]; however, the inability to phenotypically characterize CD8+ Treg or define their Ag specificity in subsequent years questioned the credibility of these studies. Work from our laboratory in recent years has addressed several issues related to immune regulation by CD8+ T cells. First, CD8+ Treg are induced when large numbers of apoptotic cells are presented to the immune system. We were only able to observe tolerance in the model system used in the present study when at least 5 × 105 OT-II T cells were seeded prior to OVA323–339 injection, suggesting that there needs to be a sufficient amount of apoptotic debris available during/after contraction to stimulate the generation of the CD8+ Treg population. We did not see the induction of tolerance in mice free of OT-II T cells following soluble peptide Ag injection (see Fig. 2A), and we were also unable to detect the expansion of any endogenous, OVA323–339-specific CD4+ T cells in these mice (data not shown). These data would suggest that soluble, peptide-induced tolerance may not be obtainable in a more physiologically relevant scenario, likely as the frequency of an endogenous T cell population specific for a particular Ag can be exceedingly low [41]. Importantly, the behavior of the adoptively transferred monoclonal population of adoptively transferred OT-II T cells after systemic peptide injection was similar to that described for polyclonal T cells expressing the relevant TCR Vβ chains after systemic injection of staphylococcal enterotoxin B [42, 43]. The activated T cells increase rapidly in number in the lymphoid tissues, and then, many of the cells die by Fas/FasL-dependent apoptosis [44, 45], leaving behind a functionally unresponsive pool of cells and the establishment of immunological tolerance [42, 46]. Similar events occur during sepsis, which results in active immunoregulation [47, 48], and we showed recently in an experimental model of sepsis that the ensuing immunological unresponsiveness was TRAIL-dependent [49]. Such tolerance mechanisms are likely not invoked during normal lymphocyte turnover, as they would certainly prevent immunity as well as any secondary responses.
One advantage of the soluble peptide/TCR-tg T cell tolerance model system used in the present study is that a defined Ag and monoclonal population of TCR-tg CD4+ T cells was used that allowed us to investigate the molecular and functional nature of the CD8+ Treg that mediate tolerance. It was also interesting to see that use of a defined soluble peptide Ag (OVA323–339 in our model) was able to tolerize animals to whole OVA protein. One would predict that the endogenous T cell response to OVA would be polyclonal and responsive to multiple epitopes within the whole OVA protein. However, OVA323–339 is the immunodominant, I-Ab-restricted epitope within OVA, and there are, on average, only 16 endogenous OVA323–339:I-Ab-specific CD4+ T cells in the spleen and LN of a mouse [41]. Further, the endogenous, OVA-responsive CD4+ T cells primarily express Vβ4, Vβ5, or Vβ8. Therefore, it is possible that a CD4+ T cell response to other potential MHC II-restricted epitopes within OVA would be too weak to detect. As a result of the restricted Vβ use, it is also possible that there is enough TCR sequence homology within the OVA-responsive polyclonal CD4+ T cell population that the TRAIL-expressing CD8+ Treg induced in our peripheral deletion model can eliminate the entire OVA-responsive polyclonal CD4+ T cell population. It is important to note that we are not the first to describe the phenomenon of peptide-induced peripheral deletion, inducing tolerance to whole proteins [50–53], but the data included herein are the first to show that a functional TRAIL/DR5 pathway must be in place for tolerance to be observed.
Second, immune unresponsiveness occurs in a TRAIL-dependent manner, which is used by CD8+ T cells to inhibit immunity by killing CD4+ T cells responding to the Ag associated with the induction of apoptosis. Our previous studies determined that Fas/FasL-mediated apoptosis of the OVA323–339-stimulated TCR-tg T cells was critical for DTH tolerance, as well as being required for the induction of the regulatory mechanism that deleted the second population of OVA323–339-specific T cells [13]. As there were similar OVA323–339-induced OT-II T cell expansion and contraction in B6, Trail-/, and Dr5−/− mice, it would appear that TRAIL has no apparent role in peripheral deletion (see Supplemental Fig. 2); however, TRAIL expression is necessary for the immune system to control immunity in the presence of large number of apoptotic cells. This is similar to what has been observed when CD8+ T cells are activated in the absence of CD4+ T cell help [23, 54]. In the present study, the CD8+ T cells make TRAIL, leading to cellular cytotoxicity and the establishment and maintenance of peripheral tolerance. Thus, the current report was able to build on our previous data and delineate the effector molecule used by the CD8+ T cells that regulate immunity (as measured by the lack of DTH and presence of in vivo deletion of a second population of naïve OT-II T cells). The data would then suggest that the tolerance established after soluble peptide-induced peripheral T cell deletion requires the cooperative induction of apoptosis by two distinct death receptor pathways at two different stages: Fas/FasL-dependent, peptide-induced peripheral deletion of the initial TCR-tg T cell population, followed by TRAIL/DR5-dependent CD8+ Treg function against any residual endogenous or exogenously supplied, Ag-specific, TCR-expressing T cells.
Peripheral tolerance to self-Ag is likely maintained because of the persistence of Ag, as several reports have shown the reversal of peripheral T cell tolerance in the absence of relevant Ag [55–58]. These facts are relevant to the third important finding from the data presented herein—that the tolerance was transient. Similar results were reported by Pape et al. [11] and more recently, in the report by Meiler et al. [55], detailing the seasonal tolerance to bee venom seen in bee workers. It is unknown, though, whether CD8+ Treg were involved in these two reports.
Fourth, regulation appears to be directed toward the TCR, preventing responses by T cells that share the same Ag specificity. As the apoptotic OT-II T cells are being cleared, it is presumed that the protein components of the dead/dying cells are cross-presented to CD8+ T cells [59]. Thus, the apoptotic OT-II T cells (generated by γ-irradiation or peptide-induced peripheral deletion) function as a T cell vaccine that stimulates tolerance via an anti-idiotype response. This theory is supported by studies performed over 20 years ago, where immunization with specific T cells or peptides corresponding to the TCR variable region led to dramatic immunoregulatory effects that prevented autoimmunity [60–63]. It is our hope that the data in this report will stimulate further interest in the underappreciated role of CD8+ Treg cells in the induction of tolerance. Future studies that identify and characterize these CD8+ Treg cells will be pivotal in designing therapeutic interventions for induction or abrogation of peripheral tolerance.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants AI 077565 (T.S.G.), CA109446 (T.S.G.), CA81261 (S.P.S.), EY06765 (T.A.F.), EY015570 (T.A.F.), and EY02687 (Department of Ophthalmology and Visual Science core grant, Washington University School of Medicine, St. Louis, MO, USA) and a University of Iowa Carver College of Medicine Medical Research initiative grant (Iowa City, IA, USA) to T.S.G. The authors thank Erik Brincks and Lyse Norian for helpful discussions.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- B6 mice
- C57BL/6 mice
- DR5
- mouse TRAIL-R
- FasL
- Fas ligand
- HEL
- hen egg lysozyme
- OVA323–339
- aa 323–339 (ISQAVHAAHAEINEAGR) of OVA
- Treg
- regulatory T cell(s)
- tg
- transgenic
AUTHORSHIP
The following authors designed and performed experiments: P.G., T.A.K., and T.S.G.; analyzed and interpreted data: P.G., T.A.F., and T.S.G.; provided vital reagents: S.P.S.; and wrote the manuscript: P.G., T.A.F., and T.S.G.
REFERENCES
- 1.Wells H. G. (1911) Studies on the chemistry of anaphylaxis. (III). Experiments with isolated proteins, especially those of the hen′s egg. J. Infect. Dis. 9, 147–171 [Google Scholar]
- 2.Gershon R. K., Kondo K. (1971) Infectious immunological tolerance. Immunology 21, 903–914 [PMC free article] [PubMed] [Google Scholar]
- 3.Wing K., Sakaguchi S. (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 [DOI] [PubMed] [Google Scholar]
- 4.Mueller D. L. (2010) Mechanisms maintaining peripheral tolerance. Nat. Immunol. 11, 21–27 [DOI] [PubMed] [Google Scholar]
- 5.Ferguson T. A., Stuart P. M., Herndon J. M., Griffith T. S. (2003) Apoptosis, tolerance, and regulatory T cells—old wine, new wineskins. Immunol. Rev. 193, 111–123 [DOI] [PubMed] [Google Scholar]
- 6.Griffith T. S., Yu X., Herndon J. M., Green D. R., Ferguson T. A. (1996) CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 5, 7–16 [DOI] [PubMed] [Google Scholar]
- 7.Hugues S., Mougneau E., Ferlin W., Jeske D., Hofman P., Homann D., Beaudoin L., Schrike C., Von Herrath M., Lehuen A., Glaichenhaus N. (2002) Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic β cells. Immunity 16, 169–181 [DOI] [PubMed] [Google Scholar]
- 8.Reynoso E. D., Elpek K. G., Francisco L., Bronson R., Bellemare-Pelletier A., Sharpe A. H., Freeman G. J., Turley S. J. (2009) Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol. 182, 2102–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green D. R., Ferguson T., Zitvogel L., Kroemer G. (2009) Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kepp O., Tesniere A., Schlemmer F., Michaud M., Senovilla L., Zitvogel L., Kroemer G. (2009) Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 14, 364–375 [DOI] [PubMed] [Google Scholar]
- 11.Pape K. A., Merica R., Mondino A., Khoruts A., Jenkins M. K. (1998) Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 160, 4719–4729 [PubMed] [Google Scholar]
- 12.Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. (1994) Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1, 327–339 [DOI] [PubMed] [Google Scholar]
- 13.Herndon J. M., Stuart P. M., Ferguson T. A. (2005) Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J. Immunol. 174, 4098–4104 [DOI] [PubMed] [Google Scholar]
- 14.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Herndon J. M., Zhang H., Griffith T. S., Ferguson T. A. (1998) Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J. Exp. Med. 188, 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., Frank M. E., Jin W., Wahl S. M. (2001) TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725 [DOI] [PubMed] [Google Scholar]
- 17.Steinman R. M., Turley S., Mellman I., Inaba K. (2000) The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191, 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert M. L., Jegathesan M., Darnell R. B. (2001) Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 19.Griffith T. S., Kazama H., VanOosten R. L., Earle J. K., Jr., Herndon J. M., Green D. R., Ferguson T. A. (2007) Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J. Immunol. 178, 2679–2687 [DOI] [PubMed] [Google Scholar]
- 20.Marrack P., Kappler J. (2004) Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 [DOI] [PubMed] [Google Scholar]
- 21.Bennett S. R., Carbone F. R., Karamalis F., Miller J. F., Heath W. R. (1997) Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenberger S. P., Toes R. E., van der Voort E. I., Offringa R., Melief C. J. (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483 [DOI] [PubMed] [Google Scholar]
- 23.Janssen E. M., Droin N. M., Lemmens E. E., Pinkoski M. J., Bensinger S. J., Ehst B. D., Griffith T. S., Green D. R., Schoenberger S. P. (2005) CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 [DOI] [PubMed] [Google Scholar]
- 24.Kurts C., Heath W. R., Carbone F. R., Allison J., Miller J. F., Kosaka H. (1996) Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedger L. M., Glaccum M. B., Schuh J. C., Kanaly S. T., Williamson E., Kayagaki N., Yun T., Smolak P., Le T., Goodwin R., Gliniak B. (2002) Characterization of the in vivo function of TNF-α-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 32, 2246–2254 [DOI] [PubMed] [Google Scholar]
- 26.Finnberg N., Gruber J. J., Fei P., Rudolph D., Bric A., Kim S. H., Burns T. F., Ajuha H., Page R., Wu G. S., Chen Y., McKenna W. G., Bernhard E., Lowe S., Mak T., El-Deiry W. S. (2005) DR5 knockout mice are compromised in radiation-induced apoptosis. Mol. Cell. Biol. 25, 2000–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L., Fisher G., Miller R. E., Peschon J., Lynch D. H., Lenardo M. J. (1995) Induction of apoptosis in mature T cells by tumor necrosis factor. Nature 377, 348–351 [DOI] [PubMed] [Google Scholar]
- 28.Lenardo M., Chan K. M., Hornung F., McFarland H., Siegel R., Wang J., Zheng L. (1999) Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17, 221–253 [DOI] [PubMed] [Google Scholar]
- 29.Hughes P., Bouillet P., Strasser A. (2006) Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases. Curr. Dir. Autoimmun. 9, 74–94 [DOI] [PubMed] [Google Scholar]
- 30.Strasser A., Puthalakath H., Bouillet P., Huang D. C., O′Connor L., O′Reilly L. A., Cullen L., Cory S., Adams J. M. (2000) The role of Bim, a proapoptotic BH3-only member of the Bcl-2 family in cell-death control. Ann. N. Y. Acad. Sci. 917, 541–548 [DOI] [PubMed] [Google Scholar]
- 31.Gurung P., Kucaba T. A., Ferguson T. A., Griffith T. S. (2009) Activation-induced CD154 expression abrogates tolerance induced by apoptotic cells. J. Immunol. 183, 6114–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffith T. S., Lynch D. H. (1998) TRAIL: a molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 10, 559–563 [DOI] [PubMed] [Google Scholar]
- 33.Wu G. S., Burns T. F., Zhan Y., Alnemri E. S., El-Deiry W. S. (1999) Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 59, 2770–2775 [PubMed] [Google Scholar]
- 34.Brincks E. L., Katewa A., Kucaba T. A., Griffith T. S., Legge K. L. (2008) CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 181, 4918–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas A. K., Lohr J., Knoechel B., Nagabhushanam V. (2004) T cell tolerance and autoimmunity. Autoimmun. Rev. 3, 471–475 [DOI] [PubMed] [Google Scholar]
- 36.Chiller J. M., Habicht G. S., Weigle W. O. (1971) Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science 171, 813–815 [DOI] [PubMed] [Google Scholar]
- 37.Dresser D. W. (1961) Effectiveness of lipid and lipidophilic substances as adjuvants. Nature 191, 1169–1171 [DOI] [PubMed] [Google Scholar]
- 38.Dresser D. W. (1962) Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology 5, 378–388 [PMC free article] [PubMed] [Google Scholar]
- 39.Dresser D. W. (1962) Specific inhibition of antibody production. I. Protein-over loading paralysis. Immunology 5, 161–168 [PMC free article] [PubMed] [Google Scholar]
- 40.Battisto J. R., Bloom B. R. (1966) Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature 212, 156–157 [DOI] [PubMed] [Google Scholar]
- 41.Moon J. J., Chu H. H., Pepper M., McSorley S. J., Jameson S. C., Kedl R. M., Jenkins M. K. (2007) Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawabe Y., Ochi A. (1991) Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature 349, 245–248 [DOI] [PubMed] [Google Scholar]
- 43.Webb S., Morris C., Sprent J. (1990) Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell 63, 1249–1256 [DOI] [PubMed] [Google Scholar]
- 44.Bonfoco E., Stuart P. M., Brunner T., Lin T., Griffith T. S., Gao Y., Nakajima H., Henkart P. A., Ferguson T. A., Green D. R. (1998) Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity 9, 711–720 [DOI] [PubMed] [Google Scholar]
- 45.Mogil R. J., Radvanyi L., Gonzalez-Quintial R., Miller R., Mills G., Theofilopoulos A. N., Green D. R. (1995) Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int. Immunol. 7, 1451–1458 [DOI] [PubMed] [Google Scholar]
- 46.Holly M., Lin Y. S., Rogers T. J. (1988) Induction of suppressor cells by staphylococcal enterotoxin B: identification of a suppressor cell circuit in the generation of suppressor-effector cells. Immunology 64, 643–648 [PMC free article] [PubMed] [Google Scholar]
- 47.Hotchkiss R. S., Karl I. E. (2003) The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 [DOI] [PubMed] [Google Scholar]
- 48.Ayala A., Chung C. S., Song G. Y., Chaudry I. H. (2001) IL-10 mediation of activation-induced TH1 cell apoptosis and lymphoid dysfunction in polymicrobial sepsis. Cytokine 14, 37–48 [DOI] [PubMed] [Google Scholar]
- 49.Unsinger J., Kazama H., McDonough J. S., Griffith T. S., Hotchkiss R. S., Ferguson T. A. (2010) Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J. Immunol. 184, 6766–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aichele P., Brduscha-Riem K., Oehen S., Odermatt B., Zinkernagel R. M., Hengartner H., Pircher H. (1997) Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity 6, 519–529 [DOI] [PubMed] [Google Scholar]
- 51.Aichele P., Brduscha-Riem K., Zinkernagel R. M., Hengartner H., Pircher H. (1995) T cell priming versus T cell tolerance induced by synthetic peptides. J. Exp. Med. 182, 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aichele P., Kyburz D., Ohashi P. S., Odermatt B., Zinkernagel R. M., Hengartner H., Pircher H. (1994) Peptide-induced T-cell tolerance to prevent autoimmune diabetes in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 91, 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kyburz D., Aichele P., Speiser D. E., Hengartner H., Zinkernagel R. M., Pircher H. (1993) T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23, 1956–1962 [DOI] [PubMed] [Google Scholar]
- 54.Kuerten S., Asaad R. J., Schoenberger S. P., Angelov D. N., Lehmann P. V., Tary-Lehmann M. (2008) The TRAIL of helpless CD8+ T cells in HIV infection. AIDS Res. Hum. Retroviruses 24, 1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meiler F., Zumkehr J., Klunker S., Ruckert B., Akdis C. A., Akdis M. (2008) In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 205, 2887–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migita K., Ochi A. (1993) The fate of anergic T cells in vivo. J. Immunol. 150, 763–770 [PubMed] [Google Scholar]
- 57.Ramsdell F., Fowlkes B. J. (1992) Maintenance of in vivo tolerance by persistence of antigen. Science 257, 1130–1134 [DOI] [PubMed] [Google Scholar]
- 58.Rocha B., Tanchot C., Von Boehmer H. (1993) Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J. Exp. Med. 177, 1517–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belz G. T., Shortman K., Bevan M. J., Heath W. R. (2005) CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 175, 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. (1989) Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science 246, 668–670 [DOI] [PubMed] [Google Scholar]
- 61.Lider O., Reshef T., Beraud E., Ben-Nun A., Cohen I. R. (1988) Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science 239, 181–183 [DOI] [PubMed] [Google Scholar]
- 62.Sun D., Qin Y., Chluba J., Epplen J. T., Wekerle H. (1988) Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T–T cell interactions. Nature 332, 843–845 [DOI] [PubMed] [Google Scholar]
- 63.Vandenbark A. A., Hashim G., Offner H. (1989) Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature 341, 541–544 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.