Abstract
Renal cytochrome P450 (CYP)-derived epoxyeicosatrienoic acids (EETs) regulate sodium transport and blood pressure. Although endothelial CYP-derived EETs are potent vasodilators, their contribution to the regulation of blood pressure remains unclear. Consequently, we developed transgenic mice with endothelial expression of the human CYP2J2 and CYP2C8 epoxygenases to increase endothelial EET biosynthesis. Compared to wild-type littermate controls, an attenuated afferent arteriole constrictor response to endothelin-1 and enhanced dilator response to acetylcholine was observed in CYP2J2 and CYP2C8 transgenic mice. CYP2J2 and CYP2C8 transgenic mice demonstrated modestly, but not significantly, lower mean arterial pressure under basal conditions compared to wild-type controls. However, mean arterial pressure was significantly lower in both CYP2J2 and CYP2C8 transgenic mice during coadministration of N-nitro-l-arginine methyl ester and indomethacin. In a separate experiment, a high-salt diet and subcutaneous angiotensin II was administered over 4 wk. The angiotensin/high-salt-induced increase in systolic blood pressure, proteinuria, and glomerular injury was significantly attenuated in CYP2J2 and CYP2C8 transgenic mice compared to wild-type controls. Collectively, these data demonstrate that increased endothelial CYP epoxygenase expression attenuates afferent arteriolar constrictor reactivity and hypertension-induced increases in blood pressure and renal injury in mice. We conclude that endothelial CYP epoxygenase function contributes to the regulation of blood pressure.—Lee, C. R., Imig, J. D., Edin, M. E., Foley, J., DeGraff, L. M., Bradbury, J. A., Graves, J. P., Lih, F. B., Clark, J., Myers, P., Perrow, A. L., Lepp, A. N., Kannon, M. A., Ronnekleiv, O. K., Alkayed, N. J., Falck, J. R., Tomer, K. B., Zeldin, D. C. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice.
Keywords: CYP2J2, CYP2C8, epoxygenase, EETs, transgenic
Cytochrome P450 (CYP) epoxygenase enzymes from the CYP2J and CYP2C subfamilies catalyze the oxidative metabolism of arachidonic acid to epoxyeicosatrienoic acids (EETs), which regulate various biological processes (1, 2). It is well-established that EETs are synthesized in the endothelium and possess potent vasodilatory effects in numerous vascular beds, including coronary and renal afferent arterioles, which are mediated via activation of calcium-sensitive potassium channels (BKCa) and smooth muscle cell hyperpolarization (3–6). Consequently, CYP-derived EETs are regarded as one of the primary endothelium-derived hyperpolarizing factors (EDHFs) (7). In addition, EETs contribute to the regulation of natriuresis through inhibition of sodium reabsorption in the proximal tubule and sodium and water reabsorption in the collecting duct (2, 8). Soluble epoxide hydrolase (sEH, Ephx2) rapidly hydrolyzes EETs to their corresponding dihydroxyeicosatrienoic acid (DHET) metabolites (9). Pharmacological inhibition of sEH and targeted disruption of Ephx2 increases circulating EET levels, lowers blood pressure, and attenuates renal injury in various rodent models of hypertension, which demonstrates the integral role of sEH in blood pressure regulation (10–14).
Renal CYP2J and CYP2C expression and EET biosynthesis are altered in various models of hypertension (8). Pharmacological inhibition of renal CYP epoxygenase function significantly reduced urinary sodium excretion and increased blood pressure in rats fed a high-salt diet (15). Mice with targeted disruption of Cyp2j5, a metabolically active and abundant renal CYP epoxygenase, demonstrated enhanced afferent arteriole responses to vasoconstrictors, enhanced proximal tubule transport rates, and higher blood pressure under basal conditions (16). Similarly, mice with targeted disruption of the CYP ω-hydroxylase Cyp4a10 demonstrated salt-sensitive hypertension secondary to increased renal epithelial sodium channel (ENaC) activity and sodium reabsorption (17). Interestingly, Cyp4a10−/− mice exhibited a reduction in renal CYP epoxygenase metabolic activity, and their hypertensive phenotype was rescued by pharmacological induction of renal Cyp2c44 expression and EET biosynthesis (17). Importantly, these investigations have implicated renal CYP epoxygenase function in the regulation of blood pressure. However, the impact of endothelial CYP epoxygenase function on blood pressure regulation and the pathogenesis of hypertension remain unclear. Consequently, we developed transgenic (Tr) mice with endothelial expression of the primary CYP epoxygenases responsible for EET biosynthesis in humans, CYP2J2 and CYP2C8 (18, 19), and characterized afferent arteriolar responses to vasoactive substances, blood pressure under basal and hypertensive conditions, and hypertension-induced renal injury.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma (St. Louis, MO, USA) unless otherwise noted.
Generation of Tie2-CYP2J2 and Tie2-CYP2C8 Tr mice
Tr mice, which express either human CYP2J2 or CYP2C8 in endothelial cells, were developed on a C57BL/6 background. The human CYP2J2 (GenBank accession number NM_000775) and CYP2C8 (GenBank NM_000770) cDNA sequences were subcloned downstream of the murine Tie2 promoter and upstream of the Tie2 full enhancer sequences (generously provided by Dr. Tom Sato, University of Texas Southwestern Medical Center, Dallas, TX, USA) to drive endothelial expression (Fig. 1A) (20).
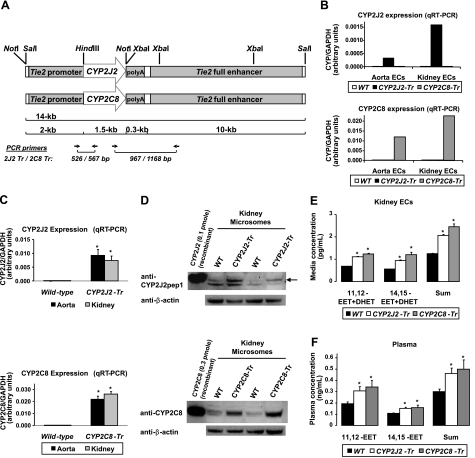
Figure 1.
Development and initial characterization of Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice. A) Diagram of the Tr constructs. B, C) Expression of human CYP2J2 and CYP2C8 mRNA by quantitative RT-PCR in primary aortic and renal endothelial cells (B; n=1 endothelial cell pool/genotype group) and aorta and kidney tissue homogenates (C; n=11–12 mice/group) is significantly higher in Tr mice compared to wild-type (WT) littermates. *P < 0.001 vs. WT. D) Representative immunoblot of microsomal fractions isolated from whole-kidney homogenates from Tie2-CYP2J2-Tr (lines 0–8), Tie2-CYP2C8-Tr (lines 0–7), and WT littermates demonstrate higher CYP2J2 and CYP2C8 protein expression in Tr mice compared to WT littermates. Recombinant CYP2J2 and CYP2C8 protein are included as positive controls. E, F) Primary renal endothelial cells were isolated from Tie2-CYP2J2-Tr (lines 0–8), Tie2-CYP2C8-Tr (lines 0–7), and WT littermates (n=3 isolations/group) and stimulated with A23187. Concentrations of 11,12- and 14,15-EET and DHET (stable EET metabolite), and the regioisomer sum total, are significantly higher in Tr mice in primary endothelial cell media (E) and plasma (F) compared to WT littermates (n=5–6/group). *P < 0.05 vs. WT.
The full-length human CYP2J2 (1.8 kb) and CYP2C8 (1.8 kb) cDNA sequences were previously subcloned into the pBluescript SK (+) vector (Stratagene, La Jolla, CA, USA) by RT-PCR (18, 19). The CYP2J2 and CYP2C8 cDNA sequences were PCR amplified with specific forward (CYP2J2: 5′-CGCTCTAGAACTAGTGGATC-3′; CYP2C8: 5′-AAGAAGAGAAGGCTTCAATGG-3′) and reverse (CYP2J2: 5′-TCTTCTTGCTTTCCTTGCCC-3′; CYP2C8: 5′-AGGTGATAGCAGATCAGCAG-3′) primers, and then subcloned into the intermediate pCR2.1-TOPO vector using the TOPO TA Cloning system (Invitrogen, Carlsbad, CA, USA) per the manufacturer's instructions. The CYP2J2 and CYP2C8 cDNA sequences were then PCR amplified with specific forward primers to add a 5′-HindIII site (CYP2J2: 5′-GTTACTAAGCTTGGAGCCATGCTCGCGGCG-3; CYP2C8: 5′-GTCAGCAAGCTTAGAGGCTTCAATGGAACC-3′), and a common reverse primer specific for the pCR2.1-TOPO vector (5′-GAATTGTAATACGACTCACTATAGGGCCA-3′). The italicized and bold nucleotides in each primer identify the restriction enzyme sites and translational start site, respectively. Each cDNA (∼1.6 kb) was restriction enzyme digested with HindIII and NotI, and subcloned into the HindIII/NotI sites of the pSPTg.T2FpAXK (#52) vector (generously provided by Dr. Tom Sato) downstream of the Tie2 promoter (2.1 kb) (20). The Tie2 promoter-CYP2J2/2C8 cDNA fragment was then subcloned upstream of the SV40 poly(A) (0.3 kb) and Tie2 full enhancer (10 kb) sequences, which drive constitutive endothelial expression by the Tie2 promoter (20). First, the poly(A) and Tie2 full enhancer were excised from the pT2HLacZp1I.7 (#59) vector (generously provided by Dr. Tom Sato) via a complete SalI digestion and subsequent partial XbaI digestion, in order to obtain the poly(A)-Tie2 full enhancer sequence (10.3 kb) with 5′-XbaI and 3′-SalI restriction enzyme sites, and then subcloned into the XbaI/SalI sites of the pBluescript SK (+) vector (Stratagene). Second, the Tie2 promoter-CYP2J2/2C8 cDNA (3.7 kb) fragments were PCR amplified with a common forward primer that added a 5′-NotI site to the Tie2 promoter (5′-GTTACTCAGCGGCCGCGTCGACACTAGCTTACTAAGA-3′) and a common reverse primer downstream of the CYP2J2/2C8 cDNA (5′-CTCTATCCCTGCCCTCCTGCCTTGTTTCCC-3′). The amplified products were then restriction enzyme digested with NotI, treated with calf intestinal alkaline phosphatase to prevent self-ligation, and subcloned into the NotI sites of the pBluescript SK (+) vector immediately upstream of the poly(A)-Tie2 full enhancer. The presence and orientation of the Tie2 promoter-CYP2J2/2C8 cDNA-poly(A)-Tie2 full enhancer (14 kb) constructs were confirmed by direct sequencing and restriction enzyme digestion with NotI and SalI.
Linearized constructs were agarose gel-purified, microinjected into pronuclei of single cell C57BL/6J mouse embryos, and implanted into pseudopregnant mice. Hemizygous founder pups were identified via PCR genotyping of genomic DNA, as described below. Breeding involved crossing hemizygous Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr founder mice with C57BL/6 wild-type mice in order to obtain heterozygous Tr (+/−) and wild-type littermates (−/−). All studies used adult F2- to F7-generation Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice from multiple founder lines and wild-type littermates as controls; all procedures were completed in accordance with the U.S. National Institutes of Health NIH Guide for the Care and Use of Laboratory Animals and approved by the animal care and use committees at the respective institutions.
PCR genotyping
Genomic DNA isolated from mouse tails was genotyped by PCR, using 2 primer pair sets for each construct (Fig. 1A and Supplemental Fig. S1). The first amplified a fragment extending from the 3′ end of the Tie2 promoter into the 5′ end of each cDNA, using a common forward primer (Tie2 promoter: 5′-GTCCTCATCGCATACCATAC-3′) and a reverse primer specific for each cDNA (CYP2J2: 5′-CGACAGTGCCCAGTAGGAGA-3′; CYP2C8: 5′-AATAGGAAGAGGAGTGGGGC-3′), yielding 526- and 567-bp products for the Tie2-CYP2J2 and Tie2-CYP2C8 constructs, respectively. The second amplified a fragment extending from the 3′ end of each cDNA into the 5′ end of the Tie2 enhancer, using a forward primer specific for each cDNA (CYP2J2: 5′-CATGCCCTACACCAATGCTG-3′; CYP2C8: 5′-AACTTGGTTGGCACTGTAGC-3′) and a common reverse primer (Tie2 enhancer: 5′-GGTGCCGCTGGAATCTGAACT-3′), yielding 967- and 1158-bp products, respectively. Amplification reactions were performed in a 50-μl reaction volume with 200 ng genomic DNA, 0.4 μM primer, 0.2 mM dNTPs, 10× Taq polymerase reaction buffer (5 μl), and 2.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA), and was completed as follows: 15 min at 94°C (1 cycle); 30 s at 94°C, 60 s at 66°C (Tie2 promoter primer pair) or 68°C (Tie2 enhancer primer pair), and 90 s at 72°C (30 cycles); and 15 min at 72°C (1 cycle). PCR products were separated on a 1% agarose gel.
Primary endothelial cell isolation
Aorta and kidneys were harvested for isolation of primary endothelial cells, as described (21). Briefly, 3 aortas and 6 kidneys(pooled) from each genotype group were minced and digested with fresh type-I collagenase (2 mg/ml, Worthington Biochemichal Corporation, Lakewood, NJ, USA) at 37°C for 45 min. Digested homogenates were then triturated via repeated passage through a 30-ml syringe, filtered through a 70-μm mesh cell strainer, and washed by centrifugation in PBS with 0.1% FBS. Cell pellets were resuspended and incubated with magnetic Dynabeads (Invitrogen) coated with an anti-PECAM Ab (BD Biosciences, San Jose, CA, USA). Endothelial cells were isolated by magnetic separation (repeat 5–7 times), and plated onto 0.1% gelatin flasks in DMEM containing 20% FBS, 100 μg/ml heparin, 100 μg/ml penicillin/streptomycin, and 100 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA, USA). After ∼5–9 d, confluent monolayers were trypsinized. Endothelial cells were further purified by incubation with anti-ICAM (BD Biosciences) coated Dynabeads, grown to confluence, washed, and then plated on 60 mm tissue culture dishes in serum-free medium with a Ca2+ ionophore (A23187, 10 μM×15 min) to stimulate arachidonic acid release. Cells and medium were harvested and stored at −80°C.
Real-time RT-PCR
Total RNA was isolated from lysed primary aortic and renal endothelial cells (∼1×106 cells/isolation) and whole aorta and kidney homogenates using RNeasy kits (Qiagen, Valencia, CA, USA) per the manufacturer's instructions.
Total RNA was reverse transcribed to cDNA using the ABI High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) for 10 min at 25°C, and then 120 min at 37°C. Expression of human CYP2J2 (Hs00356031_m1), human CYP2C8 (Hs00426387_m1), murine EPHX2 (Mm01313813_m1), and murine GAPDH (Mm99999915_g1) were quantified by real-time quantitative RT-PCR using Taqman Assays on Demand (Applied Biosystems). Each amplification reaction was performed in a 20 μl reaction volume with 50 ng cDNA, 20× Assay on Demand Gene Expression kit (1 μl) and 2× Taqman Universal PCR Master Mix (10 μl), and was completed as follows: 2 min at 50°C (1 cycle), 10 min at 95°C (1 cycle), 15 s at 95°C, and 60 s at 60°C (40 cycles). All reactions were performed in triplicate using the ABI Prism 7300 Sequence Detection System (Applied Biosystems). Human CYP2J2 and CYP2C8 expression were normalized to murine GAPDH using the 2−ΔCt method (22).
Immunoblotting
Microsomal fractions from whole kidney were prepared by differential centrifugation at 4°C, as described (23). Renal (70 μg) microsomes were separated by 10% NuPAGE Bis-Tris gels, transferred to nitrocellulose membranes (Invitrogen), and blocked in 5% nonfat milk in Tris-buffered saline (TBS) for 2 h. For Tie2-CYP2J2-Tr mice, membranes were incubated with a 1:1000 dilution of the anti-CYP2J2pep1 antibody in 5% nonfat milk at 4°C overnight. Anti-CYP2J2pep1 is a purified rabbit polyclonal antibody raised against a CYP2J2-specific peptide (amino acids 103–117) immunospecific for human CYP2J2 (24). For Tie2-CYP2C8-Tr mice, membranes were incubated with a 1:1000 dilution of anti-CYP2C8 antibody (kindly provided by Dr. Joyce Goldstein, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) in 3% nonfat milk at 4°C overnight. Anti-CYP2C8 is a purified rabbit polyclonal antibody raised against recombinant human CYP2C8; although, cross-reactivity with murine CYP2C isoforms exists (25). Membranes were also incubated with a 1:1000 dilution of anti-β-actin antibody (4967; Cell Signaling Technology, Danvers, MA, USA) in 3% nonfat milk at 4°C overnight. The membranes were washed in 0.05% TBS-Tween-20 and incubated with a 1:1000 (β-actin), 1:2000 (CYP2J2) or 1:5000 (CYP2C8) dilution of horseradish peroxidase conjugated bovine anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 5% nonfat milk at room temperature for 1.5 h. Immunoreactive bands were detected by chemiluminescence using the SuperSignal chemiluminescent substrate (Pierce, Rockford, IL, USA).
In situ hybridization
Fragments of the human CYP2J2 and CYP2C8 cDNA sequences were PCR amplified with specific forward (CYP2J2: 5′-ACCGAGACAACTTGGACAAC-3′; CYP2C8: 5′-CAATCCTCGGGACTTTATCG-3′) and reverse primers (CYP2J2: 5′-ATGCCCGCTTTCCTATTGAG-3′; CYP2C8: 5′-GGACAAGGTCACTGTATCTC-3′). The resulting 406-bp (CYP2J2) and 314-bp (CYP2C8) products were then subcloned into the intermediate pCR2.1-TOPO vector using the TOPO TA Cloning system (Invitrogen) per the manufacturer's instructions. The pCR2.1-TOPO vector contains only one promoter (T7). Consequently, sense and antisense probes required two separate vectors with the respective inserts in the correct orientation, which were confirmed by direct sequencing. Radioactive antisense and sense cRNA probes were transcribed in vitro with T7 RNA polymerase from BamH1 linearized human CYP2J2 and CYP2C8 constructs in the presence of 35S-uridine 5′ (α-thio)triphosphate (35S-UTP). Residual DNA was digested with 2 U of DNase I (Ambion, Austin, TX, USA), and the sense and antisense RNA probes were purified on a G-50 Sephadex column (Amersham Biosciences, Piscataway, NJ, USA).
Kidneys were excised, sliced into 3 coronal blocks, fixed in 4% paraformaldehyde in Sorensen buffer (0.03 M, pH 7.4), rinsed overnight in 20% buffered-sucrose solution (pH 7.4), embedded in O.C.T. (Sakura Finetek, Torrance, CA, USA), and frozen in isopentane at −55°C. Coronal sections (20 μm) were cut on a cryostat, thaw-mounted onto Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA), and stored at −80°C. Perfusion fixation was also completed on a subset of mice.
Slides were postfixed in 4% paraformaldehyde in Sorensen's phosphate buffer (0.03 M, pH 7.4) for 20 min, rinsed, and treated with proteinase K (1.0 μg/ml) for 4 min at room temperature. Perfused tissue was treated with proteinase K (1.0 or 1.6 μg/ml) for 12 to 15 min. Sections were then treated with 0.1 M triethanolamine for 3 min, followed by 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min. Sections were prehybridized for 1 h at 58°C with hybridization buffer [50% formamide, 1× Denhardt's solution, 10% dextran sulfate, 100 μM dithiothreitol (DTT), 200 mM sodium chloride, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA (pH 8.0), and 125 μg/ml tRNA] and then rinsed in 2× SSC buffer. The 35S-labeled antisense and sense riboprobes were heat-denatured, diluted with hybridization buffer, and used at a final concentration of 2 × 104 cpm/μl. Subsequently, the sections were covered with glass coverslips, sealed, and hybridized in a moist chamber for 18 h at 58°C. After hybridization, the slides were washed in 2× SSC buffer at 58°C 4 times, reacted with RNase (20 μg/ml) for 30 min at 37°C, and washed in decreasing concentrations of SSC (2×, 1×, 0.5×, 0.25×) at 58°C with 3 changes of each solution. Finally, the slides were washed in 0.1× SSC with 1 mM DTT for 45 min at 58°C. The slides were dehydrated in 50, 80, and 90% ethanol with 300 mM ammonium acetate followed by 100% ethanol for 2 min, placed side by side on a flat surface together with autoradiographic 14C microscales, and exposed to Hyperfilm-βmax (Amersham Biosciences) for 6 d at 4°C. The films were developed in D19 developer for 5 min and fixed in Kodak fixer for 5 min (Eastman Kodak, Rochester, NY, USA). The slides were then dipped in Kodak NTB-2 nuclear track emulsion and exposed for 21 d at 4°C. Thereafter, slides were developed in D19 developer for 2 min, fixed in Kodak fixer for 5 min, counterstained with hematoxylin, dehydrated, and coverslipped.
Film images were scanned and digitized images processed in Adobe Photoshop (Adobe Systems, Mountain View, CA, USA) and Macromedia FreeHand (Macromedia, San Francisco, CA, USA). Contrast and brightness were adjusted in scanned images of films to compensate for uneven illumination in bright-field images. Images of emulsion-coated slides were analyzed using a Nikon E800 microscope (Nikon, Tokyo, Japan). Dark-field and bright-field images were captured using a Nikon DS-L1 digital camera and illustrated in Adobe Photoshop and Macromedia FreeHand software programs. Contrast and brightness were adjusted to match the original images seen in the microscope.
Immunohistochemistry
Whole aorta and kidney tissue were fixed in 10% neutral buffered formalin and embedded in paraffin, and serial sections (6 μm) were stained immunohistochemically. Briefly, sections were deparaffinized in xylene, hydrated through a graded series of ethanol to 1× Automation Buffer (Biomeda, Foster City, CA, USA), and then blocked for endogenous peroxidase activity with 3% (v/v) hydrogen peroxide. Enzyme-based antigen retrieval was completed using 0.05% Pronase (Dako Corp., Carpinterina, CA, USA) for 5 min at room temperature prior to 15 min of blocking with 10% normal donkey serum in 1.25% normal mouse serum (Jackson Immunoresearch Labs, West Grove, PA, USA). Following blocking for endogenous biotin using the Avidin-Biotin blocking kit (Vector Laboratories, Burlingame, CA, USA), sections were incubated with primary antibody. For Tie2-CYP2J2-Tr mice, sections were incubated with anti-CYP2J2pep1 or normal rabbit serum (1:50 dilution) for 30 min at room temperature. For Tie2-CYP2C8-Tr mice, sections were incubated with anti-CYP2C8 or normal rabbit serum (1:100 dilution) for 30 min at room temperature. Sections were rinsed in 1× Automation Buffer, and then incubated with a donkey anti-rabbit secondary antibody (Vector Laboratories) (1:500 dilution) for 30 min at room temperature. Sections were subsequently rinsed in 1× Automation Buffer, and incubated for 30 min with a prediluted streptavidin complex (BioGenex, San Ramon, CA, USA). Visualization of the antibody complex was completed using liquid 3,3′-diaminobenzidine solution (Dako) for 6 min in the dark. Slides were counterstained with Harris hematoxylin (Harelco, Gibbston, NJ, USA), rinsed in 1× Automation Buffer, dehydrated through a graded series of ethanol to xylene washes, and coverslip applied with Permount (Surgipath, Richmond, IL, USA). Stained sections were observed under a light microscope, and digital images were captured using ImageScope software (Aperio Technologies, Vista, CA, USA).
Quantification of CYP-derived eicosanoids
Epoxy (EET) and dihydroxy (DHET) metabolites of arachidonic acid were extracted in plasma and primary endothelial cell culture medium by solid-phase extraction and eluted in ethyl acetate, as described (26, 27). Oxylipids were separated by reverse-phase HPLC on a 2- × 150-mm, 5-μm Luna C18 (2) column (Phenomenex, Torrance, CA, USA) and quantified using a MDS Sciex API 3000 triple quadrupole mass spectrometer (Applied Biosystems) with negative mode electrospray ionization and multiple reaction monitoring, as described (26). The relative response ratios of each analyte were used to calculate concentrations, while correcting for surrogate losses via quantification relative to internal standards. Cell medium concentrations were normalized to cell density. In particular, the 11,12- and 14,15-regioisomers were quantified since these are the primary metabolic products synthesized by CYP2J2 and CYP2C8 (18, 19).
Afferent arteriolar responses to vasoactive compounds
The perfused juxtamedullary nephron preparation was used to assess renal microvascular reactivity in male and female mice, as described (16, 28). Briefly, after pentobarbital anesthesia (100 mg/kg, i.p.), the kidney was immediately isolated and perfused in vitro with a physiological salt solution and mixture of l-amino acids. The juxtamedullary vasculature was isolated for study and an afferent arteriole was monitored continuously by videomicroscopy. After control diameter measurements, afferent arterioles were preconstricted with phenylephrine (1 μM) and subsequently exposed to increasing concentrations of acetylcholine (0.01–1 μM), and the diameter response was quantified. In separate experiments, the diameter response to endothelin-1 (0.001–10 nM) was determined in afferent arterioles that were not preconstricted. A subset of these experiments was conducted in the presence of the putative EET receptor antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 10 μM) (29).
Blood pressure measurements
Male and female mice were fed a normal salt diet (0.4% NaCl) with free access to water. Blood pressure was measured in conscious, unrestrained mice by radiotelemetry using an indwelling transducer-tipped catheter (TA11PA-C20; Data Sciences International, St. Paul, MN, USA) surgically inserted into the right carotid artery, as described previously (16, 30). Mice were anesthetized with 2% isoflurane with a continuous flow of 100% O2. After recovery from surgery for 2 wk, measurements were obtained continuously for 2 h each morning, and then averaged to yield a daily value. Measurements were obtained for 10 d under basal conditions and for 5 d during administration of N-nitro-l-arginine methyl ester (L-NAME, 1 mg/ml) and indomethacin (20 μg/ml) in the drinking water (31, 32). Daily values were averaged for statistical comparison across genotypes.
In a separate experiment, hypertension was induced in male and female mice using an established angiotensin/high-salt model (ANG/HS) (13). On d 1, mice were anesthetized with 2% isoflurane with a continuous flow of 95%/5% O2/CO2, ALZET osmotic pumps (Durect Corp., Cupertino, CA, USA) were implanted subcutaneously, and a high-salt diet (4% NaCl) was initiated with free access to water. The osmotic pumps infused angiotensin II (400 ng/kg/min, ANG/HS), or 0.9% NaCl in a subset of controls (HS), for 4 wk. Systolic blood pressure (SBP) was measured noninvasively on a weekly basis by tail-cuff plethysmography (IITC Life Science, Woodland Hills, CA, USA), as described previously (16, 33). On d 27, mice were placed in metabolic cages, and a 24 h urine sample was collected. On d 28, animals were anesthetized for tissue collection and then euthanized.
Renal injury assessment
In ANG/HS mice and HS controls, urinary protein and sodium concentrations were measured using the BCA protein assay kit (Pierce) and ion-selective electrodes, respectively, and total protein (mg/d) and sodium (μmol/d) excretion was calculated. Whole kidneys were excised, immediately fixed in 10% neutral buffered formalin, and embedded in paraffin for histological assessment of renal injury. Sections (2–3 μm) were stained with hematoxylin-eosin, periodic acid-Schiff reagent and periodic acid-methenamine-silver. Two observers semiquantitatively graded each section in a blinded fashion for the extent of glomerular injury. In each section, 50 glomeruli were graded according to the following scale: 0 = no injury; 1 = mild (≥25% area of injury); 2 = moderate (≥50% area of injury); 3 = extensive (≥75% area of injury); and 4 = severe (100% area of injury); an average score was calculated for each section. Four sections were scored per mouse and averaged to yield a final score for each mouse.
Statistical analysis
All data are expressed as means ± se. Data were compared statistically across genotype using a Student's t test or 1-way ANOVA followed by a post hoc Student's t test, where applicable. The afferent arteriolar dose-responses were compared using a 1-way ANOVA for repeated measures followed by a post hoc Duncan's multiple range test. Values of P < 0.05 were considered statistically significant.
RESULTS
Generation of Tie2-CYP2J2 and Tie2-CYP2C8 Tr mice
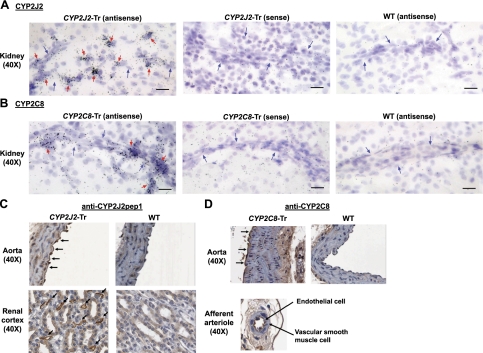
Heterozygous F1 progeny were identified in 6 Tie2-CYP2J2 and 6 Tie2-CYP2C8 founder lines. After a subsequent cross of heterozygous F1 progeny from each line with wild-type C57BL/6 mice, 6 and 5 lines, respectively, produced heterozygous F2 progeny with germline incorporation of the transgene. Quantitative real-time RT-PCR demonstrated that 3 Tie2-CYP2J2 founder lines (lines 5, 6, and 8) and 3 Tie2-CYP2C8 founder lines (lines 3, 7, and 8) yielded F2 progeny with transgene expression. Relative to wild-type littermates, Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice exhibited significantly higher CYP2J2 and CYP2C8 mRNA levels, respectively, in isolated primary aortic and renal endothelial cells (Fig. 1B) and aorta and kidney tissue homogenates (Fig. 1C). Although each founder line demonstrated significantly higher transgene mRNA levels compared to wild type, interline differences in mRNA levels were observed (Supplemental Fig. S2). Immunoblotting demonstrated higher CYP2J2 and CYP2C8 protein expression compared to wild-type littermates, respectively, in kidney microsomal fractions (Fig. 1D). In situ hybridization in kidney (Fig. 2A, B; Supplemental Figs. S3 and S4) and immunohistochemical staining in aorta and kidney (Fig. 2C, D) demonstrated endothelial CYP2J2 and CYP2C8 expression in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice, respectively, while wild-type littermate controls exhibited minimal to no expression. No significant differences in EPHX2 mRNA levels were observed in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type littermate controls in either aorta or kidney (data not shown).
Figure 2.
Endothelial CYP2J2 and CYP2C8 expression in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice. A, B) Endothelial CYP2J2 (A) and CYP2C8 (B) mRNA expression was evaluated in coronal sections through the central region of the kidney by in situ hybridization in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice, respectively, and wild-type (WT) littermate controls. Panels show representative bright-field autoradiograph images from emulsion coated slides using antisense and sense CYP2J2 and CYP2C8 probes. Black dots indicate hybridization (binding) of the probe to tissue RNA. Blue arrows indicate the presence of a vessel. Red arrows identify autoradiographic grains indicative of endothelial cell labeling. Scale bars = 20 μm. C, D) CYP2J2 (C) and CYP2C8 (D) immunostaining was completed using the anti-CYP2J2pep1 and anti-CYP2C8 antibodies, respectively. Panels show images from representative sections of aorta and renal cortex. Arrows indicate endothelial cell staining. No immunostaining was observed with normal rabbit serum in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, or WT mice (not shown).
Primary renal endothelial cells isolated from Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice released significantly higher concentrations of 11,12- and 14,15-EET and DHET into medium compared to cells isolated from wild-type controls (Fig. 1E), demonstrating higher endothelial CYP epoxygenase metabolic activity in Tr mice consistent with CYP2J2 and CYP2C8 expression. Significantly higher plasma 11,12- and 14,15-EET concentrations were also observed in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice compared to wild-type littermates (Fig. 1F). Although variable plasma EET levels were observed from experiment to experiment, these data are representative of the relative circulating EET levels observed in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type mice, which was consistent across founder lines.
Subsequent breeding of heterozygous F2 Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice with wild-type C57BL/6 mice demonstrated mendelian inheritance of the transgene, producing Tr (+/−) and wild-type (−/−) pups at an approximate ratio of 1:1. Male and female Tr mice are fertile with no impaired reproductive capacity, demonstrate no developmental defects, and have a normal life span, which suggests that endothelial CYP2J2 and CYP2C8 expression does not adversely affect development or survival.
Afferent arteriolar responses
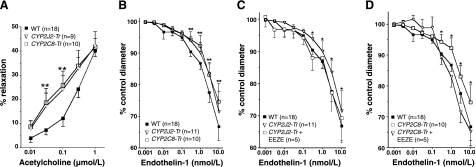
Afferent arteriolar responses to acetylcholine and endothelin-1 were assessed in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type littermate controls (Fig. 3). No significant differences in the afferent arteriolar responses were observed in male and female mice or between founder lines. Administration of phenylephrine equivalently decreased afferent arteriole diameter in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type control mice from 18.3 ± 0.7 to 13.6 ± 0.7 μm (n=9), 17.3 ± 0.7 to 11.4 ± 0.8 μm (n=10), and 18.4 ± 0.5 to 12.7 ± 0.7 μm (n=18), respectively. Following preconstriction with phenylephrine, an enhanced afferent arteriolar dilator response to acetylcholine was observed in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice compared to wild-type controls at 0.03 and 0.1 μM (P<0.05 vs. wild-type, Fig. 3A). However, no difference in the maximal afferent arteriolar relaxation to 1 μM acetylcholine was observed across the 3 groups. Endothelin-1-evoked afferent arteriolar vasoconstrictor responses were significantly attenuated in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice compared to wild-type (Fig. 3B). This attenuation was abolished in the presence of the putative EET receptor antagonist 14,15-EEZE (Fig. 3C, D), demonstrating the contribution of CYP-derived EETs to the observed vascular phenotype.
Figure 3.
Afferent arteriolar responses to acetylcholine and endothelin-1 in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice. Afferent arteriolar responses to acetylcholine (A), endothelin-1 (B), and endothelin-1 in the presence of 14,15-EEZE (C, D) in Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type (WT) littermate control mice. Arteriole diameters at each dose are expressed as percentage of baseline (control). Number of mice in each group is indicated. *P < 0.05 vs. WT; **P < 0.05 for both Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr vs. WT.
Hypertension and renal injury
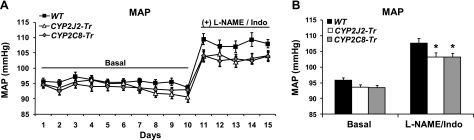
Tie2-CYP2J2-Tr (93.6±0.9 mmHg) and Tie2-CYP2C8-Tr (93.5±0.7 mmHg) mice demonstrated modestly lower average daily mean arterial pressure (MAP) under basal conditions compared to wild-type controls (95.9±0.8 mmHg); however, these differences were not statistically significant (Fig. 4). Administration of L-NAME and indomethacin significantly increased average daily MAP in wild-type mice (95.9±0.8 vs. 107.7±1.5 mmHg; P<0.001). However, Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice exhibited significantly lower MAP (103.1±1.4 and 103.2±1.1 mmHg, respectively) compared to wild-type controls (P<0.05 vs. wild-type, Fig. 4). Gender and founder line did not impact the differences observed across genotype groups.
Figure 4.
Blood pressure in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice under basal conditions and in the presence of nitric oxide synthase and cyclooxygenase inhibition. A) Daily mean arterial pressure (MAP) in Tie2-CYP2J2-Tr (n=21), Tie2-CYP2C8-Tr (n=19), and wild-type (WT) littermate control (n=29) mice was invasively measured by an indwelling catheter under basal conditions and during administration of L-NAME (1 mg/ml) and indomethacin (20 μg/ml) in the drinking water. B) Averaged MAP in each group under basal (ANOVA; P=0.061) and L-NAME/Indo-treated (ANOVA; P=0.036) conditions. *P < 0.05 vs. WT.
Baseline SBP measured by tail-cuff plethysmography was also lower, but not significantly lower, in Tie2-CYP2J2-Tr (96±5 mmHg) and Tie2-CYP2C8-Tr (95±3 mmHg) mice compared to wild-type controls (105±2 mmHg). Compared to baseline, SBP increased 2 wk (32±4 mmHg) and 4 wk (44±5 mmHg) following ANG/HS administration in wild-type mice. At 2 wk, ANG/HS-induced hypertension was significantly attenuated in Tie2-CYP2J2-Tr (22±3 mmHg; P<0.05 vs. wild-type), but not Tie2-CYP2C8-Tr (28±4 mmHg, P=NS vs. wild-type) mice. At 4 wk, a significant attenuation was observed in both Tie2-CYP2J2-Tr (27±4 mmHg, P<0.05) and Tie2-CYP2C8-Tr (26±4 mmHg, P<0.05) mice compared to wild-type (Fig. 5A). Compared to a normal salt diet, HS increased urinary sodium excretion in wild-type mice (42±15 vs. 661±77 μmol/d, respectively). Consistent with prior reports (13), coadministration of ANG/HS attenuated this increase in wild-type mice (325±90 μmol/d). Modestly higher urinary sodium excretion was observed in ANG/HS-treated Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice (414±55 and 461±64 μmol/d, respectively); however, these differences were not statistically significant (P=NS vs. ANG/HS wild-type).
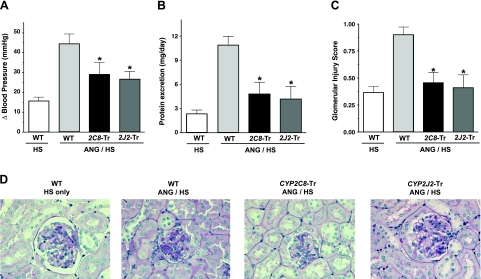
Figure 5.
Blood pressure and renal injury in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice in the presence of angiotensin/high-salt-induced hypertension. A) In the angiotensin/high-salt (ANG/HS) hypertension model, systolic blood pressure was measured noninvasively by tail-cuff plethysmography. Changes (ΔBP) from baseline at 4 wk in Tie2-CYP2J2-Tr (ANG/HS, n=6), Tie2-CYP2C8-Tr (ANG/HS, n=7), and wild-type (WT) littermate mice (ANG/HS, n=10) are provided. Control WT mice treated with in high-salt (HS) alone (n=11) are also included. B, C) On d 28, urinary protein excretion over 24 h (B) and histological glomerular injury scores (C) were measured in each group (n=5–7/group). *P < 0.05 vs. WT mice treated with ANG/HS. D) Representative images of renal histological sections in each group demonstrate the relative degree of glomerular injury.
At 4 wk, proteinuria increased 3.5-fold in ANG/HS- compared to HS-treated wild-type mice (P<0.05). However, the ANG/HS hypertension-induced increase in proteinuria was significantly attenuated in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice compared to wild-type controls (P<0.05, Fig. 5B). ANG/HS hypertension also resulted in mild glomerular injury in wild-type mice, consistent with prior reports demonstrating that C57BL/6 mice are less susceptible to hypertension-induced glomerular injury (34). However, this condition was significantly ameliorated by endothelial expression of CYP2J2 or CYP2C8 (P<0.05, Fig. 5C, D).
DISCUSSION
It is well established that CYP epoxygenase-derived EETs are synthesized in the endothelium, hyperpolarize vascular smooth muscle cells and possess potent vasodilatory effects in numerous vascular beds (7), and sEH inhibition significantly increases cellular and circulating EET levels, lowers blood pressure, and attenuates renal injury in various models of hypertension (9). More recently, the integral role of renal CYP epoxygenase function to the regulation of renal sodium transport and blood pressure has been demonstrated in mice with targeted disruption of Cyp2j5 and Cyp4a10 (16, 17). However, the specific contribution of endothelial CYP epoxygenase function to the regulation of blood pressure has remained unclear.
Consequently, we developed Tr mice that exhibit constitutive endothelial expression of the human CYP epoxygenases, CYP2J2 and CYP2C8, and increased endothelial EET biosynthesis. Compared to wild-type littermate controls, both Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice exhibited a significantly attenuated constrictor response to endothelin-1 and enhanced dilator response to acetylcholine in afferent arterioles, consistent with prior studies evaluating the impact of CYP epoxygenase metabolic activity and EETs on renal microvascular reactivity (5, 16, 35, 36). Importantly, the attenuated endothelin-1-evoked vasoconstrictor response was abolished by the putative EET receptor antagonist 14,15-EEZE, directly implicating CYP epoxygenase-derived EETs to the observed phenotype. Moreover, these data validated the utility of Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice as a model system for characterizing the functional impact of endothelial CYP epoxygenase-derived EETs on the regulation of physiological and pathophysiological processes. The principal finding reported herein is that Tr expression of the human CYP2J2 or CYP2C8 epoxygenases in the endothelium significantly lowers blood pressure in two distinct models of experimental hypertension in mice, demonstrating that endothelial CYP epoxygenase function contributes to the regulation of blood pressure.
Specifically, we observed that induction of hypertension and renal injury via ANG/HS was significantly and similarly attenuated in both Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice compared to wild-type littermate controls, consistent with the blood pressure lowering and renal protective effects associated with sEH inhibition in angiotensin II hypertension (11–13, 37). Prior studies have demonstrated that renal microvessel CYP2C and CYP2J expression is suppressed and sEH expression is induced in ANG/HS hypertension (38, 39). Similarly, double-transgenic rats (dTGRs) that overexpress the human renin and angiotensinogen genes exhibit suppressed renal CYP2C and CYP2J protein expression and renal microsomal EET formation (40, 41). Treatment with fenofibrate (a PPAR-α activator) and etanercept (a soluble TNF-α receptor blocker) restore renal CYP2C expression and epoxygenase metabolic activity in these hypertensive models, while in parallel lowering blood pressure and attenuating renal injury (38, 41). Moreover, induction of renal Cyp2c44 expression and EET biosynthesis with Wy14643 (a PPAR-α activator) lowered blood pressure and rescued the salt-sensitive hypertensive phenotype in Cyp4a10−/− mice (17). Although these studies further implicated renal CYP epoxygenase function in the pathogenesis of angiotensin II and salt-sensitive hypertension, the specific contribution of renal vascular, tubular, and cortical CYP2C/2J function on blood pressure regulation has remained unknown. Importantly, our investigation is the first to directly demonstrate that endothelial CYP epoxygenase function regulates blood pressure in the presence of hypertension.
Although the increase in blood pressure elicited by ANG/HS over 4 wk was not completely abrogated in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice, the partial restoration observed is consistent with the magnitude of blood pressure lowering achieved with sEH inhibition in prior studies (11–13, 37). Due to the lack of available pharmacological tools conducive to chronic in vivo dosing, we could not directly implicate EETs in the observed blood pressure phenotype. However, when considered in conjunction with the aforementioned renal microvascular reactivity experiments, these data collectively demonstrate that vascular CYP epoxygenase function contributes to the blood pressure lowering effects associated with potentiation of endothelial EET biosynthesis. Moreover, these data suggest that the impact of endothelial cell-derived EETs on the regulation of renal microvascular reactivity and blood flow is the most likely mechanism underlying the observed antihypertensive effects, independent of the known effects EETs elicit on renal tubular transport (2, 8). Although no significant differences in urinary sodium excretion were observed among Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type mice in the presence of ANG/HS, we cannot exclude the possibility that increased endothelial EET biosynthesis also modulated tubular transport in a paracrine fashion. Future studies remain necessary to dissect the respective contribution of CYP-derived EET-mediated alterations in vascular resistance and natriuresis to the regulation of blood pressure, and determine whether these effects can additively attenuate hypertension. Recent work has demonstrated the presence of Tie2 expression in distinct monocyte lineages, and that Tie2-driven transgene expression is not exclusively limited to endothelial cells (42). Consequently, we cannot exclude the possibility that CYP epoxygenase expression in other cell types has contributed to the observed phenotype. In addition, since CYP-derived EETs also possess potent antiinflammatory effects (43), our current investigation cannot exclude the possibility that the antiinflammatory effects associated with CYP epoxygenase overexpression, in addition to the blood pressure lowering effects, contributed to the observed attenuation of renal injury.
Statistically significant differences in basal blood pressure were not observed among Tie2-CYP2J2-Tr, Tie2-CYP2C8-Tr, and wild-type mice, consistent with recent reports in mice with targeted Ephx2 disruption (14, 37). However, blood pressure was significantly lower in both Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice after induction of hypertension by pharmacological nitric oxide synthase and cyclooxygenase inhibition. These findings are consistent with the EDHF effects of CYP-derived EETs, which are most pronounced in the presence of inhibitors of nitric oxide and prostacyclin biosynthesis in multiple vascular beds, including the afferent arteriole (3–5). L-NAME-induced hypertension is driven predominantly by reduced endothelial nitric oxide synthesis, impaired endothelial function, and increased peripheral vascular resistance (44), including increased renal microvascular reactivity (5). Consequently, these findings demonstrate further that endothelial CYP epoxygenase function can directly contribute to the regulation of blood pressure via its peripheral vascular resistance-lowering effects.
Despite a similar antihypertensive effect in angiotensin II hypertension, targeted Ephx2 disruption and sEH inhibitor administration failed to lower blood pressure in mice with L-NAME-induced hypertension in a recent study (37). It is important to note that sEH inhibition reduces the hydrolysis of EETs to DHETs in multiple cell types, Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice exhibit increased levels of EETs and DHETs in the endothelium, and DHETs possess vasodilatory effects of comparable potency to EETs in certain vascular beds (6, 37). Although prior reports have demonstrated that 11,12-DHET has no effect on renal microvascular function in rats (36) and DHET-mediated vasodilation in murine mesenteric arteries is nitric oxide-dependent (37), higher total EET and DHET levels in the afferent arteriole might have contributed to the antihypertensive phenotype observed in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice. Importantly, the specific mechanisms underlying these disparate results remain unclear and require further investigation.
Another important finding was that endothelial expression of either CYP2J2 or CYP2C8 yielded equivalent effects on renal microvascular reactivity, blood pressure under basal and hypertensive conditions, and hypertension-induced renal injury, demonstrating that the source of endothelial-derived EETs (CYP2J vs. CYP2C) does not impact afferent arteriolar function or blood pressure regulation. In contrast to mice with targeted disruption of Cyp2j5 (16), no discernable gender differences in phenotype were observed. Although the specific mechanism is unknown, these differences are most likely related to the use of a Tr model in which endothelial CYP expression is constitutively and similarly driven in both male and female mice, compared to deletion of a gene known to be regulated by sex hormones (45).
A series of recent studies has also demonstrated that overexpression of endothelial CYP4A2, a CYP ω-hydroxylase that catalyzes the formation of 20-HETE, elicits endothelial dysfunction, hypertension, and renal injury in rats via increased oxidative stress and eNOS uncoupling (46–48), providing direct evidence that endothelial CYP ω-hydroxylase function also contributes to the regulation of blood pressure. Taken together with the current investigation, these data demonstrate collectively that endothelial CYP-derived eicosanoids can regulate blood pressure in the setting of hypertension. Importantly, patients with essential hypertension and renovascular disease exhibit higher 20-HETE and lower total EET+DHET plasma levels compared to healthy controls, demonstrating the potential functional relevance of vascular CYP-mediated eicosanoid metabolism to the pathogenesis of hypertension in humans (49). In addition, genetic polymorphisms the human CYP epoxygenases (CYP2J2 and CYP2C8) and CYP ω-hydroxylases (CYP4A11 and CYP4F2) have been associated with hypertension and cardiovascular disease risk in humans and demonstrate the potential clinical importance of CYP-derived eicosanoids in the pathogenesis of vascular disease (50–53). However, direct functional interactions between endothelial CYP epoxygenases and ω-hydroxylases in the regulation of blood pressure and hypertension-induced renal injury remain unclear and require further study. Indeed, dual potentiation and inhibition of CYP-mediated EET and 20-HETE biosynthesis, respectively, offer substantial potential as an antihypertensive and renal protective therapeutic strategy and warrant further study.
In summary, we have developed novel Tr mice with endothelial expression of the human CYP2J2 and CYP2C8 epoxygenases and increased endothelial EET biosynthesis, which exhibit enhanced afferent arteriolar dilation, lower blood pressure and attenuated hypertension-induced renal injury compared to wild-type littermate controls. These data collectively demonstrate that endothelial CYP epoxygenase function can regulate blood pressure and provide important functional insight into the potential therapeutic utility of antihypertensive strategies that increase CYP-derived EETs. Future studies in Tie2-CYP2J2-Tr and Tie2-CYP2C8-Tr mice may lead to a better understanding to the role of endothelial CYP-derived eicosanoids in the regulation of vascular function in vivo and pathogenesis of hypertension.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical assistance of Dr. Taiping Jia in completion of the in situ hybridization studies, and Drs. Anton Jetten and Robert Langenbach for their helpful comments during the preparation of this manuscript.
This publication was made possible by a Beginning Grant-in-Aid from the American Heart Association to C.R.L.; U.S. National Institutes of Health (NIH) grant DK38826 and an Advancing a Healthier Wisconsin grant to J.D.I.; NIH grant P01 NS049210 to O.K.R. and N.J.A.; NIH grant GM31278 and support from the Robert A. Welch Foundation to J.R.F., and funds from the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIEHS) to K.B.T. (Z01 ES050167) and D.C.Z. (Z01 ES025034). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH. D.C.Z. is a coinventor on U.S. Patent 6,531,506 B1 (issued March 11, 2003), Inhibition of Epoxide Hydrolases for the Treatment of Hypertension, and on U.S. Patent 6,916,843 B1 (issued July 12, 2005), Anti-inflammatory Actions of Cytochrome P450 Epoxygenase-Derived Eicosanoids. J.D.I. is a coinventor on U.S. Patent 7,550,617 (issued June 23, 2009), Compositions and Methods for the Treatment of Renal and Cardiovascular Disease. No other authors have conflicts of interest to disclose.
REFERENCES
- 1.Zeldin D. C. (2001) Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 276, 36059–36062 [DOI] [PubMed] [Google Scholar]
- 2.Roman R. J. (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82, 131–185 [DOI] [PubMed] [Google Scholar]
- 3.Campbell W. B., Gebremedhin D., Pratt P. F., Harder D. R. (1996) Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 78, 415–423 [DOI] [PubMed] [Google Scholar]
- 4.Fisslthaler B., Popp R., Kiss L., Potente M., Harder D. R., Fleming I., Busse R. (1999) Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401, 493–497 [DOI] [PubMed] [Google Scholar]
- 5.Imig J. D., Falck J. R., Wei S., Capdevila J. H. (2001) Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J. Vasc. Res. 38, 247–255 [DOI] [PubMed] [Google Scholar]
- 6.Larsen B. T., Miura H., Hatoum O. A., Campbell W. B., Hammock B. D., Zeldin D. C., Falck J. R., Gutterman D. D. (2006) Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am. J. Physiol. Heart Circ. Physiol. 290, H491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell W. B., Falck J. R. (2007) Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49, 590–596 [DOI] [PubMed] [Google Scholar]
- 8.Capdevila J. H., Falck J. R., Imig J. D. (2007) Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 72, 683–689 [DOI] [PubMed] [Google Scholar]
- 9.Imig J. D., Hammock B. D. (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8, 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Z., Xu F., Huse L. M., Morisseau C., Draper A. J., Newman J. W., Parker C., Graham L., Engler M. M., Hammock B. D., Zeldin D. C., Kroetz D. L. (2000) Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 87, 992–998 [DOI] [PubMed] [Google Scholar]
- 11.Imig J. D., Zhao X., Capdevila J. H., Morisseau C., Hammock B. D. (2002) Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39, 690–694 [DOI] [PubMed] [Google Scholar]
- 12.Zhao X., Yamamoto T., Newman J. W., Kim I. H., Watanabe T., Hammock B. D., Stewart J., Pollock J. S., Pollock D. M., Imig J. D. (2004) Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J. Am. Soc. Nephrol. 15, 1244–1253 [PubMed] [Google Scholar]
- 13.Imig J. D., Zhao X., Zaharis C. Z., Olearczyk J. J., Pollock D. M., Newman J. W., Kim I. H., Watanabe T., Hammock B. D. (2005) An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46, 975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manhiani M., Quigley J. E., Knight S. F., Tasoobshirazi S., Moore T., Brands M. W., Hammock B. D., Imig J. D. (2009) Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am. J. Physiol. Renal Physiol. 297, F740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makita K., Takahashi K., Karara A., Jacobson H. R., Falck J. R., Capdevila J. H. (1994) Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J. Clin. Invest. 94, 2414–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athirakul K., Bradbury J. A., Graves J. P., DeGraff L. M., Ma J., Zhao Y., Couse J. F., Quigley R., Harder D. R., Zhao X., Imig J. D., Pedersen T. L., Newman J. W., Hammock B. D., Conley A. J., Korach K. S., Coffman T. M., Zeldin D. C. (2008) Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 22, 4096–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa K., Holla V. R., Wei Y., Wang W. H., Gatica A., Wei S., Mei S., Miller C. M., Cha D. R., Price E., Jr., Zent R., Pozzi A., Breyer M. D., Guan Y., Falck J. R., Waterman M. R., Capdevila J. H. (2006) Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J. Clin. Invest. 116, 1696–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S., Moomaw C. R., Tomer K. B., Falck J. R., Zeldin D. C. (1996) Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. 271, 3460–3468 [DOI] [PubMed] [Google Scholar]
- 19.Zeldin D. C., DuBois R. N., Falck J. R., Capdevila J. H. (1995) Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322, 76–86 [DOI] [PubMed] [Google Scholar]
- 20.Schlaeger T. M., Bartunkova S., Lawitts J. A., Teichmann G., Risau W., Deutsch U., Sato T. N. (1997) Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 94, 3058–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim Y. C., Garcia-Cardena G., Allport J. R., Zervoglos M., Connolly A. J., Gimbrone M. A., Jr., Luscinskas F. W. (2003) Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am. J. Pathol. 162, 1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 23.Lee C. R., Bottone F. G., Jr., Krahn J. M., Li L., Mohrenweiser H. W., Cook M. E., Petrovich R. M., Bell D. A., Eling T. E., Zeldin D. C. (2007) Identification and functional characterization of polymorphisms in human cyclooxygenase-1 (PTGS1). Pharmacogenet. Genomics 17, 145–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King L. M., Ma J., Srettabunjong S., Graves J., Bradbury J. A., Li L., Spiecker M., Liao J. K., Mohrenweiser H., Zeldin D. C. (2002) Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol. Pharmacol. 61, 840–852 [DOI] [PubMed] [Google Scholar]
- 25.Delozier T. C., Kissling G. E., Coulter S. J., Dai D., Foley J. F., Bradbury J. A., Murphy E., Steenbergen C., Zeldin D. C., Goldstein J. A. (2007) Detection of human CYP2C8, CYP2C9, and CYP2J2 in cardiovascular tissues. Drug Metab. Dispos. 35, 682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman J. W., Watanabe T., Hammock B. D. (2002) The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J. Lipid Res. 43, 1563–1578 [DOI] [PubMed] [Google Scholar]
- 27.Seubert J. M., Sinal C. J., Graves J., DeGraff L. M., Bradbury J. A., Lee C. R., Goralski K., Carey M. A., Luria A., Newman J. W., Hammock B. D., Falck J. R., Roberts H., Rockman H. A., Murphy E., Zeldin D. C. (2006) Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ. Res. 99, 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imig J. D., Breyer M. D., Breyer R. M. (2002) Contribution of prostaglandin EP(2) receptors to renal microvascular reactivity in mice. Am. J. Physiol. Renal Physiol. 283, F415–422 [DOI] [PubMed] [Google Scholar]
- 29.Gauthier K. M., Deeter C., Krishna U. M., Reddy Y. K., Bondlela M., Falck J. R., Campbell W. B. (2002) 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 90, 1028–1036 [DOI] [PubMed] [Google Scholar]
- 30.Mills P. A., Huetteman D. A., Brockway B. P., Zwiers L. M., Gelsema A. J., Schwartz R. S., Kramer K. (2000) A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J. Appl. Physiol. 88, 1537–1544 [DOI] [PubMed] [Google Scholar]
- 31.Ohashi Y., Kawashima S., Hirata K., Yamashita T., Ishida T., Inoue N., Sakoda T., Kurihara H., Yazaki Y., Yokoyama M. (1998) Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J. Clin. Invest. 102, 2061–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peebles R. S., Jr., Dworski R., Collins R. D., Jarzecka K., Mitchell D. B., Graham B. S., Sheller J. R. (2000) Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am. J. Respir. Crit. Care. Med. 162, 676–681 [DOI] [PubMed] [Google Scholar]
- 33.Krege J. H., Hodgin J. B., Hagaman J. R., Smithies O. (1995) A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25, 1111–1115 [DOI] [PubMed] [Google Scholar]
- 34.Hartner A., Cordasic N., Klanke B., Veelken R., Hilgers K. F. (2003) Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol. Dial. Transplant 18, 1999–2004 [DOI] [PubMed] [Google Scholar]
- 35.Imig J. D., Falck J. R., Inscho E. W. (1999) Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br. J. Pharmacol. 127, 1399–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imig J. D., Navar L. G., Roman R. J., Reddy K. K., Falck J. R. (1996) Actions of epoxygenase metabolites on the preglomerular vasculature. J. Am. Soc. Nephrol. 7, 2364–2370 [DOI] [PubMed] [Google Scholar]
- 37.Hercule H. C., Schunck W. H., Gross V., Seringer J., Leung F. P., Weldon S. M., da Costa Goncalves A., Huang Y., Luft F. C., Gollasch M. (2009) Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler. Thromb. Vasc. Biol. 29, 54–60 [DOI] [PubMed] [Google Scholar]
- 38.Elmarakby A. A., Quigley J. E., Pollock D. M., Imig J. D. (2006) Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47, 557–562 [DOI] [PubMed] [Google Scholar]
- 39.Zhao X., Pollock D. M., Inscho E. W., Zeldin D. C., Imig J. D. (2003) Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41, 709–714 [DOI] [PubMed] [Google Scholar]
- 40.Kaergel E., Muller D. N., Honeck H., Theuer J., Shagdarsuren E., Mullally A., Luft F. C., Schunck W. H. (2002) P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension 40, 273–279 [DOI] [PubMed] [Google Scholar]
- 41.Muller D. N., Theuer J., Shagdarsuren E., Kaergel E., Honeck H., Park J. K., Markovic M., Barbosa-Sicard E., Dechend R., Wellner M., Kirsch T., Fiebeler A., Rothe M., Haller H., Luft F. C., Schunck W. H. (2004) A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am. J. Pathol. 164, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Palma M., Venneri M. A., Galli R., Sergi Sergi L., Politi L. S., Sampaolesi M., Naldini L. (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 [DOI] [PubMed] [Google Scholar]
- 43.Deng Y., Theken K. N., Lee C. R. (2010) Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 48, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torok J. (2008) Participation of nitric oxide in different models of experimental hypertension. Physiol. Res. 57, 813–825 [DOI] [PubMed] [Google Scholar]
- 45.Ma J., Graves J., Bradbury J. A., Zhao Y., Swope D. L., King L., Qu W., Clark J., Myers P., Walker V., Lindzey J., Korach K. S., Zeldin D. C. (2004) Regulation of mouse renal CYP2J5 expression by sex hormones. Mol. Pharmacol. 65, 730–743 [DOI] [PubMed] [Google Scholar]
- 46.Wang J. S., Singh H., Zhang F., Ishizuka T., Deng H., Kemp R., Wolin M. S., Hintze T. H., Abraham N. G., Nasjletti A., Laniado-Schwartzman M. (2006) Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ. Res. 98, 962–969 [DOI] [PubMed] [Google Scholar]
- 47.Singh H., Cheng J., Deng H., Kemp R., Ishizuka T., Nasjletti A., Schwartzman M. L. (2007) Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50, 123–129 [DOI] [PubMed] [Google Scholar]
- 48.Inoue K., Sodhi K., Puri N., Gotlinger K. H., Cao J., Rezzani R., Falck J. R., Abraham N. G., Laniado-Schwartzman M. (2009) Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am. J. Physiol. Renal Physiol. 297, F875–F884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minuz P., Jiang H., Fava C., Turolo L., Tacconelli S., Ricci M., Patrignani P., Morganti A., Lechi A., McGiff J. C. (2008) Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension 51, 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King L. M., Gainer J. V., David G. L., Dai D., Goldstein J. A., Brown N. J., Zeldin D. C. (2005) Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet. Genomics 15, 7–13 [DOI] [PubMed] [Google Scholar]
- 51.Theken K. N., Lee C. R. (2007) Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics 8, 1369–1383 [DOI] [PubMed] [Google Scholar]
- 52.Gainer J. V., Bellamine A., Dawson E. P., Womble K. E., Grant S. W., Wang Y., Cupples L. A., Guo C. Y., Demissie S., O'Donnell C. J., Brown N. J., Waterman M. R., Capdevila J. H. (2005) Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111, 63–69 [DOI] [PubMed] [Google Scholar]
- 53.Fava C., Montagnana M., Almgren P., Rosberg L., Lippi G., Hedblad B., Engstrom G., Berglund G., Minuz P., Melander O. (2008) The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension 52, 373–380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.