Abstract
Evidence was presented that thyrotropin [thyroid-stimulating hormone (TSH)]-stimulated persistent cAMP signaling is dependent on receptor (with G-protein α subunits and adenylyl cyclase) internalization. Because it is not clear whether G proteins and adenylyl cyclase internalize with receptors, we tested whether persistent cAMP signaling by TSH receptor (TSHR) is dependent on internalization. We measured persistent TSHR signaling as an accumulation of cAMP in HEK-EM293 cells permanently expressing human TSHRs incubated with isobutylmethylxanthine for 30 min after washing the cells to remove unbound TSH, and TSHR internalization by fluorescence microscopy using Alexa-tagged TSH and binding assays using 125I-TSH. TSHRs, but not the closely related lutropin or follitropin receptors, exhibit persistent cAMP signaling. TSHRs were not internalized by 30 min incubation with unlabeled TSH; however, expression of β-arrestin-2 promoted TSHR internalization that was inhibited by dynasore, a dynamin inhibitor. Expression of β-arrestin-2 had no effect on TSHR cAMP signaling, dynasore inhibited TSHR cAMP signaling in the absence or presence of TSHR internalization, and expression of a dominant-negative mutant dynamin, which inhibited internalization, had no effect on persistent cAMP signaling. Persistent cAMP signaling was specifically inhibited by a small molecule TSHR antagonist. We conclude that TSHRs do not have to be internalized to exhibit persistent cAMP signaling.—Neumann, S., Geras-Raaka, E., Marcus-Samuels, B., Gershengorn, M. C. Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization.
Keywords: G-protein-coupled receptors, receptor-mediated endocytosis, sustained signaling, β-arrestin-2, dynamin
G-protein-coupled receptors (GPCRs) are traditionally thought to signal at the cell surface where an agonist binds and the activated GPCR couples to a G protein, which in turn activates an effector enzyme. In one signaling pathway, agonist-activated GPCR couples to the stimulatory G protein (Gs), which in turn activates adenylyl cyclase (AC) to generate the intracellular messenger molecule cAMP. After activation, GPCRs usually are internalized (or endocytosed), principally via clathrin-coated pits, and desensitized with regard to cAMP signaling, leading to a transient signaling response (1). (Other pathways, for example, signaling by GPCRs via mitogen-activated protein kinases, appear to occur during the internalization process; see ref. 2.) The mechanism of cAMP signaling by the thyrotropin [thyroid-stimulating hormone (TSH)] receptor (TSHR) has been thought to conform to this general outline in that cAMP production occurs at the cell surface. Recently, however, the parathyroid hormone receptor (3) and TSHR (4) were shown to exhibit persistent cAMP signaling, and evidence was presented that persistent cAMP signaling was dependent on receptor [and Gs α subunit (Gαs) and AC] endocytosis. The S1P1 receptor, which couples to the inhibitory G protein (Gi), also exhibits persistent signaling (5). Based on these studies a new paradigm for cAMP signaling by GPCRs has been proposed that agonist-activated GPCRs, Gα, and AC are internalized together and continue to signal in an endosomal compartment within cells (for review, see ref. 6).
Although the phenomenon that activated GPCRs internalize is well established, it is not clear whether the GPCR, Gαs or Gαi, and AC internalize as a complex as proposed (3–6). For example, it has been reported that the β2 adrenergic receptor and Gαs cycle constitutively via clathrin-independent endocytosis (7). On activation, however, Gαs remains associated with the cell surface membrane and recycling endosomes, whereas the receptor enters the cell via clathrin-dependent endocytosis and was found in late endosomes (7). In another study of β2 adrenergic receptors and Gαs it was shown that on activation the receptor is internalized via clathrin-dependent endocytosis but that Gαs moves to membrane rafts and is internalized via a clathrin-independent mechanism into a compartment different from that which contains the receptor (8). By contrast, it has been reported that on activation the glucagon receptor and Gαs are both internalized and are both found in an endosome/lysosome compartment (9). Thus, the fates of GPCRs and Gαs after receptor activation are not clear and may be receptor- and cell-type specific.
Because it is not clear whether Gαs and AC internalize along with agonist-bound GPCRs, we tested whether persistent cAMP signaling by TSHR is dependent on internalization. We show that HEK293 cells stably expressing human TSHRs (HEKTSHR cells), in which TSHR internalization does not occur, exhibit persistent production of cAMP even after TSH has been removed from the medium. Moreover, when HEKTSHR cells are made to overexpress β-arrestin 2 (βArr2), which is known to be a mediator of TSHR internalization, TSHR internalization is readily measured but persistent cAMP production is not affected. When internalization is inhibited by dynasore, a dynamin inhibitor, or by expression of a dominant-negative dynamin (K44A), persistent signaling is affected no differently in HEKTSHR cells that exhibit TSHR internalization and those that do not. We conclude, therefore, that TSHR receptor internalization is not required for persistent cAMP signaling.
MATERIALS AND METHODS
Culture of HEK-EM 293 cell lines and transient transfection
HEK-EM 293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 10 μg/ml streptomycin (Life Technologies Corp., Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator. The generation of a stable HEK-EM 293 cell line expressing TSHRs, lutropin [luteinizing hormone (LH)] receptors [LH/chorionic gonadotropin receptors (LHCGRs)], or follitropin [follicle-stimulating hormone (FSH)] receptors (FSHRs) was described previously (10). Cells were transiently transfected with wild-type TSHR, βArr2, or K44A (both kindly provided by Dr. Marc Caron, Duke University Medical Center, Durham, NC, USA) in 24-well plates (7.5×104 cells/well) with 0.2 μg DNA/well using FuGene 6 reagent (Roche, Indianapolis, IN, USA). Experiments were performed 48 or 72 h after transfection.
Determination of immediate or persistent cAMP production
HEKTSHR cells or HEK-EM 293 cells stably expressing LHCGRs or FSHRs were seeded into 24-well plates at a density of 2.2 × 105 cells/well in DMEM containing 10% FBS. Cells were cultured for 24–48 h before incubation for 30 min in HBSS/10 mM HEPES, pH 7.4. To determine the immediate effects of TSH stimulation, cAMP production was measured in cells incubated for 30 min at 37°C in a humidified incubator in HBSS/HEPES containing 1 mM 3-isobutyl-1-methylxanthine (IBMX; Sigma, St. Louis, MO, USA) in the presence or absence of 10 or 100 mU/ml bovine TSH (bTSH, Sigma) as described previously (10). To determine the persistent effect of TSH on cAMP production, cells were incubated without (control) or with TSH for 30 min (“pretreatment”), washed with 0.5 ml ice-cold acetic acid/Na acetate buffer, pH 2.8, or ice-cold HBSS/HEPES 3 times and then with HBSS/HEPES at RT and incubated in HBSS/HEPES at 37°C. At the times indicated after the washes, cells were incubated in HBSS/HEPES with 1 mM IBMX or 1 mM IBMX and 100 mU/ml bTSH at 37°C for 30 min (“treatment”). Following aspiration of the incubation buffers, cells were lysed using lysis buffer of the cAMP-Screen Direct System (Applied Biosystems, Foster City, CA, USA), and the cAMP content of the cell lysate was determined using the method described in the manufacturer's protocol. The chemiluminescence signal was measured in a Victor V 1420 Multilabel Counter (Perkin Elmer, Waltham, MA, USA).
125I-TSH binding assays
To estimate the number of cell surface TSHRs, HEKTSHR cells were seeded in 24-well plates at a density of 2.2 × 105 cells/well. Cell surface binding was measured by incubation in 0.25 ml ice-cold binding buffer (HBSS/HEPES containing 2.5% milk powder and 0.2% BSA) with 2–6 × 104 cpm bovine 125I-TSH (Brahms Aktiengesellschaft, Hennigsdorf, Germany) for 3 h. Total binding was measured in the absence of unlabeled TSH and nonspecific binding in the presence of 100 mU/ml unlabeled bovine TSH. Cells were washed 3 times with 0.5 ml ice-cold HBSS and lysed with 0.5 ml 0.4 N NaOH, and the cell-associated radioactivity was counted in a 1470 Wallac Wizard γ counter (Perkin Elmer).
To measure acid-resistant binding, cells were incubated in binding buffer for 30 min at 37°C and then washed 3 times with 0.5 ml ice-cold HBSS or with ice-cold acetic acid/Na acetate buffer, pH 2.8. Percent acid-resistant binding was calculated as ×100 (specific binding after acid wash/specific binding after HBSS wash). Although it has traditionally been assumed that acid-resistant binding of ligands to GPCRs monitors internalized receptors, it has recently been shown that this is not the case (11), and we find this as well (see Results).
Fluorescence microscopy
Alexa Fluor 546-modified bovine TSH (Alexa-TSH) was synthesized by conjugating 1 mg of bovine TSH (Sigma-Aldrich) with Alexa Fluor 546 reactive dye (Invitrogen, Carlsbad, CA, USA) and purified with Bio-Rad BioGel P-30 fine-size resin to separate labeled protein (1 ml total volume) from unincorporated dye. Imaging was performed on cell monolayers. Cells were plated onto poly-d-lysine-coated coverglass culture dishes (MatTek, Ashland, MA, USA) at a density of 104 cells/cm2 and allowed to adhere overnight. Monolayers were washed twice with HEPES/HBSS and then blocked with HBSS containing 4% bovine serum albumin for 15 min at 37°C. For binding, Alexa-TSH (10 μl) was diluted with 500 μl of blocking buffer, added to monolayer cultures, and incubated for 30 min at 37°C.
Confocal (<0.5 μm) images were acquired on a Zeiss 510 NLO/Meta system, using a Plan-Apochromat ×40 1.3 objective (Carl Zeiss, Oberkochen, Germany), and are representative of slices at midpoint cell thickness. Detector gains and microscope parameters remained unchanged throughout all experimental conditions.
Inhibition of internalization
Internalization was inhibited in 2 ways. In the first, we used the dynamin inhibitor dynasore (Sigma). For 125I-TSH binding, cells were incubated in binding buffer for 30 min at 37°C without (control) or with varying doses of dynasore and then incubated in binding buffer without or with dynasore and 125I-TSH. Dynasore in doses above 10 μM in HBSS/HEPES and above 400 μM in binding buffer were toxic to HEKTSHR cells. After 30 min, total and acid-resistant binding were measured. For cAMP signaling, cells were incubated in binding buffer for 30 min at 37°C without (control) or with varying doses of dynasore and then incubated in binding buffer without or with dynasore and IBMX or IBMX and TSH for 30 min (“immediate signaling”). For measurement of persistent signaling, after 30 min with dynasore alone and then 30 min with dynasore and TSH, cells were incubated in HBSS/HEPES for 2–3 h, after which IBMX or IBMX and TSH were added for 30 min. cAMP levels in cell lysates were measured.
Inhibition of persistent signaling by a TSHR antagonist
To determine whether persistent cAMP signaling was dependent on persistent activation of TSHR, we measured the effects of a recently developed allosteric TSHR antagonist (S2) (12). Analog S2 was added to persistently signaling HEKTSHR cells for 30 min prior to and during the addition of IBMX or IBMX and TSH. Cellular cAMP levels were measured after 30 min.
Statistical analyses
Significance was determined by t test or ANOVA; P < 0.05 was considered significant.
RESULTS
Persistent cAMP signaling by TSHRs but not by LHCGRs or FSHRs
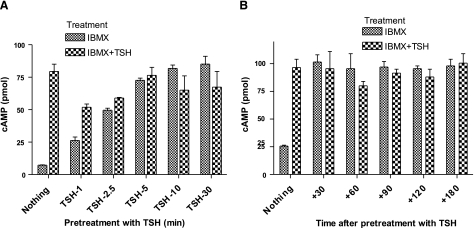
Persistent signaling was measured as the amount of cAMP accumulated in HEKTSHR cells incubated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) after the cells had been exposed to TSH and washed with acid buffer to remove TSH from the incubation medium and from acid-sensitive binding sites on the cell surface. Thus, we measured persistent signaling under conditions in which TSHRs were not continually exposed to TSH so that persistent signaling was caused by pretreatment with TSH. TSHR-mediated persistent cAMP production was found when cells were washed with neutral buffer also (not shown) in experiments similar in design to those reported by Calebiro et al. (4). In the series of experiments illustrated in Fig. 1, TSH stimulated an immediate increase in cAMP production of 4- to 12-fold. There was a time dependence of incubation with TSH prior to acid wash to attain maximum persistent signaling (Fig. 1A). Incubation for 1 or 2.5 min led to increasing levels of persistent signaling that was maximal after 5 min. The level of persistent signaling was not increased by readdition of TSH once the maximum cAMP production was attained; TSH stimulated increases in cAMP in cells in which maximum persistent signaling had not been attained. Cells incubated with TSH for 30 min exhibited persistent cAMP signaling for more than 3 h (Fig. 1B). Persistent cAMP signaling was found in HEKTSHR cells in the absence of IBMX also (see Fig. S1).
Figure 1.
Persistent cAMP signaling by TSHRs. A) Time course of acquisition of persistent signaling. HEKTSHR cells were incubated without (nothing) or with 10 mU/ml TSH at 37°C. At the times indicated, cells were washed 3 times with ice-cold acid buffer, followed by 1 wash with HBSS/HEPES, and then incubated in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in the cell lysates. Bars represent mean ± range of duplicate measurements in 1 of 2 experiments. B) Persistence of cAMP signaling. HEKTSHR cells were incubated without (nothing) or with 10 mU/ml TSH. After 30 min, cells were washed 3 times with ice-cold acid buffer, followed by 1 wash with HBSS/HEPES. Immediately after the washes, cells were incubated in HBSS/HEPES at 37°C. At 30, 60, 90, 120, and 180 min after the washes, cells were incubated in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in the cell lysates. Bars represent mean ± range of duplicate measurements in 1 of 4 experiments.
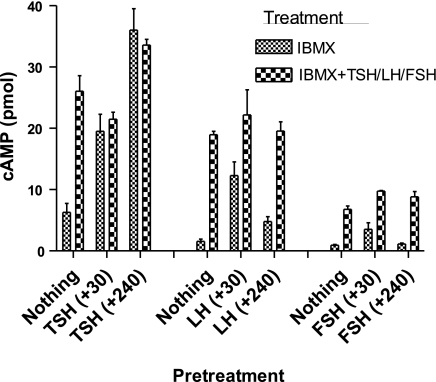
To show that persistent cAMP signaling was a property of the GPCR and not of the cells expressing these receptors, we measured persistent signaling in HEK-EM 293 cells stably expressing LHCGRs or FSHRs, the 2 other glycoprotein hormone receptors that are most homologous to TSHR. Cells were pretreated with LH or FSH for 30 min, washed with acid buffer, and persistent signaling was measured for up to 4.5 h. Figure 2 shows that both LHCGR and FSHR exhibit transient, low levels of persistent signaling that are virtually abolished by 4 h and that LH- and FSH-stimulated increases in cAMP production can be measured during this period. By comparison with LHCGR and FSHR, the well-established basal (or agonist-independent) cAMP signaling by TSHR (13) is evident in Fig. 2 (leftmost bar for each receptor) also.
Figure 2.
Comparison of persistent cAMP signaling in cells stably expressing TSHRs, LHCGRs, or FSHRs. HEK-EM 293 cells stably expressing TSHRs, LHCGRs, or FSHRs were incubated without (nothing) or with 10 mU/ml TSH, 100 ng/ml LH, or 200 ng/ml FSH, respectively. After 30 min, cells were washed 3 times with ice-cold acid buffer, followed by 1 wash with HBSS/HEPES. At the times indicated (+min), cells were incubated in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH, 100 ng/ml LH, or 200 ng/ml FSH, respectively, at 37°C for 30 min. Cells were lysed, and cAMP was measured in cell lysates. Bars repreasent mean ± range of duplicate measurements in 2 experiments.
Persistent cAMP signaling by TSHR is independent of internalization
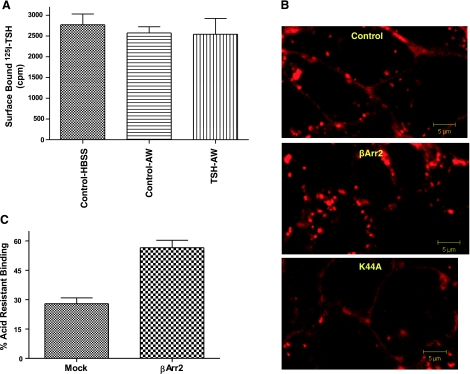
Having shown specific, persistent cAMP signaling for TSHR, we next determined whether persistent signaling was dependent on TSHR internalization. GPCR internalization usually leads to receptor down-regulation (2). We measured the level of TSHRs on the surface of HEKTSHR cells using 125I-TSH binding after a 30-min incubation with a maximally effective concentration of TSH (Fig. 3A). Cells incubated with TSH were washed with acid buffer to remove cell surface-bound TSH. There was no decrease in TSHR levels after TSH. We visualized TSH-bound TSHRs in the cell using Alexa Fluor 546-tagged TSH (Alexa-TSH). There was no apparent TSHR internalization in HEKTSHR cells, as has been reported previously (14) (Fig. 3B). However, we cannot exclude that a minor population of TSHRs were internalized. In contrast, when HEKTSHR cells were made to express βArr2 there was marked internalization of Alexa-TSH-bound TSHRs (Fig. 3B). This observation was further supported by measuring the levels of acid-resistant 125I-TSH binding. The acquisition of acid-resistant binding was time dependent and reached a maximum after 30 min that was maintained for at least 2 h (Fig. S2). Although not all acid-resistant ligand binding reflects internalized ligand-bound GPCRs (11), a correlation exists when internalization is induced. As predicted by the results in Fig. 3B, there was a 2-fold increase in acid-resistant 125I-TSH binding in HEKTSHR cells expressing βArr2 compared to mock-transfected cells (Fig. 3C). Thus it appears that HEKTSHR cells do not internalize TSH-bound TSHRs but can be made to internalize TSHRs after expression of βArr2. These findings suggest, therefore, that persistent cAMP signaling by TSHR does not require TSHR internalization.
Figure 3.
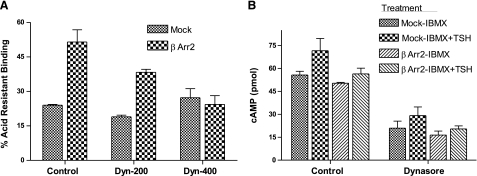
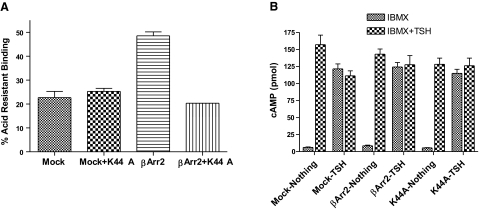
βArrestin2 (βArr2) induces TSH-mediated TSHR internalization in HEKTSHR cells. A) TSH does not down-regulate TSHRs. HEKTSHR cells were incubated without (control) or with 100 mU/ml TSH. After 30 min, cell surface TSHRs were estimated by binding 125I-TSH (6×104 cpm) to cells in the cold for 3 h. Nonspecific binding was subtracted. Bars repressent mean ± range of duplicate measurements of specific binding in 2 experiments. AW, acid wash. B) βArr2 induces TSHR internalization stimulated by Alexa-TSH. HEKTSHR cells were transfected to transiently express GFP without or with βArr2 or with K44A. After 48 h, cells were incubated with Alexa-TSH for 30 min at 37°C. Images were acquired as described in Materials and Methods. Micrographs are representative of slices at the midpoint of the cell thickness. C) βArr2 increases acid-resistant binding to TSHRs. HEKTSHR cells were transfected with empty plasmid (mock) or with plasmid to express βArr2. After 48 h, the cells were incubated with 125I-TSH at 37°C for 30 min. The cells were washed 3 times with ice-cold HBSS (total binding) or ice-cold acid buffer (acid-resistant binding) and the cell radioactivity counted. Percentage acid-resistant binding was calculated as 100 × (specific binding after acid wash/specific binding after HBSS wash). Bars represent mean ± sd of triplicate determinations in 2 experiments.
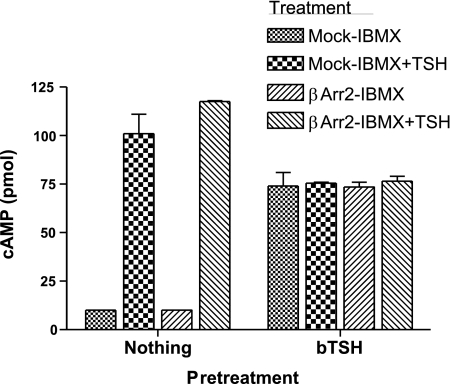
If persistent cAMP signaling were dependent on internalization it is predicted that persistent signaling would increase when internalization was induced and decreased when internalization was inhibited. We, therefore, compared signaling in mock-transfected and βArr2-expressing HEKTSHR cells in which internalization was induced (Fig. 4). There was no effect of βArr2 expression on immediate or persistent cAMP signaling. We inhibited internalization by preincubating cells with dynasore, which is a dynamin inhibitor (15). As expected, dynasore inhibited acid-resistant 125I-TSH binding in a dose-dependent manner in βArr2-expressing cells but had no effect in mock-transfected cells (Fig. 5A). Dynasore did not affect immediate TSH-stimulated production of cAMP (not shown) but inhibited persistent cAMP signaling to the same extent in both mock-transfected cells and in βArr2-expressing cells (Fig. 5B). Thus, dynasore inhibited persistent cAMP signaling in a manner independent of internalization. To specifically inhibit TSHR internalization we expressed a dominant negative dynamin mutant protein (K44A) that inhibits GPCR internalization (16). Expression of K44A had no effect on acid-resistant 125I-TSH binding in cells not expressing βArr2 but inhibited 125I-TSH binding in βArr2-expressing cells (Fig. 6A). Similarly, expression of K44A had little effect on HEKTSHR cell-associated Alexa-TSH (3B). Importantly, expression of K44A had no effect on persistent cAMP production by TSHR in cells not expressing or expressing βArr2 (Fig. 6B). These findings show that inhibition of internalization does not affect persistent cAMP signaling by TSHR.
Figure 4.
βArrestin2 (βArr2) has no effect on persistent cAMP signaling. HEKTSHR cells were transfected with empty plasmid (mock) or with plasmid to express βArr2. After 48 h, the cells were incubated without (nothing) or with 100 mU/ml TSH at 37°C. After 30 min, the cells were washed 3 times with ice-cold acid buffer, followed by 1 wash with HBSS/HEPES. Immediately after the washes, cells were incubated in HBSS/HEPES at 37°C. At 120 min after the washes, cells were incubated in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in cell lysates. Bars represent mean ± range of duplicate measurements in 1 of 3 experiments.
Figure 5.
Dynasore nonspecifically inhibits TSHR persistent cAMP signaling. A) Dynasore inhibits TSHR internalization in βArr2-expressing HEKTSHR cells. HEKTSHR cells were transfected with empty plasmid (mock) or with plasmid to express βArr2. After 48 h, cells were incubated without (control) or with 200 or 400 μM dynasore in binding buffer. After 30 min, cells were incubated in binding buffer with dynasore and 125I-TSH at 37°C for 30 min. Cells were washed 3 times with ice-cold HBSS (total binding) or ice-cold acid buffer (acid-resistant binding), and cell radioactivity was counted. Percentage acid-resistant binding was calculated as 100 × (specific binding after acid wash/specific binding after HBSS wash). Bars represent mean ± range of duplicate determinations in one of 2 experiments. B) Dynasore inhibits persistent TSHR cAMP signaling in a manner independent of internalization. HEKTSHR cells were transfected with empty plasmid (mock) or with plasmid to express βArr2. After 48 h, cells were incubated without (control) or with 400 μM dynasore in binding buffer. After 30 min, cells were incubated in binding buffer with dynasore and 100 mU/ml TSH at 37°C. After an additional 30 min, cells were incubated in HBSS/HEPES for 2 h and then in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in cell lysates. Bars represent mean ± range of duplicate measurements in 3 experiments.
Figure 6.
A dominant negative dynamin (K44A) inhibits βArr2-induced internalization but has no effect on persistent cAMP signaling by TSHR. A) Effect of K44A on acid-resistant 125I-TSH binding. HEKTSHR cells were transfected with empty plasmid (mock), plasmid to express K44A, plasmid to express βArr2, or plasmids to coexpress K44A and βArr2. After 48 h, cells were incubated in binding buffer with 125I-TSH at 37°C for 30 min. Cells were washed 3 times with ice-cold HBSS (total binding) or ice-cold acid buffer (acid-resistant binding), and cell radioactivity was counted. Percentage acid-resistant binding was calculated as 100 × (specific binding after acid wash/specific binding after HBSS wash). Bars represent mean ± range of duplicate determinations in 1 of 2 experiments. B) K44A has no effect on persistent cAMP signaling by TSHR. HEKTSHR cells were transfected with empty plasmid (mock), plasmid to express K44A, plasmid to express βArr2, or plasmids to coexpress K44A and βArr2. After 72 h, cells were incubated in HBSS/HEPES with 100 mU/ml TSH at 37°C. After 30 min, cells were incubated in HBSS/HEPES for 2 h and then in HBSS/HEPES with 1 mM IBMX or IBMX and 100 mU/ml TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in cell lysates. Bars represent mean ± se of duplicate measurements in 3 experiments.
Persistent cAMP signaling by TSHR can be inhibited by a TSHR antagonist
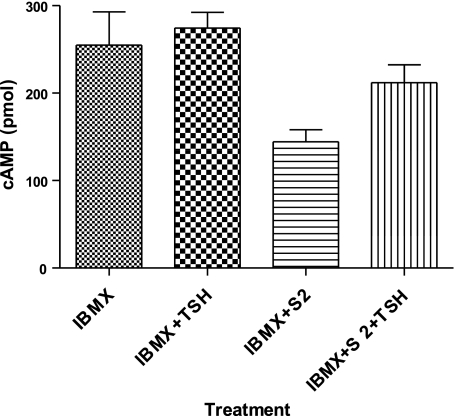
Although it was likely that the persistent cAMP signaling we observed was mediated by persistent TSHR activation, we tested this using a specific, small molecule, allosteric TSHR antagonist [S2, 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one; NCGC 00161856] that we recently discovered (12). Figure 7 shows that addition of 30 μM S2, a concentration that maximally inhibited acute stimulation of cAMP production by TSH by 60%, inhibited persistent cAMP signaling by TSHR. Moreover, addition of TSH in the presence of S2 caused an increase in cAMP production, whereas, as noted above, TSH did not increase cAMP production in the absence of S2. These data show that persistent signaling is due to persistent activation of TSHR and that when persistent TSHR activity is decreased, addition of TSH can further increase its activity.
Figure 7.
A small molecule TSHR antagonist added 2 h after TSH was removed inhibits persistent cAMP signaling by TSHR. HEKTSHR cells were incubated with 100 mU/ml TSH at 37°C. After 30 min, cells were washed 3 times with ice-cold acid buffer, followed by 1 wash with HBSS/HEPES. Immediately after the washes, cells were incubated in HBSS/HEPES at 37°C. After an additional 120 min, cells were incubated in HBSS/HEPES with 1 mM IBMX; IBMX and 100 mU/ml TSH; IBMX and 30 μM S2; or IBMX, S2, and TSH at 37°C for 30 min. Cells were lysed, and cAMP was measured in cell lysates. Bars represent mean ± range of duplicate measurements in 1 of 4 experiments.
DISCUSSION
Herein we present the following observations that allow us to conclude that persistent cAMP signaling by TSHR is independent of internalization. 1) We showed that HEKTSHR cells, in which TSHR internalization does not occur, exhibited persistent production of cAMP after stimulation by TSH. 2) When TSHR internalization was made to occur in HEKTSHR cells by overexpression of βArr2, persistent cAMP production was not affected. 3) When TSHR internalization was inhibited by dynasore, persistent signaling was affected no differently in HEKTSHR cells that exhibit TSHR internalization and those that did not. 4) Expression of K44A did not affect persistent signaling in HEKTSHR cells or in HEKTSHR cells expressing βArr2, even though K44A inhibited TSHR internalization in βArr2-expressing HEKTSHR cells. These findings show that persistent cAMP production by HEKTSHR cells stimulated with TSH occurs in the absence or presence of internalization.
We also found that persistent cAMP signaling in HEKTSHR cells stimulated with TSH for 30 min and measured 2 h after TSH was removed was inhibited by a small molecule TSHR antagonist (12). This observation shows that persistent cAMP production is dependent on continued TSHR activation and not on independent, persistent activation of Gαs or AC. This is similar to the finding of inhibition of β-adrenergic receptor signaling 3–4 min after the addition of isoproterenol by the antagonist propranolol (4).
An interesting finding in our study was that persistent cAMP production continued for several hours after TSH had been removed from the medium and the cells were washed with acid buffer as long as stimulation was with a maximally effective dose of TSH for a period > 5 min. In the previous studies of TSHR (4) and parathyroid hormone receptor (3), persistent cAMP stimulation was followed for <1 h after hormone removal, and there was no attempt to remove cell surface receptor-bound hormone. Moreover, we found that readdition of TSH to cells exhibiting persistent cAMP signaling did not further increase cAMP production. This could have been because the receptors were maximally activated or fully occupied by TSH, or were in a compartment that TSH could not enter. However, we found that inhibition of persistent signaling by S2 allowed TSHRs to respond to readdition of TSH. Although S2 as a small organic compound may readily enter cells (and thereby inhibit TSHR signaling), TSH is a 30-kDa glycoprotein hormone that enters cells primarily, if not exclusively, by receptor-mediated endocytosis. Thus, these findings suggest that the activated receptor during persistent signaling is in a readily accessible pool in which TSH can bind to TSHR rather than in an intracellular vesicular compartment. These findings, therefore, support the conclusion that persistently signaling TSHRs are at the cell surface.
An interesting question is whether persistently signaling TSHRs are persistently occupied by TSH or whether TSH has dissociated from them but the receptor remains in a more active conformation than TSHRs that have not been bound by TSH. Unfortunately, we cannot distinguish between these 2 possibilities because the antagonist we used, S2, is an inverse agonist (12) that can inhibit TSHRs that signal because they are bound by TSH and receptors that signal even though they are not bound by an agonist.
To the best of our knowledge, all studies to date to monitor persistent cAMP signaling by GPCRs have been performed in cells in tissue culture. Thus, the physiological significance of persistent cAMP signaling by GPCRs is not clear. As for TSHR signaling, it is well established that TSH levels in humans undergo a distinct circadian rhythm and that this could lead to different levels of thyroid gland function throughout the day (17). A mechanism to dampen changes in thyroid hormone levels by binding to proteins in the blood has evolved (18). It is possible that persistent signaling by TSHRs evolved as a complement to serum-binding proteins to lessen the changes in serum thyroid hormone levels through the day by prolonging the period of thyroid stimulation by TSH.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Institutes of Health (1 Z01 DK011006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Hausdorff W. P., Caron M. G., Lefkowitz R. J. (1990) Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 4, 2881–2889 [PubMed] [Google Scholar]
- 2.Hanyaloglu A. C., Von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 3.Ferrandon S., Feinstein T. N., Castro M., Wang B., Bouley R., Potts J. T., Gardella T. J., Vilardaga J. P. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calebiro D., Nikolaev V. O., Gagliani M. C., de Filippis T., Dees C., Tacchetti C., Persani L., Lohse M. J. (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 [DOI] [PubMed] [Google Scholar]
- 6.Jalink K., Moolenaar W. H. (2010) G protein-coupled receptors: the inside story. BioEssays 32, 13–16 [DOI] [PubMed] [Google Scholar]
- 7.Scarselli M., Donaldson J. G. (2009) Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J. Biol. Chem. 284, 3577–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen J. A., Yu J. Z., Donati R. J., Rasenick M. M. (2005) Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol. Pharmacol. 67, 1493–1504 [DOI] [PubMed] [Google Scholar]
- 9.Merlen C., Fabrega S., Desbuquois B., Unson C. G., Authier F. (2006) Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsalpha in rat liver. FEBS Lett. 580, 5697–5704 [DOI] [PubMed] [Google Scholar]
- 10.Neumann S., Huang W., Titus S., Krause G., Kleinau G., Alberobello A. T., Zheng W., Southall N., Inglese J., Austin C. P., Celi F. S., Gavrilova O., Thomas C. J., Raaka B. M., Gershengorn M. C. (2009) Small molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc. Natl. Acad. Sci. U. S. A. 106, 12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones B. W., Hinkle P. M. (2008) Arrestin binds to different phosphorylated regions of the thyrotropin-releasing hormone receptor with distinct functional consequences. Mol. Pharmacol. 74, 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann S., Huang W., Eliseeva E., Titus, Thomas C. J., Gershengorn M. (2010) A small molecule inverse agonist for the human TSH receptor. [E-pub ahead of print]Endocrinology PMID: 20427476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport B., McLachlan S. M. (2007) The thyrotropin receptor in Graves' disease. Thyroid 17, 911–922 [DOI] [PubMed] [Google Scholar]
- 14.Frenzel R., Voigt C., Paschke R. (2006) The human thyrotropin receptor is predominantly internalized by beta-arrestin 2. Endocrinology 147, 3114–3122 [DOI] [PubMed] [Google Scholar]
- 15.Kirchhausen T., Macia E., Pelish H. E. (2008) Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 438, 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Ferguson S. S., Barak L. S., Menard L., Caron M. G. (1996) Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J. Biol. Chem. 271, 18302–18305 [DOI] [PubMed] [Google Scholar]
- 17.Russell W., Harrison R. F., Smith N., Darzy K., Shalet S., Weetman A. P., Ross R. J. (2008) Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab. 93, 2300–2306 [DOI] [PubMed] [Google Scholar]
- 18.Robbins J., Cheng S. Y., Gershengorn M. C., Glinoer D., Cahnmann H. J., Edelnoch H. (1978) Thyroxine transport proteins of plasma: molecular properties and biosynthesis. Recent Prog. Horm. Res. 34, 477–519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.