Abstract
Mast cells contribute to allergy through IgE-dependent activation via the high-affinity IgE receptor FcεRI. The role of the FcεRIβ chain (MS4A2) in mast cell function is not understood fully, although it serves to amplify FcεRI-dependent signaling. We demonstrate the expression of a novel MS4A2 truncation lacking exon 3 in human mast cells termed MS4A2trunc. MS4A2trunc gene expression was regulated negatively by the mast cell growth factor stem cell factor (SCF), and its expression was not detected in the SCF receptor gain-of-function human mast cell line HMC-1. Unlike MS4A2, MS4A2trunc did not traffic to the cytoplasmic membrane but instead was associated with the nuclear membrane. Overexpression of MS4A2trunc induced human lung mast cell death and profoundly inhibited HMC-1 cell proliferation by inducing G2-phase cell cycle arrest and apoptosis. Thus, we have identified a novel splice variant of MS4A2 that might be important in the regulation of human mast cell proliferation and survival. This finding demonstrates that the MS4A2 gene has multiple roles, extending beyond the regulation of acute allergic responses. By understanding the mechanisms regulating its function, it might be possible to induce its expression in mast cells in vivo, which could lead to better treatments for diseases such as mastocytosis and asthma.—Cruse, G., Kaur, D., Leyland, M., Bradding, P. A novel FcεRIβ-chain truncation regulates human mast cell proliferation and survival.
Keywords: MS4A2, IgE receptor, cell cycle, G2 phase, cell membrane
Mast cells play key roles in both health and disease through their ability to release a diverse array of inflammatory mediators, chemokines, cytokines, and growth factors (1). For example, they are critical for early host defense against bacteria (2), but their chronic activation contributes to the pathophysiology of many diverse diseases, including rheumatoid disease, tissue fibrosis, allergy, and asthma (3–5). Furthermore, mast cell proliferation associated with mutations in their c-KIT (CD117) stem cell factor (SCF) receptor causes systemic mastocytosis, which can vary in severity from a minor nuisance to life-threatening mast cell leukemia for which no satisfactory therapy exists at present (6). Understanding the mechanisms regulating mast cell function in terms of mediator release, cell growth, differentiation, and survival might therefore uncover novel targets for the treatment of a number of devastating diseases.
Mast cells express the high-affinity IgE receptor (FcεRI), which mediates their activation by IgE alone or following IgE-cross-linking by allergen (1, 7, 8). This tetrameric receptor consists of an α chain that binds IgE, two signal-transducing γ chains, and in many human cells including mast cells, a β chain. This β chain is part of the membrane-spanning 4 A (MS4A) gene family (9). The actual function of the β chain of FcεRI (MS4A2) is not fully understood. Transfection experiments suggest that MS4A2 is required for receptor expression in the mouse where only tetrameric FcεRI (αβγ2) is expressed on the surface (10). Thus, MS4A2 is required for the maturation and expression of the α chain of FcεRI chaperoning it to the cytoplasmic membrane and receptor complex. However, in humans it is less clear since the human α chain can be expressed on the cell surface without MS4A2 (10), and in certain human cells trimeric FcεRI (αγ2) can be expressed (11). However, in human mast cells, only tetrameric FcεRI exists (11). Although its expression is not essential for IgE receptor signaling in human cells (12), MS4A2 is known to act as an amplifier of FcεRI-dependent signaling (13, 14), and for the γ chains of CD16 (14). This effect is achieved through its ability to bind and activate the membrane-associated tyrosine kinase, Lyn kinase, through the C-terminal noncanonical immunoreceptor tyrosine-based activation motif (ITAM) of MS4A2, which in turn recruits and phosphorylates Syk tyrosine kinase amplifying the Syk kinase phosphorylation from the canonical γ subunit ITAMs (1, 15, 16).
Interestingly, a truncation of MS4A2 has been described in human cord-blood-derived mast cells and basophils, which arises from an inclusion of intron 5 and results in an in-frame stop codon (MS4A2 variant 2) (17). This truncation decreases the expression of FcεRI, since it chaperones the α chain to endosomes for degradation instead of to the cytoplasmic membrane (17), although the effects on cell function are not known.
In this study, we have identified the expression and regulation of a novel truncated variant of MS4A2 (MS4A2trunc), which is expressed in primary human lung mast cells (HLMCs) and the slowly dividing human mast cell line LAD-2, but not the rapidly dividing human mast cell line HMC-1. We demonstrate that, unlike MS4A2, MS4A2trunc traffics to the nuclear membrane region. Transduction of MS4A2trunc induces HLMC death and profoundly inhibits proliferation of HMC-1 cells through the arrest of the cell cycle at the G2 checkpoint and initiates apoptosis. This finding suggests that the loss of expression of MS4A2trunc might represent an important step in the progression to mast cell neoplasia and that overexpression of this gene product might be useful for the treatment of mast cell-associated disease.
MATERIALS AND METHODS
Cell lines
The HMC-1 cell line was a generous gift from Dr. J. Butterfield (Mayo Clinic, Rochester, MN, USA). The cells were cultured, as described previously (18), in Iscove's medium containing 10% iron-supplemented fetal calf serum and 1.2 mM -thioglycerol. Cells were split 1:10 every 7 d and resuspended in fresh medium.
LAD-2 cells were obtained from Dr. D. Metcalfe (National Institutes of Health, Bethesda, MD, USA). The cells were cultured, as described previously (19), in StemPro-34 medium containing supplement, 1% antibiotic/antimycotic solution (all from Sigma-Aldrich, Dorset, UK) and 1% nonessential amino acids (Life Technologies, Paisley, UK) with 100 ng/ml recombinant human SCF added (R&D, Abingdon, UK). Half of the medium supplemented with SCF was changed every 7 d.
HLMC purification and culture
All human subjects gave written informed consent, and the study was approved by the Leicestershire Research Ethics Committee, UK. HLMCs were dispersed from lung tissue obtained by resection for bronchial carcinoma and purified using immunoaffinity magnetic selection using anti-CD117 (BD Biosciences, San Diego, CA, USA) as described previously (20). HLMC purity was >98% with cell viability >97% (monitored by exclusion of trypan blue). HLMCs were cultured as described previously (21).
MS4A2 cloning
Total RNA was isolated from 2 × 106 mast cells using the QiagenRNEasy kit, which was used according to the manufacturer's instructions (Qiagen, Crawley, UK). The full open reading frame (ORF) of MS4A2 was amplified using primers designed to incorporate EcoRI and BamHI restriction sites at the 5′ and 3′ ends of the clone, respectively, for directional cloning vectors. The primers used for cloning are shown in Table 1.
Table 1.
Primer sequences for molecular biology
| Primer | Sequence |
|---|---|
| MS4A2 cloning forward | 5′-GGGGAATTCATGGACACAGAAAGTAATAGGAG-3′ |
| MS4A2 cloning reverse | 5′-GGGGGATCCTTATAAATCAATGGGAGGAGAC-3′ |
| QRT-PCR MS4A2 forward | 5′-AATCTTGCTCTCCCACAGGA-3′ |
| QRT-PCR MS4A2 reverse | 5′-TGTGTTACCCCCAGGAACTC-3′ |
| QRT-PCR MS4A2trunc forward | 5′-AATCTTGCTCTCCCACAGGA-3′ |
| QRT-PCR MS4A2trunc reverse | 5′-ATAGAAAACCCCAGGAACTC-3′ |
| EGFP tagging forward | 5′-GGGGAGCTCATGGACACAGAAAGTAATAGGAG-3′ |
| EGFP tagging reverse | 5′-GGGCCGCGGTAAATCAATGGGAGGAGACATTTC-3′ |
The MS4A2 genes were cloned using the selected primers and a single tube AccessQuick™ RT-PCR System according to the manufacturer's instructions (Promega, Southampton, UK). A final concentration of 2 μM of each primer was used, and 1 μg of total RNA was added to each tube.
The protocol for RT-PCR was performed as follows: Reverse transcription was carried out at 48°C for 45 min followed by 5 min at 95°C using the gene-specific primers. Forty cycles of melting at 95°C for 1 min, annealing at 50°C for 1 min followed by extension at 72°C for 2 min were performed. A final extension step of 7 min at 72°C was performed after the 40 cycles. Loading dye (10 μl of 6×) was added to each sample, and products were run on a 1.5% agarose gel containing 0.5 μg/ml ethidium bromide for 1 h at 80V. Gels were visualized on an UV-emitting light box, and bands were excised using a clean scalpel blade. DNA from the excised bands was purified using the QIAquick Gel Extraction Kit (Qiagen) according to the manufacturer's instructions.
Purified MS4A2 cDNA was incorporated into the pGEM® T Easy Vector System according to the manufacturer's instructions (Promega). Briefly, 3 μl of cDNA was incubated with 5 μl of 2× reaction buffer, 1 μl of pGEM T easy vector, and 1 μl of T4 DNA ligase and incubated overnight at 4°C. 50 μl of highly competent JM109 Escherichia coli cells were then transformed with the MS4A2 clones and plated out on agar plates containing 100 μg/ml ampicillin, with 100 μl of IPTG and 20 μl of X-galactose added. Transformed colonies were then positively selected by colony appearance (i.e., white colonies), subcultured into a broth culture containing 100 μg/ml ampicillin, and incubated overnight with rotational agitation at 150 rpm. Cultures were then centrifuged at 5000 g for 5 min. Transformed cell pellets were lysed, and the cDNA was purified using the Wizard SV Plasmid Purification Kit according to the manufacturer's instructions (Promega). The resulting cDNA constructs were sequenced to confirm clone integrity (Protein and Nucleic Acids Chemistry Laboratory, University of Leicester).
Quantitative real-time RT-PCR
For the quantitative real-time RT-PCR, primers were designed to amplify each individual splice variant of MS4A2 specifically. Since the splice variants are a result of a loss of exon 3, the junction between exon 2 and exon 3 in the full-length variant and exon 2 and exon 4 in the truncation were targeted. Thus, for the truncation an antisense primer was designed to span the exon-exon junction of the truncation, resulting in only the truncated variant being reverse transcribed. The primers used for QPCR are shown in Table 1.
Quantitative RT-PCR was carried out using the FullVelocity® SYBR® Green QRT-PCR system (Stratagene, Amsterdam, The Netherlands) as described previously (21). Products were also run on a 1.5% agarose gel to confirm that the products were the expected length. Bands were then excised from the gel and sequenced.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
HLMCs and HMC-1 cells (4×106) from the indicated conditions were washed with cold PBS and resuspended in ice-cold RIPA lysis buffer containing protease inhibitors. The insoluble debris was removed by centrifugation at 10,000 g for 10 min. Proteins were then mixed with 2× SDS loading buffer and heated at 100°C for 10 min. Proteins were then loaded in a 12% NuPAGE Novex gel (Invitrogen) and run for 1 h at 200V. For Western blotting, the proteins were blotted onto a nitrocellulose membrane after electrophoresis. The membranes were blocked in 5% nonfat milk in PBS 0.1% Tween20 then incubated with either anti-MS4A2 (clones N-17, S-17, C-18) (Santa Cruz Biotechnologies, Heidelberg, Germany), Cdk2 phospho Thr160, Cdk1, or Cdk1 phospho Tyr15 (all from Abcam, Cambridge, UK.) overnight. HRP-conjugated secondary antibodies (Dako, Cambridge, UK) were used to visualize the bands.
Transduction of MS4A2 clones into HLMCs and HMC-1 cells
The Ad5C20Att01 virus (BioFocus DPI, Leiden, The Netherlands) was used for HLMC and HMC-1 transduction (22). The eGFP control virus (batch ID 12538) was used to optimize transduction and was dispatched at a titer of 2.11 × 108 infective units (IFU)/ml. For the optimization, a multiplicity of infection (MOI) of 1 to 50 IFU/HLMC was used. Optimization determined that a MOI of 10 IFU of the virus per HLMC was sufficient for ∼100% transduction efficiency after 48 h with minimal toxicity.
Cell survival assays–trypan blue method
HLMCs were plated at 5 × 104 cells/well, and HMC-1 cells were plated at 2.5 × 104 cells/well in 24-well plates in duplicate. HLMCs were plated in 1 ml (final volume) of DMEM 10% FBS containing 1% antibiotic/antimycotic, 1% nonessential amino acids, and 100 ng/ml of SCF. HMC-1 cells were plated in 1 ml (final volume) of Iscove's medium containing 10% iron-supplemented fetal calf serum and 1.2 mM thioglycerol. The appropriate virus was added to each condition at an MOI of 10 IFU/cell. The cells were incubated at 37°C in a humidified incubator flushed with 5% CO2 for the indicated time. At the end of the incubation, cells were removed and centrifuged at 250 g for 5 min. The cells were resuspended in 20 μl of DMEM and 20 μl of trypan blue solution was added. Cells were then counted and viability was assayed by exclusion of trypan blue dye using a hemocytometer.
Apoptosis assays
Apoptosis was assayed using propidium iodide (PI) and annexin V staining. HLMCs and HMC-1 cells were plated at 2 × 105 cells/well in 1 ml (final volume) of appropriate medium in duplicate in a 24-well plate. HLMCs were plated in DMEM 10% FBS containing 1% antibiotic/antimycotic, 1% nonessential amino acids (all from Invitrogen), and 100 ng/ml of SCF. HMC-1 cells were plated in Iscove's medium containing 10% iron-supplemented fetal calf serum and 1.2 mM thioglycerol. The appropriate adenovirus was added to each condition at an MOI of 10 IFU/cell. Cells were incubated for 24 or 48 h at 37°C in a humidified incubator flushed with 5% CO2. After the incubation, the duplicate wells were combined and the cells were centrifuged at 250 g for 5 min. The supernatant was removed, and the cell pellets were washed in 2 ml of cold PBS and split into 4 FACS tubes. The tubes were then centrifuged again at 250 g for 5 min. The cell pellets were resuspended in 195 μl of either annexin binding buffer (ABB) or HEPES buffer without calcium. FITC (1 μl) conjugated annexin V was added to the annexin control, the annexin in ABB, and the annexin plus PI conditions. The tubes were then incubated at room temperature for 15 min. Samples were then analyzed using 2-color flow cytometry FACS. For the conditions containing PI, 2 μl (0.5 μg/ml) of PI was added to the tube immediately prior to analysis on the BD FACSCanto flow cytometer (BD Biosciences).
Cell cycle analysis
For the analysis of the cell cycle, we used flow cytometry and PI after incubation with RNase A. The same cells were used as for the apoptosis assays. The remaining cells after the apoptosis assays were fixed overnight in 70% ethanol at 4°C, centrifuged at 250 g for 8 min, and resuspended in PBS 0.1% BSA and stored at 4°C until analysis. Prior to analysis, cells were washed 2× in PBS and resuspended in 100 μl of PBS. Cells were then incubated for 30 min with 100 μg/ml RNase A at room temperature, stained with 3 μg/ml PI for 30 min, and analyzed on the BD FACSCanto flow cytometer. Data was acquired on forward scatter (FSC) against light side scatter (SSC), and a secondary gate was placed around the single cell population on a pulse area vs. pulse width dot plot and analyzed for cell cycle analysis.
Proliferation assays–3H-thymidine uptake
For the 3H-thymidine uptake proliferation assays, 100 μl of HMC-1 medium or HLMC culture medium supplemented with 2× final concentration of cytokines containing 4×103 HMC-1 cells and 8×103 HLMCs, respectively, were plated out into a V bottom 96-well plate in triplicate. Either HMC-1 medium (100 μl) or HLMC culture medium (100 μl) was then added to the control wells. IFU of Ad5C20Att01 adenovirus (4×104 and 8×104; BioFocus DPI) was diluted into 100 μl of HMC-1 culture medium and HLMC culture medium, respectively, and added to the appropriate cells. Thus, the final MOI was 10 IFU/mast cell of either the GFP cDNA control virus or the custom made MS4A2trunc cDNA virus. A low control containing either HMC-1 cells in HMC-1 medium without FBS or HLMCs in culture medium without the addition of cytokines was also included.
Cells were then incubated for 72 h with the viruses at 37°C in a humidified incubator flushed with 5% CO2. After 72 h, the cells were centrifuged at 250 g for 5 min and the supernatants removed. The cell pellets were then resuspended in 200 μl of either HMC-1 medium or HLMC culture medium supplemented with cytokines containing 1 μl/ml of 3H-thymidine (Amersham) to make a final concentration of 37 kBq/well. The cells were then incubated for 16 h at 37°C in a humidified incubator flushed with 5% CO2. Following the incubation, the plates were then centrifuged at 400 g for 5 min, and the supernatant was removed and discarded. The cell pellets were washed 2× with 200 μl of PBS by centrifugation (400 g for 5 min each). Cell pellets were then resuspended in 5% TCA and incubated for 1 h at 4°C. The cells were again centrifuged at 400 g for 5 min, and the supernatant was discarded. Absolute ethanol (50 μl) was added to each well and left overnight to evaporate. The following day, 200 μl of 1% SDS was added to each well and mixed thoroughly. This was then added to 10 ml of liquid scintillant and analyzed on the Tri-Carb® scintillation analyzer (Packard Instrument Co., Downers Grove, IL, USA). Data were retrieved as counts per minute and calculated by removing the background counts (using 200 μl of 1% SDS in 10 ml of scintillant).
Generation of GFP-MS4A2 chimeric constructs
MS4A2 clones were GFP tagged using the pEGFP N1 vector from Clontech (Basingstoke, UK). This vector incorporates GFP cDNA onto the 3′ end of the cloned inserts. Therefore, the stop codon on the 3′ end of the ORF was removed, and primers were designed to incorporate SacI and SacII at the 5′ and 3′ ends, respectively (Table 1).
Constructs were amplified using the following parameters: 94°C for 5 min, followed by 25 cycles of 94°C for 1 min, 50°C for 1 min, and 68°C for 2 min, followed by a final extension step of 68°C for 7 min. cDNA (100 ng) was used for each reaction with a primer concentration of 1 μM for each. PCR products were then cloned into pGEM T Easy as described above and sequenced. MS4A2 constructs were digested with SacI and SacII and subcloned into pEGFP-N1. These EGFP-MS4A2 constructs were checked by DNA sequencing prior to use.
Transfection of GFP-tagged MS4A proteins into LAD-2 cells
LAD-2 cells were transfected using the Nuclector® machine from Amaxa (Cologne, Germany). LAD-2 cells (1×106) were centrifuged very gently at 90 g for 10 min. Cells were then washed once in PBS by centrifugation again at 90 g for 10 min. LAD-2 cells were then resuspended in 100 μl of the transfection medium designed for use with the Amaxa system containing 2 μg of the appropriate cloned cDNA. The kit used was the basic nucleofector kit for primary mammalian epithelial cells. The mixture was then placed into cuvettes supplied with the kit and inserted into the nucleofector machine. The program for aortic smooth muscle was selected and run. Cells were then plated out in 35-mm glass-bottom fluorodishes with 2 ml of DMEM without phenol red with 100 ng/ml of SCF added, and left overnight at 37°C in a humidified incubator for confocal analysis the following day.
Transfection of GFP tagged MS4A proteins into HMC-1 cells
For the transfection of HMC-1 cells, it was possible to achieve modest transfection efficiency using lipid based technologies due to the rapid replication of the HMC-1 cells. Thus for the HMC-1 cells, transfection was achieved using FuGene HD reagent (Roche, Basel, Switzerland). HMC-1 cells (1×105) were plated into 1 ml of HAM F12 medium containing 10% FBS and 1% nonessential amino acids (Invitrogen) into a 24-well culture plate. The cells were then allowed to rest in a humidified incubator set at 37°C and flushed with 5% CO2. MS4A2 cDNA (500 ng) in pEGFP N1 was mixed with 2 μl of FuGene HD reagent in 100 μl of DMEM with no added supplements and incubated for 20 min at room temperature. The mixture was then added dropwise to the cells and mixed. Analysis was then performed 24–48 h after transfection.
Confocal microscopy
Confocal microscopy was performed on the Olympus FV1000 microscope (Olympus, Tokyo, Japan) within the advanced microscopy suite in the Department of Biochemistry, University of Leicester. Images were taken with live cells in 35-mm glass-bottom fluorodishes kept at 37°C. Images were acquired under oil immersion and analyzed using Fluoview FV10-ASW 1.6 (Olympus) and ImageJ 1.37 (National Institutes of Health) software.
FcεRI expression assays
To determine the level of FcεRI expression in HLMCs, we used flow cytometry as described previously (23), using the monoclonal FcεRI antibody clone 3G6 (Upstate Biotechnology, NY, USA). HLMCs were transduced with Ad5C20Att01 adenovirus 48 h prior to analysis. For the level of expression, the log of the geometric mean fluorescence intensity was calculated for each condition, and the percentage of control was determined.
Statistical analysis
Data are presented as means ± se. Differences between paired data sets were analyzed using a 2-tailed Student's paired t test. Differences among multiple data sets were analyzed using 1-way ANOVA with Tukey's posttest. P < 0.05 was considered statistically significant. ANOVA analyses for QRT-PCR were performed on the ratio data of the normalizer gene and the gene of interest Ct values.
RESULTS
Identification of a novel truncation of MS4A2
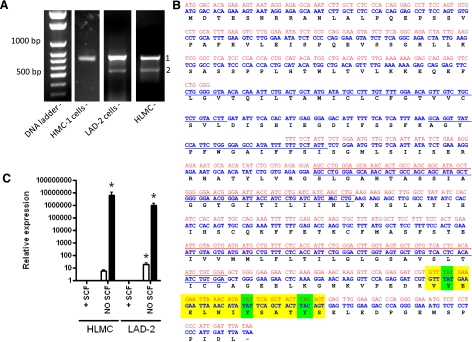
For the analysis of expression and function of MS4A2, we first cloned the full ORF of MS4A2 using RT-PCR. Bands of the expected size (753 bp) were amplified from HLMCs and the HMC-1 and LAD2 cell lines (Fig. 1A). DNA sequencing confirmed the identity of these bands as MS4A2, and no differences were found among the published sequences. However, in HLMCs and LAD2 cells, a smaller band of ∼600 bp was consistently detected (Fig. 1A). Sequencing of this band showed that it was not an artifact due to non-specific priming (Fig. 1B). We will refer to this as MS4A2trunc. Comparison of the sequence of MS4A2trunc with genomic DNA showed that this isoform lacked exon 3 of MS4A2 (Fig. 1B).
Figure 1.
Identification and regulation of a novel MS4A2 variant. A) RT-PCR cloning of MS4A2 from HMC-1 cells, LAD-2 cells, and HLMCs. Two bands were present in the LAD-2 cells and HLMCs but not in the HMC-1 cells. An expected band of ∼750 bp (band 1) and a shorter band of ∼600 bp (band 2). Each panel is from a different gel. B) Nucleotide and predicted amino acid sequence of MS4A2 (blue) and MS4A2trunc (red). Sequence remains in frame so MS4A2trunc would retain both N and C termini of MS4A2, including the noncanonical ITAM (highlighted in yellow). Predicted transmembrane regions are underscored. C) Quantitative real-time RT-PCR of MS4A2 (open bars) and MS4A2trunc expression (solid bars) in HLMCs and LAD-2 cells. Data are presented as means ± se; n = 10 from 5 separate donors for HLMCs, n = 7 for LAD-2 cells. *P < 0.001 vs. control; ANOVA with Tukey's posttest.
Using a readily available predictor of α-helical transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM/), the predicted topology of MS4A2 is that it spans the membrane 4 times with cytoplasmic N and C termini. However, the truncation of exon 3, which occurs in MS4A2trunc is predicted to span the membrane only twice (Fig. 1B) with the first and second transmembrane domains being lost. The N and C termini are still predicted to be cytoplasmic, and the protein would retain its ITAM, which performs important functions in the full-length variant.
MS4A2 gene regulation
We next analyzed the regulation of gene expression of both MS4A2 and MS4A2trunc in HLMCs and LAD-2 cells. Interestingly, in both HLMCs and LAD-2 cells, a profound up-regulation of MS4A2trunc gene expression with withdrawal of SCF was noted (Fig. 1C). Most HLMC donors had little or no expression of MS4A2trunc in the presence of SCF. With SCF withdrawal MS4A2trunc expression was induced in these donors; even in the donors that expressed low levels of MS4A2trunc when SCF was present, SCF withdrawal profoundly up-regulated MS4A2trunc expression (n=10, P<0.001, ANOVA) (Fig. 1C).
Transduction and overexpression of the MS4A2 variants in human mast cells
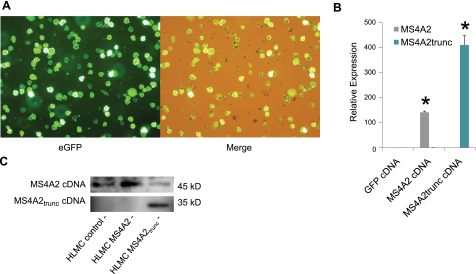
HLMCs were transduced efficiently using an adenoviral delivery system described previously (21). Using an MOI of 10 IFU/HLMC, we could achieve up to 100% transduction efficiency when using the eGFP control virus (Fig. 2A). To confirm the transduction of both MS4A2 and MS4A2trunc, we first performed QPCR on transduced HLMCs. HLMCs transduced with MS4A2 and MS4A2trunc demonstrated marked up-regulation of respective mRNA expression. Thus, the relative expression of MS4A2 after transduction with cDNA was 141.6 ± 4.9-fold that of the GFP virus control (Fig. 2B) (n=8, P<0.000001, t test). The relative expression of MS4A2trunc was more markedly up-regulated, due to a lower copy number starting point, with a relative expression of 410.3 ± 38.7-fold over the GFP virus control (Fig. 2B) (n=8, P<0.000001, t test).
Figure 2.
MS4A2 and MS4A2trunc can be successfully transduced into human mast cells. A) Adenovirus Ad5C20Att01 efficiently transduces eGFP cDNA into primary ex vivo HLMCs. Merged image (right panel) demonstrates that no GFP− cells are evident under visible light. B) Using viruses constructed to contain the full-length ORF of MS4A2 and MS4A2trunc, transduction leads to overexpression of the corresponding mRNA for each variant assessed using quantitative real-time RT-PCR. Data are means ± se of 8 experiments from 4 donors. *P < 0.0001 vs. control. C) Western blot demonstrating that transduction of the cDNA for each MS4A2 variant leads to the overexpression of the corresponding protein. Each panel is from a separate gel with different antibodies.
To confirm that overexpression of the MS4A2 variants was translated into MS4A2 protein, we next tested for MS4A2 variant protein expression using Western blotting. We could demonstrate that both MS4A2 variants were overexpressed at the protein level as expected (Fig. 2C).
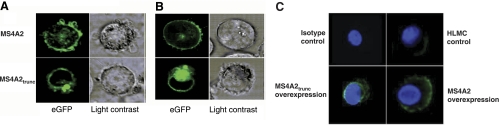
MS4A2trunc traffics preferentially to the perinuclear region
We next studied the intracellular localization of both MS4A2 and MS4A2trunc in human mast cells. As expected, transfection of chimeric GFP-MS4A2 cDNA into LAD-2 cells using nucleofection demonstrated plasma membrane localization of the protein when using confocal microscopy (Fig. 3A). However, transfection of GFP-MS4A2trunc chimeric cDNA demonstrated a preferential perinuclear localization suggesting targeting to the nuclear membrane (Fig. 3A). Similar results were seen in HMC-1 cells using the lipofection technique to transfect the cells (Fig. 3B). In addition, immunofluorescent staining of HLMCs following adenoviral transduction of MS4A2 variants without GFP tags demonstrated a similar staining pattern indicating that GFP did not affect trafficking of the proteins (Fig. 3C).
Figure 3.
MS4A2trunc localizes to the nuclear membrane rather than the cytoplasmic membrane. A) Confocal micrographs of LAD-2 cells transfected with MS4A2 (top panels) and MS4A2trunc (bottom panels) tagged with eGFP (pEGFP-N1) using nucleofection. MS4A2 localized to the cytoplasmic membrane. However, the truncated variant localized to the perinuclear region. B) Similar results were seen using lipofection of HMC-1 cells. C) A similar staining pattern was observed in HLMCs after overexpression with adenoviral transduction of untagged MS4A2 variants stained using FITC immunofluorescence. Images from Olympus FV1000 confocal laser scanning microscope analyzed using Fluoview FV10-ASW v1.6 software. View ×600 at 37°C.
Transduction of MS4A2trunc into human mast cells inhibits cell proliferation and survival
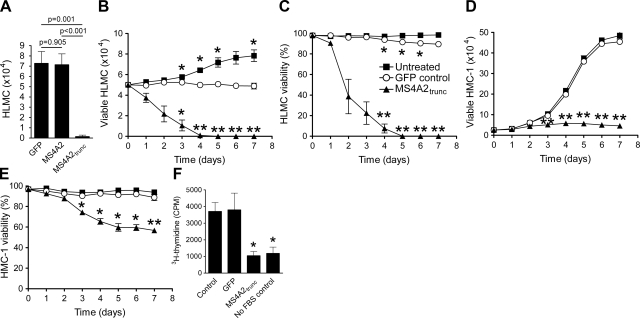
Having confirmed the overexpression of the MS4A2 variants in human mast cells, we next looked at the function of these proteins. We first examined any potential cytotoxicity of the viruses on the cells. After 7 d of culture with the viruses, no significant difference was found in HLMC numbers between the GFP virus control cells and the MS4A2 overexpression cells (Fig. 4A) (n=6, P=0.905). However, with the overexpression of MS4A2trunc, marked cell death with 0.1 ± 0.1 × 104 HLMCs in the MS4A2trunc transductions was found compared with 7.3 ± 1.2 × 104 HLMCs in the GFP control transductions (Fig. 4A) (n=6, P=0.001).
Figure 4.
MS4A2trunc inhibits proliferation and initiates cell death in human mast cells. A) Transduction of MS4A2 had no effect on HLMC viability after 7 d when compared to the GFP control virus. However, transduction of MS4A2trunc leads to cell death by d 7. B) Time course study shows that HLMCs had died by 5 d after transduction of MS4A2trunc. GFP control virus had a minor effect on cell number compared to no-transduction control but did not induce HLMC death. C) In parallel, HLMC viability declined rapidly following MS4A2trunc transduction. D) In HMC-1 cells, transduction of MS4A2trunc profoundly inhibited proliferation. E) HMC-1 viability was reduced by ∼50% by d 7. F) Antiproliferative effect of MS4A2trunc in HMC-1 was also evident when measuring 3H-thymidine incorporation into DNA. Data are means ±se from 6 (A) or 4 (B–F) experiments. *P < 0.05, **P < 0.01; ANOVA with Tukey's posttest.
We next performed a cell survival and proliferation assay to obtain a time course for the death of the HLMCs. HLMC death reached 100% by d 5 after transduction with MS4A2trunc (Fig. 4B). A small but significant effect of the GFP virus on HLMC survival and viability was found when compared to untreated cells, although this condition was much less marked than MS4A2trunc (Fig. 4B, C). Since HMC-1 cells do not natively express MS4A2trunc, we also performed these experiments on HMC-1. Interestingly, we found that exogenous expression of MS4A2trunc markedly inhibited the proliferation of HMC-1 cells when compared to untreated or GFP virus-treated cells (Fig. 4D). The addition of the GFP virus had no significant effect on HMC-1 cell proliferation or viability (Fig. 4D, E). HMC-1 cells transduced with MS4A2trunc had significantly reduced viability after d 3, which was as low as 56.7 ± 3.4% by d 7 (Fig. 4E) (n=4, P=0.006).
To confirm that MS4A2trunc was indeed antiproliferative, we next performed 3H-thymidine incorporation assays on both HLMCs and HMC-1 cells. HLMCs proliferate slowly in culture and had very low incorporation of 3H-thymidine (not shown). HMC-1 cells transduced with MS4A2trunc incorporated considerably less 3H-thymidine than either untreated or GFP virus-treated HMC-1 (Fig. 4F) (n=4, P=0.016 and 0.049, respectively).
MS4A2trunc induces apoptosis in human mast cells
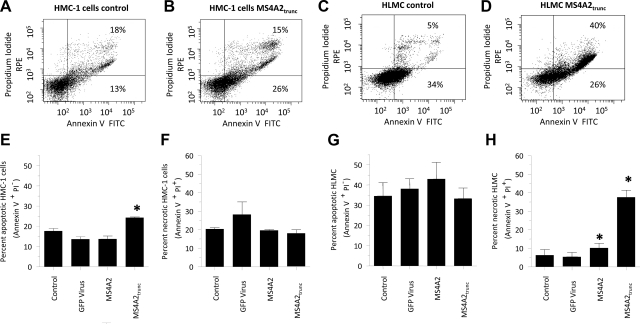
Since the expression of MS4A2trunc affects the viability of both HLMCs and HMC-1 cells, we next examined the effects of overexpression on apoptosis and cell death using annexin V and propidium iodide (PI) staining, respectively. Since the expression of GFP would induce fluorescence of the cells, the use of the GFP virus control was not compatible with the apoptosis assays. We therefore used a shRNA virus for GFP as the GFP control virus for these assays in addition to the MS4A2 virus, which would also act as an appropriate control. We found that after 48 h transduction of HMC-1 cells with either (shRNA)GFP or MS4A2 had no effect on apoptosis compared to untreated cells (Fig. 5). However, transduction of HMC-1 cells with MS4A2trunc significantly increased apoptosis (Fig. 5). Thus, we found 13.6 ± 1.2% apoptotic cells with the transduction of (shRNA)GFP virus control compared to 24.3 ± 0.3% with MS4A2trunc (Fig. 5E) (n=3, P=0.0198). No increase in PI+ HMC-1 cells was found. This indicates that although induction of apoptosis occurs with MS4A2trunc overexpression, the profound effects on HMC-1 proliferation cannot be attributed to cell necrosis. Annexin V staining in HLMCs 48 h after control transduction was relatively high due to constitutive degranulation, which is evident in mast cells (24). This finding did not increase further following transduction with MS4A2trunc, but a marked increase in the number of PI+ cells at 48 h indicating significant cell death was noted (Fig. 5H).
Figure 5.
Transduction of MS4A2trunc induces HMC-1 apoptosis and HLMC death. A) Scatter plot of HMC-1 adenoviral-treated control cells stained for annexin V (x axis) and propidium iodide (y axis) 48 h after transduction. B) Transduction of MS4A2trunc increases the proportion of annexin V+ PI− cells present at 48 h, which is indicative of apoptosis. C) At 48 h after transduction, HLMC annexin binding was relatively high in the control cells (34% stained). D) HLMC annexin V staining did not change with the addition of MS4A2trunc, but the percentage of dead PI+ cells increased markedly. E–H) Graphical representation of the means ±se of 3 separate experiments in annexin V+ PI− (E, G) and annexin V+ PI+ (F, H) HMC-1 cells (E, F) and HLMCs (G, H), demonstrating the changes in annexin V and PI expression in the different cell populations. *P < 0.05.
The antiproliferative effects of MS4A2trunc are mediated via G2 cell cycle arrest
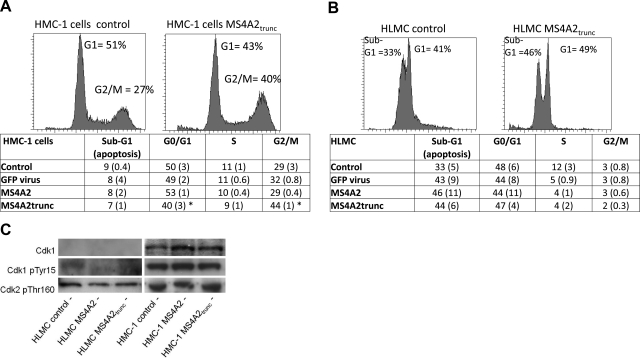
To delineate the mechanism behind the antiproliferative effects of MS4A2trunc, we next examined its effects on the cell cycle using flow cytometry. We found significantly fewer HMC-1 cells in G0/G1 phase with transduction of MS4A2trunc (39.6±3.1%) when compared to untreated cells (50.4±3.1%, n=3, P=0.040), (shRNA)GFP transduction (48.8±2.2%, n= 3, P=0.0169) and MS4A2 transduction (52.7±1.4%, n=3, P=0.0160) (Fig. 6A). This reduction in G0/G1 phase was mirrored by an increase in cells at G2/M phase. Thus, for HMC-1 cells, we found 44.2 ± 1.0% G2/M phase cells with transduction of MS4A2trunc compared to 28.8 ± 3.4% in the untreated cells (n=3, P=0.0417), 31.6 ± 0.8% in the (shRNA)GFP transductions (n=3, P=0.020), and 29.3 ± 0.4% in the MS4A2 transductions (n=3, P=0.009) (Fig. 6A).
Figure 6.
Antiproliferative effect of MS4A2trunc in HMC-1 cells is due to G2/M phase arrest. A) Transduction of MS4A2trunc in HMC-1 cells induced an increase in the number of cells in G2/M phase when compared to control. This was accompanied by a decrease in cells in G1 phase consistent with G2 arrest. Notably, this effect was not evident with either GFP control virus or overexpression of MS4A2. Data in the table are means and se (in parentheses) of 3 separate experiments undertaken 48 h after transduction. B) No significant difference in the cell cycle was evident in HLMCs, probably attributable to very few cells progressing through to S and G2/M phases. C) No difference was found among the level of Cdk1, phosphorylated Cdk1 (Tyr15), or phosphorylated Cdk2 (Thr160) with the overexpression of either MS4A2 or MS4A2trunc in HMC-1 cells.
The effect on cell cycle in the HMC-1 cells was not evident in the HLMCs (Fig. 6B). However, HLMCs replicate slowly (25, 26), and the cell cycle analysis did not appear as the typical histogram seen for the HMC-1 cells. Nevertheless, significant cell death was evident in these cells as demonstrated by populations of cells in sub-G0/G1. We found no significant differences among any of the cell cycle populations when comparing the different virus constructs. However, all constructs increased the percentage of cells in G0, which is consistent with the small increase in nonviable cells with the GFP virus in the survival assays. These assays were performed 48 h postinfection with the adenovirus, which was adequate for good protein expression. However, assays performed at 24 h had very similar results in the HLMCs and HMC-1 cells (not shown).
Because HMC-1 cells were arrested in the G2/M phase with the exogenous expression of MS4A2trunc, we next examined the regulatory phosphorylation of the cyclin-dependent kinases (Cdk). No apparent difference was found in phosphorylated Thr160 on Cdk2 in either HMC-1 cells or HLMCs (Fig. 6C). Similarly, no difference was found in either total Cdk1 or phosphorylated (Tyr15) Cdk1 in HMC-1 cells transduced with MS4A2trunc (Fig. 6C). The expression of Cdk1 was difficult to detect in the HLMCs, possibly due to the lack of HLMCs in G2/M phase as demonstrated by the cell cycle analysis (Fig. 6B).
Overexpression of MS4A2trunc has no effect on surface FcεRIα expression in HLMCs
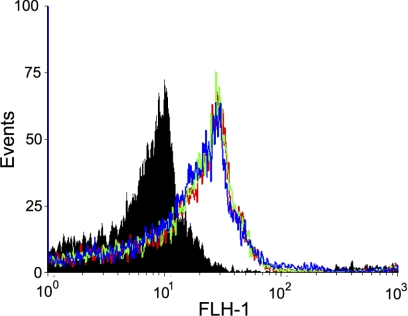
Since a truncation of MS4A2 has been reported to act as a dominant-negative for FcεRI expression in human cord-blood-derived mast cells and basophils (17), we next examined the effects of overexpression of MS4A2 and MS4A2trunc on FcεRI expression. A good level of FcεRIα chain expression was evident in HLMCs, which were cultured for 4 wk (Fig. 7). Surprisingly, this level of expression was not increased with MS4A2 overexpression. Neither was it significantly affected by overexpression of MS4A2trunc (Fig. 7, n=5).
Figure 7.
Overexpression of MS4A2trunc does not reduce FcεRI expression in HLMCs. Histogram shows the fluorescence intensity of cells stained for FcεRIα. No significant difference was found between MS4A2trunc-overexpressing cells (blue), when compared to either MS4A2-overexpressing cells (green) or virus-treated control cells (red). Solid filled histogram is the isotype control. Data are representative of 5 donors.
DISCUSSION
In this study we have identified a novel truncation of the high-affinity IgE receptor β subunit, MS4A2. Unlike the full-length variant, this novel protein (MS4A2trunc) associates with the perinuclear region rather than the cytoplasmic membrane. This cellular localization is likely to be critical for its function since overexpression of this protein induces cell cycle arrest at the G2 checkpoint, leading to loss of cell proliferation and the initiation of apoptosis. Interestingly, this truncation was expressed in HLMCs and LAD-2 cells, which are slowly replicating cells, and was not expressed in HMC-1 cells, which proliferate rapidly. The expression of MS4A2trunc was not always present at baseline in the presence of the mast cell growth and survival factor SCF, but removal of SCF induced MS4A2trunc expression. In addition, MS4A2trunc was not expressed in the rapidly proliferating HMC-1 cell line, which contains SCF-receptor-gain-of-function mutations resulting in constitutive cKIT signaling. These observations suggest that MS4A2trunc expression is suppressed by the SCF receptor cKIT and induction of expression negatively regulates the cell cycle and proliferation. Thus, loss of expression of this gene could be a step in the development of mastocytosis and mast cell neoplasia.
MS4A2 is an integral subunit of the high-affinity IgE receptor FcεRI. Although its expression is not essential for FcεRI signaling (12), MS4A2 is known to amplify signaling (13). Since the human genome project found significantly fewer genes than expected, only around 35,000 genes accounting for around 100,000 proteins (27, 28), it is now considered that alternative splicing of genes accounts for much of the genetic diversity in the human genome and that up to 60% of human genes are alternatively spliced (29–31). Indeed, Modrek et al. (30) predicted that MS4A2 variants resulting from alternative splicing existed and that they may contribute to functional regulation of the FcεRI.
Donnadieu et al. (17) were the first to report a splice variant of MS4A2 (transcript variant 2) expressed in human basophils and cord-blood-derived mast cells. This splice variant was a truncation due to the retention of intron 5, which results in the encoding of several amino acids before an in-frame stop codon (17). Thus, the C-terminal domains of MS4A2 including the immunoreceptor tyrosine-based activation motif (ITAM) were lost (17). This truncation was shown to bind to immature α chains of FcεRI in the endoplasmic reticulum, and traffic them for endosomal degradation leading to down-regulation of surface FcεRI expression. This would presumably reduce IgE-dependent mast cell activation, although this was not examined. This protein has, therefore, been proposed to act as a naturally occurring negative-regulator of FcεRI-dependent cell activation, in direct competition with native MS4A2, and as such could determine the severity of allergic disease.
MS4A2trunc identified in this study in mature tissue-derived HLMCs is generated due to a deletion of exon 3 and so retains both N and C termini, thus retaining the C-terminal MS4A2 ITAM. This deletion of exon 3 results in the putative loss of transmembrane domains 1 and 2. Notably, Singleton et al. (32) described that it is the first transmembrane domain (TM1) of MS4A2, which is required to form a complex with FcεRIα and stabilize the FcεRI tetramer. Indeed, just the TM1 region was sufficient for this. In addition, they also demonstrated that a generated mutant of MS4A2, which lacked TM1 could not form a complex with FcεRIα and had diminished association with FcεRIγ (32). This finding agrees with both the current study where MS4A2trunc—which lacks TM1—does not affect surface FcεRIα expression and does not localize to the cytoplasmic membrane and the previous study by Donnadieu et al. (17) where a truncation that retains TM1 also retains the ability to complex with FcεRIα and negatively regulates surface expression. Thus, we have identified a novel splice variant of MS4A2 which appears to have functions independent of FcεRI.
In keeping with the above observations, MS4A2trunc demonstrates novel activity for the MS4A2 gene and a profound effect on mast cell biology. Thus, MS4A2trunc overexpression almost completely inhibited the proliferation of HMC-1 cells and induced profound cell death in HLMCs. It seems likely that the mechanism driving these effects is that, unlike MS4A2, MS4A2trunc loses the ability to complex with FcεRIα through the loss of the TM1 domain and as a result localizes to the perinuclear membrane rather than the cytoplasmic membrane. MS4A2trunc induced cell cycle arrest at G2/M phase. However, we could not identify any significant regulation of Tyr15 phosphorylation on Cdk1, which acts as a gatekeeper for G2/M phase transition. Thus MS4A2trunc might elicit its effects through a novel mechanism.
Other MS4A family members have also been implicated in cellular proliferation. MS4A3, which is closely related to MS4A2, has also been shown to traffic to the nuclear membrane rather than the cytoplasmic membrane, where it binds directly to KAP, a cyclin-dependent kinase (Cdk)-associated phosphatase that dephosphorylates human Cdk2 in the nucleus attenuating cell cycle progression (33). This dephosphorylation of Cdk2 at Thr160 by KAP is facilitated by MS4A3 binding directly to the complex, which not only allows KAP greater access to dephosphorylate Thr160 on Cdk2 but also forces the dissociation and exclusion of cyclin A from this complex thus preventing G1–S phase transition (34).
MS4A1 (CD20) might act as a distinct calcium channel (or modulate an as-yet-unidentified calcium channel) in B cells, which is important in B cell activation and proliferation (35–38). Indeed, MS4A1 expression is increased in B cell malignancies, particularly non-Hodgkin B cell lymphoma (39). In addition, MS4A12 might also act as a distinct calcium channel, which is colon specific and its expression was associated with colon cancer (40). Subsequent functional knockdown of MS4A12 using siRNA in colon cancer cells significantly attenuated cell proliferation, motility and chemotactic tissue infiltration (40). Furthermore, cells expressing MS4A12 were more reactive to lower concentrations of growth factors than their counterpart cells not expressing MS4A12 (40). Further evidence of a possible role of MS4A gene family members in cancer has been demonstrated with an increased expression of MS4A8B in small cell lung cancer, although no mechanism was described (41). Thus, a major role of the MS4A family appears to be to regulate cell cycle progression and therefore cellular proliferation and survival. Our data adds the MS4A2 gene to this biological activity of the MS4A family. Taken together, it seems that the relative expression of pro- vs. antiproliferative MS4A family members and splice variants might be important in determining the proliferative potential of a cell.
It is interesting that the rapidly dividing human mast cell line HMC-1 does not express MS4A2trunc, while the slowly dividing cell line LAD2 and slowly dividing primary HLMCs do express it. This provides a functional correlate of relevance to the proposed antiproliferative effect of this protein. The suppression of MS4A2trunc gene expression by SCF might be highly relevant to the action of this cytokine and might provide an additional mechanism of action promoting SCF-dependent cell survival and proliferation. The HMC-1 cell line exhibits gain-of-function mutations at amino acid 816 and the juxtamembrane region of c-KIT, which leads to constitutive receptor activity (42). These mutations are not present in primary HLMCs or the LAD2 cell line and might explain the differential expression of MS4A2trunc in these cells and the high rate of proliferation seen in HMC-1. It is also conceivable that loss expression of MS4A2trunc through epigenetic changes represents part of the multistep sequence leading to mast cell neoplasia.
In summary, we have shown that human mast cells express a novel truncated variant of MS4A2, the β chain of the high-affinity IgE receptor. MS4A2trunc inhibits mast cell proliferation and survival, thus demonstrating that the MS4A2 gene has multiple roles, extending well beyond the regulation of acute allergic responses. By understanding the mechanisms regulating its function, it might one day be possible to induce its expression in mast cells in vivo, leading to better treatments for a number of mast cell-dependent or -associated diseases such as mastocytosis and asthma, respectively.
Acknowledgments
This study was funded in part by an unrestricted Ph.D. studentship (G.C.) from Novartis Pharmaceuticals. The authors are grateful to the Midlands Lung Tissue Consortium for the coordination of human lung tissue retrieval. The authors also thank Ryan Murray-Slater and Michelle Powell for assistance with the mast cell isolation and the Wolfson Foundation Light Microscopy Facility (Department of Biochemistry, University of Leicester) for the use of the scanning confocal microscope.
REFERENCES
- 1.Bradding P., Cruse G. (2008) Mast cells: Biological properties and role in health and allergic diseases. In Allergy and Allergic Diseases (Kay A. B., Kaplan A. P., Bousquet J., Holt P. eds) pp. 217–257, Wiley-Blackwell, Hoboken, NJ, USA [Google Scholar]
- 2.Echtenacher B., Mannel D. N., Hultner L. (1996) Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 [DOI] [PubMed] [Google Scholar]
- 3.Bradding P., Walls A. F., Holgate S. T. (2006) The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 117, 1277–1284 [DOI] [PubMed] [Google Scholar]
- 4.Tetlow L. C., Woolley D. E. (1995) Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann. Rheum. Dis. 54, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y., Liu Z., Hu W., Li L. (2005) Mast cell infiltration associated with tubulointerstitial fibrosis in chronic aristolochic acid nephropathy. Hum. Exp. Toxicol. 24, 41–47 [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe D. D. (2008) Mast cells and mastocytosis. Blood 112, 946–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruse G., Kaur D., Yang W., Duffy S. M., Brightling C. E., Bradding P. (2005) Activation of human lung mast cells by monomeric immunoglobulin E. Eur. Respir. J. 25, 858–863 [DOI] [PubMed] [Google Scholar]
- 8.Brown J. M., Wilson T. M., Metcalfe D. D. (2008) The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin. Exp. Allergy 38, 4–18 [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi K., Suzuki M., Sasaki S., Imai M. (2001) Identification of a new multigene four-transmembrane family (MS4A) related to CD20, HTm4 and β subunit of the high-affinity IgE receptor. Gene 264, 87–93 [DOI] [PubMed] [Google Scholar]
- 10.Ra C., Jouvin M. H., Kinet J. P. (1989) Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 264, 15323–15327 [PubMed] [Google Scholar]
- 11.Kinet J. P. (1999) The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu. Rev. Immunol. 17, 931–972 [DOI] [PubMed] [Google Scholar]
- 12.Alber G., Miller L., Jelsema C. L., Varin-Blank N., Metzger H. (1991) Structure-function relationships in the mast cell high affinity receptor for IgE. Role of the cytoplasmic domains and of the beta subunit. J. Biol. Chem. 266, 22613–22620 [PubMed] [Google Scholar]
- 13.Lin S., Cicala C., Scharenberg A. M., Kinet J. P. (1996) The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell 85, 985–995 [DOI] [PubMed] [Google Scholar]
- 14.Dombrowicz C., Lin S., Flamand V., Brini A. T., Koller B. H., Kinet J. P. (1998) Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity 8, 517–529 [DOI] [PubMed] [Google Scholar]
- 15.Gilfillan A. M., Tkaczyk C. (2006) Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 [DOI] [PubMed] [Google Scholar]
- 16.Rivera J., Gilfillan A. M. (2006) Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117, 1214–1225 [DOI] [PubMed] [Google Scholar]
- 17.Donnadieu E., Jouvin M. H., Rana S., Moffatt M. F., Mockford E. H., Cookson W. O., Kinet J. P. (2003) Competing functions encoded in the allergy-associated F(c) epsilonRIbeta gene. Immunity 18, 665–674 [DOI] [PubMed] [Google Scholar]
- 18.Butterfield J. H., Weiler D. A., Hunt L. W., Wynn S. R., Roche P. C. (1990) Purification of tryptase from a human mast cell line. J. Leukoc. Biol. 47, 409–419 [DOI] [PubMed] [Google Scholar]
- 19.Kirshenbaum A. S., Akin C., Wu Y., Rottem M., Goff J. P., Beaven M. A., Rao V. K., Metcalfe D. D. (2003) Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leukaemia Res. 27, 677–682 [DOI] [PubMed] [Google Scholar]
- 20.Sanmugalingam D., Wardlaw A. J., Bradding P. (2000) Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J. Leukoc. Biol. 68, 38–46 [PubMed] [Google Scholar]
- 21.Cruse G., Cockerill S., Bradding P. (2008) IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunol. 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wykes R. C., Lee M., Duffy S. M., Yang W., Seward E. P., Bradding P. (2007) Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J. Immunol. 179, 4045–4052 [DOI] [PubMed] [Google Scholar]
- 23.Kaur D., Berger P., Duffy S. M., Brightling C. E., Bradding P. (2005) Co-cultivation of mast cells and Fc epsilon RI alpha+ dendritic-like cells from human hip bone marrow. Clin. Exp. Allergy 35, 226–233 [DOI] [PubMed] [Google Scholar]
- 24.Demo S. D., Masuda E., Rossi A. B., Throndset B. T., Gerard A. L., Chan E. H., Armstrong R. J., Fox B. P., Lorens J. B., Payan D. G., Scheller R. H., Fisher J. M. (1999) Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry 36, 340–348 [DOI] [PubMed] [Google Scholar]
- 25.Cruse G., Duffy S. M., Brightling C. E., Bradding P. (2006) Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax 61, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy S. M., Lawley W. J., Kaur D., Yang W., Bradding P. (2003) Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J. Allergy Clin. Immunol. 112, 970–977 [DOI] [PubMed] [Google Scholar]
- 27.Venter J. C., Adams M. D., Myers E. W., et al. (2001) The sequence of the human genome. Science 291, 1304–1351 [DOI] [PubMed] [Google Scholar]
- 28.Lander E. S., Linton L. M., Birren B., et al. (2001) Initial sequencing and analysis of the human genome. Nature 409, 860–921 [DOI] [PubMed] [Google Scholar]
- 29.Modrek B., Lee C. (2002) A genomic view of alternative splicing. Nat. Genet. 30, 13–19 [DOI] [PubMed] [Google Scholar]
- 30.Modrek B., Resch A., Grasso C., Lee C. (2001) Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 29, 2850–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorek R., Amitai M. (2001) Piecing together the significance of splicing. Nat. Biotechnol. 19, 196. [DOI] [PubMed] [Google Scholar]
- 32.Singleton T. E., Platzer B., Dehlink E., Fiebiger E. (2009) The first transmembrane region of the β-chain stabilizes the tetrameric FcεRI complex. Mol. Immunol. 46, 2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donato J. L., Ko J., Kutok J. L., Cheng T., Shirakawa T., Mao X. Q., Beach D., Scadden D. T., Sayegh M. H., Adra C. N. (2002) Human HTm4 is a hematopoietic cell cycle regulator. J. Clin. Invest. 109, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinami M., Yano Y., Yang X., Salahuddin S., Moriyama K., Shiroishi M., Turner H., Shirakawa T., Adra C. N. (2005) Binding of HTm4 to cyclin-dependent kinase (Cdk)-associated phosphatase (KAP).Cdk2.cyclin A complex enhances the phosphatase activity of KAP, dissociates cyclin A, and facilitates KAP dephosphorylation of Cdk2. J. Biol. Chem. 280, 17235–17242 [DOI] [PubMed] [Google Scholar]
- 35.Tedder T. F., Engel P. (1994) CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol. Today 15, 450–454 [DOI] [PubMed] [Google Scholar]
- 36.Li H., Ayer L. M., Lytton J., Deans J. P. (2003) Store-operated cation entry mediated by CD20 in membrane rafts. J. Biol. Chem. 278, 42427–42434 [DOI] [PubMed] [Google Scholar]
- 37.Bubien J. K., Zhou L. J., Bell P. D., Frizzell R. A., Tedder T. F. (1993) Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J. Cell. Biol. 121, 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanzaki M., Shibata H., Mogami H., Kojima I. (1995) Expression of calcium-permeable cation channel CD20 accelerates progression through the G1 phase in Balb/c 3T3 cells. J. Biol. Chem. 270, 13099–13104 [DOI] [PubMed] [Google Scholar]
- 39.Anderson K. C., Bates M. P., Slaughenhoupt B. L., Pinkus G. S., Schlossman S. F., Nadler L. M. (1984) Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood 63, 1424–1433 [PubMed] [Google Scholar]
- 40.Koslowski M., Sahin U., Dhaene K., Huber C., Tureci O. (2008) MS4A12 is a colon-selective store-operated calcium channel promoting malignant cell processes. Cancer Res. 68, 3458–3466 [DOI] [PubMed] [Google Scholar]
- 41.Bangur C. S., Johnson J. C., Switzer A., Wang Y. H., Hill B., Fanger G. R., Wang T., Retter M. W. (2004) Identification and characterization of L985P, a CD20 related family member over-expressed in small cell lung carcinoma. Int. J. Oncol. 25, 1583–1590 [PubMed] [Google Scholar]
- 42.Furitsu T., Tsujimura T., Tono T., Ikeda H., Kitayama H., Koshimizu U., Sugahara H., Butterfield J. H., Ashman L. K., Kanayama Y. (1993) Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Invest. 92, 1736–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]