Abstract
The polycystic kidney disease 1-like 3 (PKD1L3) and polycystic kidney disease 2-like 1 (PKD2L1) proteins have been proposed to form heteromers that function as sour taste receptors in mammals. Here, we show that PKD1L3 and PKD2L1 interact through their transmembrane domains, and not through the coiled-coil domain, by coimmunoprecipitation experiments using a series of deletion mutants. Deletion mutants lacking the critical interaction region were not transported to the cell surface and remained in the cytoplasm, whereas PKD1L3 and PKD2L1 proteins were expressed at the cell surface when both are transfected. Calcium imaging analysis revealed that neither the coiled-coil domain nor the EF-hand domain located in the C-terminal cytoplasmic tail of PKD2L1 was required for response on stimulation with an acidic solution. Finally, PKD2L1 did not localize to the taste pore but was distributed throughout the cytoplasm in taste cells of circumvallate and foliate papillae in PKD1L3−/− mice, whereas it localized to the taste pore in wild-type mice. Collectively, these results suggest that the interaction between PKD1L3 and PKD2L1 through their transmembrane domains is essential for proper trafficking of the channels to the cell surface in taste cells of circumvallate and foliate papillae and in cultured cells.—Ishimaru, Y., Katano, Y., Yamamoto, K., Akiba, M., Misaka, T., Roberts, R. W., Asakura, T., Matsunami, H., Abe, K. Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae.
Keywords: association, trafficking, sour taste receptor, polycystic kidney disease
The polycystic kidney disease 1-like 3 (PKD1L3) and polycystic kidney disease 2-like 1 (PKD2L1) proteins belong to the polycystic kidney disease (PKD) family, which includes 8 members (1). PKD1L3 and PKD2L1 are robustly coexpressed in subsets of taste receptor cells (type III taste cells) of circumvallate and foliate papillae (2–5). Type III taste cells are distinct from sweet, bitter, and umami (savory) sensing cells (type II taste cells), which express signal transduction molecules such as two families of taste receptors, T1Rs and T2Rs, phospholipase C-β2 (PLC-β2), inositol 1,4,5-triphosphate receptor type 3 (IP3R3), and transient receptor potential melastatin 5 (TRPM5) (6–10). In a heterologous expression system using human embryonic kidney (HEK) 293T cells, PKD1L3 and PKD2L1 interact with each other, and this interaction is required for their functional cell surface expression (3). HEK293T cells expressing both PKD1L3 and PKD2L1 respond specifically to acid stimuli, showing a unique “off-response” property (3, 11). This phenomenon means that the PKD1L3/PKD2L1 channel is gated open only after the removal of an acid stimulus, but the initial acid exposure is essential. The “off-responses” on acid stimulus were clearly observed in native taste cells from circumvallate, but not fungiform papillae, using Ca2+-imaging and patch-clamp methods (12). In addition, genetic ablation of PKD2L1-expressing cells results in the elimination of gustatory nerve response to sour stimuli, indicating that cells expressing PKD2L1 function as sour taste detectors (2). Collectively, these results suggest that PKD1L3/PKD2L1 may play a significant role, possibly as taste receptors, in sour taste sensation (13–15).
Mutations in PKD1 and PKD2, the founding members of the PKD family, cause autosomal dominant PKD (ADPKD) (1, 16). PKD1 and PKD2 interact through the coiled-coil domains in their C-terminal cytoplasmic tails (17–19), and this association as a heteromer appears to be required for formation of a functional receptor/channel (20). Further, PKD1 and PKD2 have been proposed to function as mechanical flow sensors in both epithelial and endothelial cells (21, 22). PKD2 inhibits stretch-activated ion channels via the actin cytoskeleton, and this inhibition is reversed by coexpression with PKD1, indicating that the PKD1/PKD2 ratio regulates pressure sensing (23).
PKD1L3 is a large protein with a very long N-terminal extracellular domain, followed by 11-transmembrane spanning domains that include a 6-transmembrane TRP-like channel domain at the C terminus (24). Like PKD1, PKD1L3 contains a C-type lectin domain and a G-protein-coupled receptor proteolytic site (GPS) in its N-terminal extracellular region, as well as a polycystin-1-lipoxygenase-α toxin (PLAT)/lipoxygenase homology 2 (LH2) domain in its first intracellular loop (24). Unlike PKD1, however, the C-terminal cytoplasmic tail of PKD1L3 is composed of only ∼50 amino acid residues without a coiled-coil domain. PKD2L1 has 6 transmembrane domains, similar to other members of the TRP channel family. PKD2L1 contains 2 endoplasmic reticulum (ER) retention signals, a putative Ca2+ binding EF-hand domain, and a predicted coiled-coil domain in its C-terminal cytoplasmic tail (25).
To investigate in more detail the molecular mechanisms underlying the interaction between PKD1L3 and PKD2L1, the trafficking of these channels to the cell surface, and their function, we performed coimmunoprecipitation, cell surface expression, and calcium imaging analyses using a series of PKD1L3 and PKD2L1 deletion mutants. We further generated PKD1L3-knockout mice in which the genomic region required for PKD1L3 interaction with PKD2L1 is deleted, and analyzed the subcellular localization of PKD2L1 in taste cells. We showed that the interaction between PKD1L3 and PKD2L1 through their transmembrane domains is important for localizing PKD2L1 to the taste pores in vivo.
MATERIALS AND METHODS
Vector construction
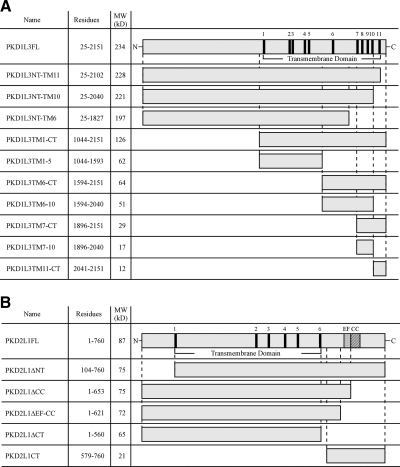
Expression vectors for deletion mutants were constructed by subcloning PCR products, amplified from the putative mature PKD1L3 (R25-Y2151) and full-length PKD2L1 (M1-S760) (3) proteins using Pyrobest DNA polymerase (Takara Bio Inc., Shiga, Japan), into the pDisplay (Invitrogen, San Diego, CA, USA) or pCI (Promega, Madison, WI, USA) expression vectors. PKD1L3FL (R25-Y2151), PKD1L3NT-TM11 (R25-S2102), PKD1L3NT-TM10 (R25-N2040), and PKD1L3NT-TM6 (R25-T1827) were inserted into pDisplay to generate N-terminally HA-tagged proteins (Fig. 1). PKD1L3TM1-CT (L1044-Y2151), PKD1L3TM1-5 (L1044-L1593), PKD1L3TM7-CT (W1896-Y2151), PKD1L3TM7-10 (W1896-N2040), and PKD1L3TM11-CT (L2041-Y2151) were inserted into pDisplay, and another HA tag was added at their C-terminal ends (Fig. 1). An HA tag was inserted at the C-terminal end of PKD1L3TM6-CT (M1594-Y2151) and PKD1L3TM6-10 (M1594-N2040) in pCI. A FLAG tag was inserted at the N terminus of PKD2L1FL and the PKD2L1 deletion mutants in pCI.
Figure 1.
Schematic representation of the domain organization of PKD1L3, PKD2L1 and their deletion constructs. Schematic representations show the PKD1L3 (A) and PKD2L1 (B) deletion constructs tested in this study. Black boxes indicate predicted transmembrane (TM) domains. EF, Ca2+-binding EF-hand domain; CC, coiled-coil domain.
Cell culture and immunoprecipitation
HEK293T cells were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS (Invitrogen). Lipofectamine 2000 (Invitrogen) was used for transfection. Immunoprecipitations were performed essentially as described previously (26); protease inhibitor cocktail (Sigma-Aldrich) was used. Rabbit anti-HA antibody (Sigma-Aldrich), anti-FLAG M2 monoclonal antibody (Sigma-Aldrich), and anti-PKD2L1 antiserum (3) were used as primary antibodies, and AP-conjugated anti-rabbit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-mouse (Vector Laboratories, Burlingame, CA, USA) antibodies were used as secondary antibodies. NBT and BCIP (Sigma-Aldrich) were used for detecting proteins on PVDF membranes. HRP-conjugated anti-rabbit antibody and ECL Plus Western blotting Detection Reagents (GE Healthcare, Little Chalfont, UK) were used to detect HA signals of C-terminally truncated PKD1L3 mutants (Fig. 2, lanes 1–4).
Figure 2.
Regions required for PKD1L3 and PKD2L1 to interact. Coimmunoprecipitation assays were performed using PKD1L3, PKD2L1, and their deletion mutants. A) Control Western blot analysis indicating expression of PKD1L3, PKD2L1, and their deletion mutants. i) C-terminally truncated PKD1L3 mutants and full-length PKD2L1. ii) PKD1L3 deletion mutants and full-length PKD2L1. iii) PKD1L3TM6-CT and C- or N-terminally truncated PKD2L1 mutants. iv) Full-length PKD2L1 and PKD2L1ΔCT. B) i) Interaction between C-terminally truncated PKD1L3 mutants and PKD2L1. When HA-tagged PKD1L3 or C-terminally truncated PKD1L3 mutants were precipitated, FLAG-tagged PKD2L1 proteins coprecipitated (lanes 1–3). Less FLAG-tagged PKD2L1 protein coprecipitated with the PKD1L3NT-TM6 protein (lane 4). We could not detect any signals for HA-tagged PKD1L3FL in either the lysis (A) or the immunoprecipitation, likely because of low transfer efficiency of high-molecular-mass protein (∼234 kDa) from gels to PVDF membranes. Immunostaining analysis under permeabilized conditions demonstrated that protein expression levels were comparable among PKD1L3FL, PKD1L3NT-TM11, PKD1L3NT-TM10, and PKD1L3NT-TM6 (Fig. 3). ii) Region of PKD1L3 that is required for interaction with PKD2L1. When HA-tagged PKD1L3 deletion mutants were precipitated, FLAG-tagged PKD2L1 proteins were coprecipitated (lanes 5–10). Little FLAG-tagged PKD2L1 protein coprecipitated with the PKD1L3TM11-CT protein (lane 11). iii) Region of PKD2L1 that is required for heteromeric interaction with PKD1L3. When HA-tagged PKD1L3TM6-CT was precipitated, FLAG-tagged PKD2L1 N-terminal or C-terminal truncated proteins coprecipitated (lanes 12–14, 16); in contrast, little PKD2L1CT protein coprecipitated (lane 15). iv) Region of PKD2L1 that is required for homomeric interaction. When HA-tagged PKD2L1 or PKD2L1ΔCT was precipitated, FLAG-tagged PKD2L1 or PKD2L1ΔCT protein coprecipitated (lanes 17–19). Solid arrowheads indicate PKD1L3 deletion mutants; open arrowheads indicate PKD2L1 or PKD2L1 deletion mutants; brackets indicate high-molecular-mass oligomers. Data are representative of ≥3 independent experiments.
Cell surface expression analysis
Cell surface expression analysis was performed essentially as described previously (26). For live-cell staining, cells were incubated (16 h after transfection) in M10-containing rabbit anti-HA antibody and 15 mM NaN3 at 4°C for 1 h. After being washed, the cells were incubated with Cy3-conjugated anti-rabbit IgG (Jackson Immunologicals, West Grove, PA, USA), washed, and mounted. To confirm protein expression within the cells, they were fixed with 4% paraformaldehyde, permeabilized with ice-cold methanol, and subjected to immunostaining.
Calcium imaging analysis
Calcium imaging analysis was performed essentially as described previously (27). Briefly, cells were incubated with 5 μM Fura-2 AM (Invitrogen) for 30 min at room temperature. For calcium imaging, cells were seeded onto 35-mm glass dishes, and plasmid DNA encoding PKD1L3, PKD2L1, or their deletion mutants together with the red fluorescent protein DsRed2 (pDsRed2-N1; Takara Bio), was transfected into the cells with Lipofectamine 2000 and incubated for 30–42 h before dye loading. Cells were exposed to a constant flow of bath solution (Hank's buffer containing 10 mM HEPES; Invitrogen). Bath solution containing 25 mM citric acid solution (pH 2.7) was applied to cells for ∼6 s by changing the solution with a perfusion device. We used a computer-controlled filter changer (Lambda 10-2; Sutter Instruments, San Rafael, CA, USA), a MicroMax charged-coupled device (CCD) camera (Princeton Instruments, Trenton, NJ, USA), and an inverted fluorescence microscope (IX-70; Olympus, Tokyo, Japan) (excitation at 340 and 380 nm; emission at 510 nm). MetaFluor software (Molecular Devices, Sunnyvale, CA, USA) was used for data acquisition. Data were collected at 4-s intervals and analyzed with Microsoft Excel (Microsoft, Redmond, WA, USA). The resultant changes in the intracellular calcium ion concentration ([Ca2+]i) were measured by analyzing 100 randomly selected DsRed2-positive cells, which were regarded as transfected cells. The changes are presented as the ratio of the fluorescence intensities at 2 excitation wavelengths (F340/F380). The cells were regarded as responsive when the increase in the F340/F380 ratio was >0.2.
Generation of PKD1L3-knockout mice
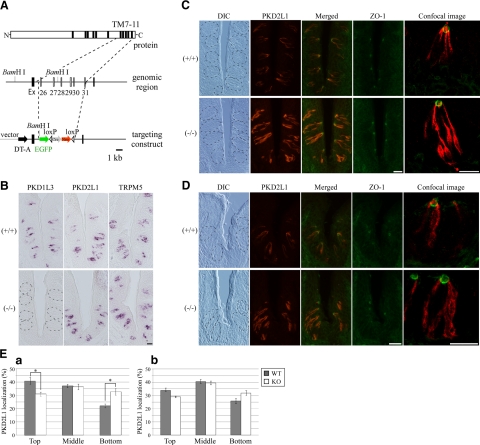
All animal experiments were approved by the Animal Care and Use Committees at The University of Tokyo and Duke University. The strategy for generating PKD1L3-knockout mice is illustrated in Fig. 5A. In the knockout mice, the genomic region that contains exons 26 to 31, encoding transmembrane (TM) motif 7 to 11 of PKD1L3, was replaced by internal ribosomal entry site (IRES)-enhanced green fluorescent protein (EGFP) and followed by the loxP sequence. Fragments used for the left and right arms were amplified by PCR using BAC clone RP23–178P21 (BACPAC Resources Center), derived from C57BL/6 mice, as a template. The neomycin resistance gene in the ACN cassette (28) and the diphtheria toxin A-chain (DT-A) gene were used for positive and negative selection, respectively. The targeting vector was electroporated into the EF1 embryonic stem cell line, which is a 129SvEv/C57B6 hybrid, and the colonies were selected in G418-containing medium. Genomic DNA was digested by BamH I and hybridized with a 500-bp external probe on Southern blots. Three targeted ES cell clones were injected into C57BL/6 blastocysts, and chimeric mice were bred with C57BL/6 mice. The PCR primers for genotyping were 5′-cagtcacccacctcaggggc-3′ and 5′-ggccagtcagtggtaaccaatc-3′ for the wild-type allele and 5′-ttctccactacagtcctggatg-3′ and 5′-cttgctcaccatggttgtg-3′ for the mutated allele.
Figure 5.
Localization of PKD2L1 protein in PKD1L3-knockout mice. A) Schematic representation showing the structure of the PKD1L3 gene and the strategy for generating knockout mice. Targeting construct deleted predicted transmembrane (TM) motifs 7 to 11. Ex, exon; Cre, Cre recombinase gene; Neo, neomycin resistant gene; loxP, loxP site; DT-A, diphtheria toxin A-chain gene; EGF, enhanced green fluorescent protein. B) In situ hybridization experiments demonstrating complete loss of PKD1L3 expression in the taste buds of the circumvallate papillae of PKD1L3−/− mice and robust expression in wild-type mice. C, D) Sections of circumvallate (C) or foliate (D) papillae in PKD1L3−/− mice or wild-type littermates were incubated with anti-PKD2L1 (red) and ZO-1 (green) antibodies. PKD2L1 was mainly distributed in the upper half of taste cells, with intensely accumulated signals at the taste pore in circumvallate papillae of wild-type mice, while much stronger PKD2L1 immunoreactivity was observed throughout the cytoplasm in PKD1L3−/− mice. Dotted lines on differential interference contrast (DIC) images indicate approximate area of the taste buds. Stained images were obtained with a confocal laser-scanning microscope at high magnification. E) Signal intensity in each third of taste bud was quantified using the ImageJ program in circumvallate (a) and foliate (b) papillae. Percentage of PKD2L1 protein distributed in the top third of taste cells in circumvallate and foliate papillae was larger in wild-type mice than in PKD1L3−/− mice. Bars represent means ± se (n=3). *P < 0.05; Student's t test. Scale bars = 20 μm.
In situ hybridization and immunocytochemistry
In situ hybridization and immunocytochemistry were performed essentially as described by Ishimaru et al. (3). The probe for PKD1L3 contained the region encoding from TM9 to the C terminus (V1982-Y2151). Anti-PKD2L1 antiserum (3) and an anti-ZO-1 monoclonal antibody (Invitrogen) (29) were used as primary antibodies, and Alexa Fluor 555-conjugated anti-rabbit antibody and Alexa Fluor 488-conjugated anti-mouse antibody (Invitrogen) were used as secondary antibodies, respectively. Stained images were obtained with a fluorescence microscope (BX51; Olympus) equipped with a cooled CCD digital camera (DP71; Olympus) or a confocal laser scanning microscope at higher magnification (FV500; Olympus). The total signal intensity in each one-third area of taste bud along the top-bottom axis was quantified using the ImageJ program (http://rsb.info.nih.gov/ij/) (Fig. 5E).
RESULTS
Identification of regions involved in heteromeric interaction between PKD1L3 and PKD2L1 and homomeric interaction of PKD2L1
We previously demonstrated that PKD1L3 and PKD2L1 must interact for their functional cell surface expression in HEK293T cells (3). Coimmunoprecipitation assays revealed that both the entire mature PKD1L3 protein (R25-Y2151) and the deletion constructs TM1-CT (H1069-Y2151) and TM6-CT (M1594-Y2151) interact with full-length PKD2L1 (3, 11). In addition, full-length PKD2L1 also engages in homomeric interactions (3). To narrow down the regions responsible for the interactions in both proteins, we generated a series of deletion mutants (Fig. 1). In this manuscript, we refer to the region consisting of transmembrane motifs, extracellular loops, and intracellular loops as the transmembrane domain.
We initially investigated the region of PKD1L3 that is required for interaction with PKD2L1 by immunoprecipitation assays. FLAG-tagged PKD2L1FL was coimmunoprecipitated with the HA-tagged PKD1L3FL or deletion mutants truncated from the C terminus, such as PKD1L3NT-TM11 and PKD1L3NT-TM10 (Fig. 2, lanes 1–3). In contrast, much weaker signals were detected for PKD1L3NT-TM6 (Fig. 2, lane 4). These results demonstrate that the region from TM7 to the C terminus of PKD1L3 is important for its interaction with PKD2L1, although other parts may also be involved in this association to some extent. To narrow the region of PKD1L3 necessary for interaction with PKD2L1, we further examined whether FLAG-tagged PKD2L1FL was coimmunoprecipitated with the HA-tagged deletion mutants lacking larger regions (Fig. 2, lanes 5–11). FLAG-tagged PKD2L1FL coimmunoprecipitated with either PKD1L3TM6-CT or PKD1L3TM6–10 (Fig. 2, lanes 7, 8). Weaker but significant signals were detected for PKD1L3TM7-CT and PKD1L3TM7–10, while faint signals were detected for PKD1L3TM1-CT and PKD1L3TM1–5 (Fig. 2, lanes 5, 6, 9, 10). In contrast, no signals were detected in attempts to immunoprecipitate the FLAG-tagged PKD2L1FL with the HA-tagged PKD1L3TM11-CT (Fig. 2, lane 11). Collectively, these results indicate that the PKD1L3 region from TM7 to TM10 (W1896-N2040) plays a pivotal role in its heteromeric interaction with PKD2L1, although other regions (including TM1 to TM5) may also weakly interact with PKD2L1.
Next, we investigated the region of PKD2L1 that is required for heteromeric interaction with PKD1L3. The PKD1L3TM6-CT construct was used in these experiments because it showed as strong an interaction with the full-length PKD2L1 as the entire mature PKD1L3 protein (PKD1L3FL, ∼234 kDa), which was undetectable, likely due to low transfer efficiency to the PVDF membrane (Fig. 2, lane 1). Similar to PKD2L1FL, FLAG-tagged PKD2L1ΔCC, PKD2L1ΔEF-CC, PKD2L1ΔCT, and PKD2L1ΔNT coimmunoprecipitated with HA-tagged PKD1L3TM6-CT (Fig. 2, lanes 12–14, 16). In contrast, no signals were detected in attempts to immunoprecipitate the FLAG-tagged C-terminal cytoplasmic region (PKD2L1CT) with HA-tagged PKD1L3TM6-CT (Fig. 2, lane 15). These results demonstrate that the transmembrane domain (L104-I560) of PKD2L1 is sufficient for its interaction with PKD1L3.
We further examined the region of PKD2L1 that is required for homomeric interaction. As in the case of FLAG-tagged PKD2L1FL and HA-tagged PKD2L1FL, FLAG-tagged PKD2L1ΔCT coimmunoprecipitated with either HA-tagged PKD2L1FL or PKD2L1ΔCT (Fig. 2, lanes 17–19). Similarly, FLAG-tagged PKD2L1ΔCC coimmunoprecipitated with either HA-tagged PKD2L1FL or PKD2L1ΔCC (data not shown). These results demonstrate that the C-terminal cytoplasmic tail (N561–S760), including the coiled-coil domain, is not necessary for PKD2L1 homomeric interactions.
Trafficking of PKD1L3 and PKD2L1 deletion mutants to the cell surface
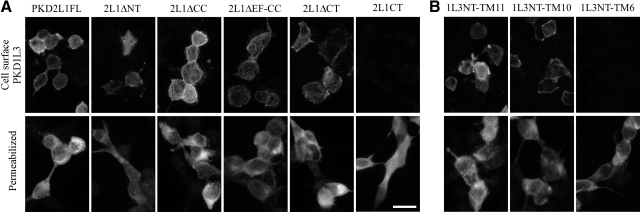
Neither PKD1L3 nor PKD2L1 is efficiently trafficked to the cell surface when either is expressed alone in HEK293T cells (3). To test whether the cell surface localization of PKD1L3/PKD2L1 channels depends on their heteromerization, we first analyzed the cell surface expression of PKD1L3 in the presence of the PKD2L1 deletion mutants. PKD1L3 was tagged with HA at its N-terminal extracellular domain. Similar to the full-length PKD2L1, robust cell surface localization of PKD1L3 was observed when PKD1L3 was expressed with PKD2L1ΔCC, PKD2L1ΔEF-CC, or PKD2L1ΔCT (Fig. 3A). In the case of PKD2L1ΔNT, weaker but significant cell surface signals were observed in a small number of cells, probably because of the low expression of PKD2L1ΔNT protein, as revealed by Western blot analysis using anti-PKD2L1 and anti-FLAG antibodies (Fig. 2, lane 16; data not shown). In contrast, when PKD1L3 was expressed with PKD2L1CT, little cell surface localization was observed; however, intense signals were detected when cells were permeabilized and stained, indicating high expression of PKD1L3 protein within the cells. These results demonstrate that deleting the region that is dispensable for the interaction between PKD1L3 and PKD2L1, including the N-terminal or C-terminal cytoplasmic regions of PKD2L1, has little effect on the ability of PKD1L3/PKD2L1 channels to be transported to the cell surface.
Figure 3.
Association of PKD1L3 and PKD2L1 proteins is required for their cell surface localization. HEK293T cells expressing HA-tagged PKD1L3 together with PKD2L1 or PKD2L1 deletion mutants (A) and those expressing HA-tagged C-terminal truncated PKD1L3 mutants in the presence of PKD2L1 (B) were stained with anti-HA antibodies under nonpermeabilized (top panels) or permeabilized conditions (bottom panels). Scale bar = 20 μm. Data are representative of ≥3 independent experiments.
We next assessed the cell surface localization of C-terminally truncated PKD1L3 mutants in the presence of full-length PKD2L1. All of the PKD1L3 mutants were tagged with HA at their N-terminal extracellular domains. When PKD1L3NT-TM11 or PKD1L3NT-TM10 was expressed with PKD2L1FL, robust cell surface localization was observed (Fig. 3B), as in the case of the entire mature PKD1L3 protein (PKD1L3FL). In contrast, when PKD1L3NT-TM6 was expressed with PKD2L1FL, little cell surface localization was observed, whereas intense signals were detected when cells were permeabilized and stained, indicating that PKD1L3NT-TM6 protein was robustly expressed in the cells. These results demonstrate that the region ranging from TM7 to the C terminus of PKD1L3, which is critical for the interaction between PKD1L3 and PKD2L1 (Fig. 2), is also necessary for proper trafficking of PKD1L3/PKD2L1 channels to the cell surface. Collectively, cell surface localization analysis revealed that the interaction between PKD1L3 and PKD2L1 through transmembrane domains is necessary for proper trafficking of PKD1L3/PKD2L1 channels to the cell surface.
Neither the coiled-coil domain nor the EF-hand domain in the C-terminal cytoplasmic tail of PKD2L1 is required for responding to acid stimulation
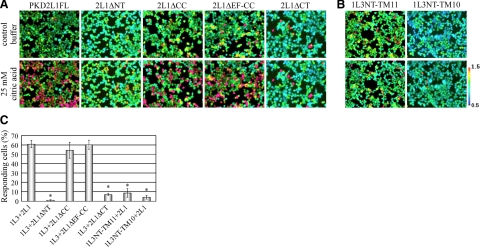
HEK293T cells transiently expressing both PKD1L3 and PKD2L1 respond when an acid solution (pH<3) is applied and subsequently washed out (3, 11). To investigate which regions in both proteins are necessary for forming a functional channel, we performed calcium imaging experiments using HEK293T cells transiently expressing PKD1L3 and a series of deletion mutants lacking the N-terminal or C-terminal cytoplasmic regions of PKD2L1, which are still transported to the cell surface (Fig. 4). Intriguingly, cells expressing PKD1L3 and the PKD2L1 deletion mutant lacking either the coiled-coil domain (PKD2L1ΔCC) or both the coiled-coil domain and the EF-hand domain (PKD2L1ΔEF-CC) responded to 25 mM citric acid solution, similar to the full-length PKD2L1 (Fig. 4). In contrast, when the PKD2L1 deletion mutant lacking the entire C-terminal cytoplasmic tail (PKD2L1ΔCT) was coexpressed with PKD1L3, little response was observed on stimulation with 25 mM citric acid, despite robust cell surface localization. In the case of PKD2L1ΔNT, no response was observed, likely due to weak cell surface localization. Collectively, these results demonstrate that neither the coiled-coil domain nor the EF-hand domain in the C-terminal cytoplasmic tail of PKD2L1 is required for forming a functional channel.
Figure 4.
Regions required for responding to acid stimuli. A) Representative ratiometric images of Fura-2-loaded HEK293T cells. HEK293T cells expressing PKD1L3 together with PKD2L1 or PKD2L1 deletion mutants were stimulated with control buffer (top panels) or buffer containing 25 mM citric acid (pH 2.7) (bottom panels). Color scale bar indicates the ratiometric value of Fura-2 340/380, from 0.5 (cyan) to 1.5 (red) as pseudocolors. B) HEK293T cells expressing PKD2L1 together with truncated PKD1L3 mutants were stimulated with control buffer (top panels) or buffer containing 25 mM citric acid (bottom panels). C) Percentage of responding cells of 100 DsRed2-positive cells. Bars represent means ± se (n=3). *P < 0.05 vs. control; Student's t test.
On the other hand, cells expressing the PKD1L3 deletion mutant that lacked the entire C-terminal cytoplasmic tail (PKD1L3NT-TM11) and full-length PKD2L1 did not respond to 25 mM citric acid solution (Fig. 4), suggesting that the C-terminal cytoplasmic tail of PKD1L3 may be important for forming a functional channel but not for trafficking to the cell surface (Fig. 3).
Protein localization of PKD2L1 at taste pores is regulated by PKD1L3 in taste cells in the posterior gustatory area on the tongue
Immunohistochemistry using an anti-PKD2L1 antibody demonstrated that PKD2L1 localizes to the taste pore at the apical tip of a subset of taste cells, with weaker labeling throughout the cytoplasm (3, 29). Cell surface biotinylation assays revealed that cell-surface PKD2L1 protein was dramatically increased in the presence of PKD1L3 in HEK293T cells. To examine whether the subcellular localization of PKD2L1 protein is regulated by PKD1L3 in taste cells, we generated PKD1L3-knockout mice and performed immunostaining using an anti-PKD2L1 antibody on sections of circumvallate or foliate papillae, where both PKD1L3 and PKD2L1 are normally coexpressed in the same subsets of taste cells.
We generated PKD1L3-knockout mice, in which 6 exons encoding TM motifs 7 to 11, which are critical for interaction with PKD2L1, were deleted (Fig. 5A). In situ hybridization experiments using the region encoding TM9 to the C terminus of PKD1L3 (V1982-Y2151) as a probe showed that PKD1L3 expression was completely lost in the taste buds of PKD1L3−/− mice (Fig. 5B). When the region encoding TM2 to the C terminus (G1176-Y2151) was used as a probe, strong signals were detected in the taste buds of PKD1L3−/− mice (data not shown), demonstrating that partial PKD1L3 mRNA is transcribed in PKD1L3−/− mice. No significant differences were observed between PKD1L3−/− mice and wild-type littermates when probes for PKD2L1 and TRPM5, a TRP channel family member required for normal bitter, sweet, and umami sensation, were used (Fig. 5B).
Immunohistochemistry using an anti-PKD2L1 antibody demonstrated that PKD2L1 is mainly distributed in the upper half of taste cells, with intensely accumulated signals at the taste pore in circumvallate papillae of wild-type mice; much stronger PKD2L1-immunoreactivity was observed throughout the cytoplasm in PKD1L3−/− mice (Fig. 5C, E). ZO-1 is a component of tight junctions that separates the apical and basolateral plasma membrane domains (29). Double staining using antibodies against PKD2L1 and ZO-1 revealed that the PKD2L1 signal on the apical side appeared to be weaker in PKD1L3−/− mice than in wild-type littermates (Fig. 5C). A similar difference was also observed in foliate papillae between the PKD1L3−/− mice and wild-type littermates (Fig. 5D, E). These results indicate that the subcellular localization of PKD2L1 is regulated by PKD1L3 in both taste cells and cultured cells.
DISCUSSION
In the present study, we demonstrated that the transmembrane domains of PKD1L3 and PKD2L1, but not their C-terminal cytoplasmic tails, are necessary for their heteromeric interaction and proper trafficking to the cell surface in HEK293T cells, as well as the homomeric interaction of PKD2L1. Calcium imaging analysis revealed that neither the Ca2+-binding EF-hand domain nor the coiled-coil domain in the C-terminal cytoplasmic tail of PKD2L1 was required for responding to acid stimuli, whereas deleting the short C-terminal cytoplasmic tail of PKD1L3 resulted in a much weaker response. In PKD1L3-knockout mice, PKD2L1 was not localized to the taste pore but robustly distributed throughout the cytoplasm in circumvallate and foliate papillae, indicating the physiological correlation between PKD1L3 and PKD2L1 in taste cells of the posterior gustatory areas on the tongue.
Our coimmunoprecipitation assays revealed that the transmembrane domains, ranging from TM7 to 10 (W1896-N2040) of PKD1L3 and TM1 to 6 (L104-I560) of PKD2L1, are involved in these proteins' heteromeric interaction (Fig. 2). This interaction is responsible for proper trafficking of the channels to the cell surface (Fig. 3). In addition, the C-terminal cytoplasmic tail (N561-S760) is not necessary for PKD2L1 homomeric interactions. In contrast, PKD1 and PKD2 (the founding members of PKD family) interact with each other through the coiled-coil domains in their C-terminal cytoplasmic tails to form a functional receptor/channel (17, 18, 20). The homomeric interactions of PKD2 also occur through its coiled-coil domain (17, 18). PKD1L3 probably interact with PKD2L1 in a different manner from PKD1 because PKD1L3 and other PKD1 family members (e.g., PKD1L2 and PKDREJ) contain a short C-terminal cytoplasmic tail lacking the coiled-coil domain (1, 24, 30). On the other hand, the coiled-coil domain located in the C-terminal cytoplasmic tail of PKD2L1 has been assumed to be necessary for the interaction because this domain is well conserved among all three PKD2 family members (i.e., PKD2, PKD2L1, and PKD2L2) (1). In addition, the C-terminal coiled-coil domains are responsible for trafficking of PKD2L1 to the cell surface by PKD1, although the physiological significance of a PKD1/PKD2L1 complex remains unclear (31). The coiled-coil domain in the C-terminal cytoplasmic tail is also conserved among another subfamily of TRP channels, the TRP melastatins (TRPMs) (32). For example, TRPM8 forms functional channels by assembling as a multimer using its C-terminal coiled-coil domain (33, 34). The transmembrane domain of TRPM8 is also sufficient for assembly into tetramers (35). Our results indicate that the transmembrane domain of PKD2L1, but not its C-terminal cytoplasmic tail, is important for interaction and proper trafficking with PKD1L3, as well as channel function (see below).
We previously showed that HEK293T cells transiently expressing both PKD1L3 and PKD2L1 respond robustly after application and subsequent removal of various acidic solutions (including strong and weak acids), whereas cells expressing either PKD1L3 or PKD2L1 alone showed little response (3, 11, 27). On the other hand, previous studies report that human or murine PKD2L1 forms a Ca2+-permeable nonselective cation channel by itself in Xenopus oocytes, planar lipid bilayers, and HEK293 cells in the absence of PKD1L3 (36–40). PKD2L1 also independently functions as a voltage-dependent, pH- and volume-sensitive plasma membrane cation channel in HEK293 cells (37).
To examine which regions of both proteins are important for responding to acid stimuli, we performed calcium imaging analysis using deletion mutants that are still transported to the cell surface (Fig. 4). The deletion mutants lacking the entire C-terminal cytoplasmic tail of PKD1L3 (PKD1L3NT-TM11) or PKD2L1 (PKD2L1ΔCT) showed much weaker responses, if any, despite proper trafficking to the cell surface, demonstrating that these regions are necessary for forming functional channels. Intriguingly, when the PKD2L1 deletion mutant lacking the coiled-coil domain (PKD2L1ΔCC) or both the coiled-coil domain and the EF-hand domain (PKD2L1ΔEF-CC) was coexpressed with PKD1L3, cells responded to acid solutions as robustly as they responded when coexpressed with full-length PKD2L1. Consistent with our results, PKD2L1 splicing variants and artificial truncation mutants lacking the EF-hand domain retain basal conductance to cations in the absence of calcium and channel activation by calcium (41). These results suggest that the EF-hand domain and other parts of the C-terminal cytoplasmic region (T622-S760) of PKD2L1 are not key determinants of channel activation.
To investigate whether PKD1L3 and PKD2L1 form heteromers in vivo, we generated PKD1L3-knockout mice, in which the genomic region encoding TM motifs 7 to 11 was deleted, and examined the subcellular localization of PKD2L1. In the absence of this PKD1L3 region, PKD2L1 protein was robustly distributed throughout the cytoplasm, whereas PKD2L1 protein was mainly observed in the top half of elongated taste cells in wild-type mice (Fig. 5). These results suggest that PKD1L3 and PKD2L1 interact with each other to form a functional channel in the posterior gustatory area on the tongue (e.g., circumvallate and foliate papillae), where PKD1L3 and PKD2L1 are coexpressed in the same subsets of taste cells (2, 3, 5). Because PKD2L1 contains two ER retention signals in its C-terminal cytoplasmic tail, the interaction between PKD1L3 and PKD2L1 may result in the transport of PKD2L1 from the ER to the cell surface. In contrast, PKD1L3 is not expressed in taste cells of the fungiform papillae and palate or in pH-sensing neurons surrounding the central canal of the spinal cord, where PKD2L1 is robustly expressed (refs. 2, 3, 5 and unpublished results). In these tissues, PKD2L1 may solely function in homomeric assemblies. Alternatively, it may form a channel, with different properties, together with other nonidentified molecules, including other TRP family members.
In summary, the interaction between PKD1L3 and PKD2L1, which occurs via their transmembrane domains, is essential for proper trafficking of the channels to the cell surface in taste cells of the posterior gustatory area on the tongue, as well as in cultured cells.
Acknowledgments
The authors are grateful to Cheryl Bock and Mei Lang Flowers (Duke Transgenic Mouse Facility) for microinjection of the DNA construct into embryonic stem cells and to Frederick W. Alt (Harvard Medical School, Boston, MA, USA) for the EF1 embryonic stem cell line. The authors also thank Qiuyi Chi and Taeko Fukuda for expert technical assistance and members of the Taste Science and Abe laboratories for critical readings of the manuscript.
This work was supported in part by Japan Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid for Young Scientists (B) 20780089 to Y.I. and Grants-in-Aid for Scientific Research 16108004 and 20380183 to K.A.; a Kao Foundation for Arts and Sciences grant to Y.I.; a Nestlé Nutrition Council grant to Y.I.; a Salt Science Research Foundation grant (0915) to Y.I; a U.S. National Institutes of Health grant (R21 DC008967) to H.M.; and a Research and Development Program for New Bioindustry Initiatives grant to K.A.
REFERENCES
- 1.Delmas P., Padilla F., Osorio N., Coste B., Raoux M., Crest M. (2004) Polycystins, calcium signaling, and human diseases. Biochem. Biophys. Res. Commun. 322, 1374–1383 [DOI] [PubMed] [Google Scholar]
- 2.Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Trankner D., Ryba N. J., Zuker C. S. (2006) The cells and logic for mammalian sour taste detection. Nature 442, 934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. U. S. A. 103, 12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kataoka S., Yang R., Ishimaru Y., Matsunami H., Sevigny J., Kinnamon J. C., Finger T. E. (2008) The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses 33, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LopezJimenez N. D., Cavenagh M. M., Sainz E., Cruz-Ithier M. A., Battey J. F., Sullivan S. L. (2006) Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 98, 68–77 [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi M. A., Abe K., Emori Y. (2001) IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem. Senses 26, 259–265 [DOI] [PubMed] [Google Scholar]
- 7.Hoon M. A., Adler E., Lindemeier J., Battey J. F., Ryba N. J., Zuker C. S. (1999) Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96, 541–551 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Hoon M. A., Chandrashekar J., Mueller K. L., Cook B., Wu D., Zuker C. S., Ryba N. J. (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 [DOI] [PubMed] [Google Scholar]
- 9.Clapp T. R., Stone L. M., Margolskee R. F., Kinnamon S. C. (2001) Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez C. A., Huang L., Rong M., Kozak J. A., Preuss A. K., Zhang H., Max M., Margolskee R. F. (2002) A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 5, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 11.Inada H., Kawabata F., Ishimaru Y., Fushiki T., Matsunami H., Tominaga M. (2008) Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 9, 690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi H., Yamanaka A., Uchida K., Shibasaki K., Sokabe T., Maruyama Y., Yanagawa Y., Murakami S., Tominaga M. (2010) Activation of polycystic kidney disease-2-like 1 (PKD2L1)/PKD1L3 complex by acid in mouse taste cells. J. Biol. Chem. 285, 17277–17281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishimaru Y., Matsunami H. (2009) Transient receptor potential (TRP) channels and taste sensation. J. Dent. Res. 88, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishimaru Y. (2009) Molecular mechanisms of taste transduction in vertebrates. Odontology 97, 1–7 [DOI] [PubMed] [Google Scholar]
- 15.Yarmolinsky D. A., Zuker C. S., Ryba N. J. (2009) Common sense about taste: from mammals to insects. Cell 139, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauli S. M., Zhou J. (2004) Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26, 844–856 [DOI] [PubMed] [Google Scholar]
- 17.Qian F., Germino F. J., Cai Y., Zhang X., Somlo S., Germino G. G. (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179–183 [DOI] [PubMed] [Google Scholar]
- 18.Tsiokas L., Kim E., Arnould T., Sukhatme V. P., Walz G. (1997) Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. U. S. A. 94, 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casuscelli J., Schmidt S., DeGray B., Petri E. T., Celic A., Folta-Stogniew E., Ehrlich B. E., Boggon T. J. (2009) Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2. Am. J. Physiol. Renal Physiol. 297, F1310–F1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990–994 [DOI] [PubMed] [Google Scholar]
- 21.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 22.Nauli S. M., Kawanabe Y., Kaminski J. J., Pearce W. J., Ingber D. E., Zhou J. (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharif-Naeini R., Folgering J. H., Bichet D., Duprat F., Lauritzen I., Arhatte M., Jodar M., Dedman A., Chatelain F. C., Schulte U., Retailleau K., Loufrani L., Patel A., Sachs F., Delmas P., Peters D. J., Honore E. (2009) Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 [DOI] [PubMed] [Google Scholar]
- 24.Li A., Tian X., Sung S. W., Somlo S. (2003) Identification of two novel polycystic kidney disease-1-like genes in human and mouse genomes. Genomics 81, 596–608 [DOI] [PubMed] [Google Scholar]
- 25.Nomura H., Turco A. E., Pei Y., Kalaydjieva L., Schiavello T., Weremowicz S., Ji W., Morton C. C., Meisler M., Reeders S. T., Zhou J. (1998) Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J. Biol. Chem. 273, 25967–25973 [DOI] [PubMed] [Google Scholar]
- 26.Saito H., Kubota M., Roberts R. W., Chi Q., Matsunami H. (2004) RTP family members induce functional expression of mammalian odorant receptors. Cell 119, 679–691 [DOI] [PubMed] [Google Scholar]
- 27.Ishii S., Misaka T., Kishi M., Kaga T., Ishimaru Y., Abe K. (2009) Acetic acid activates PKD1L3-PKD2L1 channel–a candidate sour taste receptor. Biochem. Biophys. Res. Commun. 385, 346–350 [DOI] [PubMed] [Google Scholar]
- 28.Bunting M., Bernstein K. E., Greer J. M., Capecchi M. R., Thomas K. R. (1999) Targeting genes for self-excision in the germ line. Genes Dev. 13, 1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao N., Lu M., Echeverri F., Laita B., Kalabat D., Williams M. E., Hevezi P., Zlotnik A., Moyer B. D. (2009) Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes J., Ward C. J., Aspinwall R., Butler R., Harris P. C. (1999) Identification of a human homologue of the sea urchin receptor for egg jelly: a polycystic kidney disease-like protein. Hum. Mol. Genet. 8, 543–549 [DOI] [PubMed] [Google Scholar]
- 31.Murakami M., Ohba T., Xu F., Shida S., Satoh E., Ono K., Miyoshi I., Watanabe H., Ito H., Iijima T. (2005) Genomic organization and functional analysis of murine PKD2L1. J. Biol. Chem. 280, 5626–5635 [DOI] [PubMed] [Google Scholar]
- 32.Montell C. (2005) The TRP superfamily of cation channels. Sci. STKE 2005, re3. [DOI] [PubMed] [Google Scholar]
- 33.Erler I., Al-Ansary D. M., Wissenbach U., Wagner T. F., Flockerzi V., Niemeyer B. A. (2006) Trafficking and assembly of the cold-sensitive TRPM8 channel. J. Biol. Chem. 281, 38396–38404 [DOI] [PubMed] [Google Scholar]
- 34.Tsuruda PR, Julius D., Minor DL., Jr. (2006) Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phelps C. B., Gaudet R. (2007) The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J. Biol. Chem. 282, 36474–36480 [DOI] [PubMed] [Google Scholar]
- 36.Dai X. Q., Karpinski E., Chen X. Z. (2006) Permeation and inhibition of polycystin-L channel by monovalent organic cations. Biochim. Biophys. Acta 1758, 197–205 [DOI] [PubMed] [Google Scholar]
- 37.Shimizu T., Janssens A., Voets T., Nilius B. (2009) Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflügers Arch. 457, 795–807 [DOI] [PubMed] [Google Scholar]
- 38.Chen X. Z., Vassilev P. M., Basora N., Peng J. B., Nomura H., Segal Y., Brown E. M., Reeders S. T., Hediger M. A., Zhou J. (1999) Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401, 383–386 [DOI] [PubMed] [Google Scholar]
- 39.Dai X. Q., Ramji A., Liu Y., Li Q., Karpinski E., Chen X. Z. (2007) Inhibition of TRPP3 channel by amiloride and analogs. Mol. Pharmacol. 72, 1576–1585 [DOI] [PubMed] [Google Scholar]
- 40.Li Q., Dai X. Q., Shen P. Y., Wu Y., Long W., Chen C. X., Hussain Z., Wang S., Chen X. Z. (2007) Direct binding of alpha-actinin enhances TRPP3 channel activity. J. Neurochem. 103, 2391–2400 [DOI] [PubMed] [Google Scholar]
- 41.Li Q., Liu Y., Zhao W., Chen X. Z. (2002) The calcium-binding EF-hand in polycystin-L is not a domain for channel activation and ensuing inactivation. FEBS Lett. 516, 270–278 [DOI] [PubMed] [Google Scholar]