Abstract
The family of CLC proteins comprises both Cl− channels and Cl−/H+ exchange transporters with varying degrees of voltage dependence. The human CLC-5 is an electrogenic voltage-dependent 2Cl−/1H+ exchanger that gives rise to strongly outwardly rectifying currents when expressed. We conducted whole-cell recordings from HEK293 cells transiently transfected with either wild-type CLC-5 or a permeation-deficient mutant, E268A. With E268A CLC-5 we recorded transient voltage-dependent currents that represent the gating currents associated with CLC-5 activation and had kinetics that could be described by voltage-dependent forward and reverse transition rates. In extracellular solutions rich in Cl− or Br−, CLC-5 exhibited a gating charge of 1.3, but this was reduced to 0.9 in solutions comprising the impermeant anions aspartate, methanesulfonate, sulfate, or HEPES. Extracellular ion depletion by local perfusion with isotonic mannitol failed to reduce the gating charge further. Lowering intracellular pH from 7.4 to 5.4 did not shift the voltage-dependence of the gating currents, but reducing and increasing intracellular Cl− shifted the charge-voltage relationship to more negative and positive potentials, respectively. Our data suggest that voltage sensing is an intrinsic property of the CLC-5 protein and that permeant anions, particularly Cl−, modulate a voltage-dependent transition to an activated state from which Cl−/H+ exchange can occur.—Smith, A. J., Lippiat, J. D. Voltage-dependent charge movement associated with activation of the CLC-5 2Cl−/1H+ exchanger.
Keywords: chloride channel, chloride transport, gating charge, voltage sensor
Related members of a multigene family are expected to have similar properties and physiological roles. This is not the case, however, for the CLC family of proteins, originally referred to as a family of voltage-gated chloride channels (reviewed in ref. 1). The Torpedo electroplax CLC-0 and its mammalian homologue, the skeletal muscle CLC-1, are indeed outwardly rectifying chloride channels. In contrast, CLC-2 is an inwardly rectifying chloride channel, while the epithelial CLC-Ka and Kb Cl− channels exhibit very little voltage dependence. CLC-4 and 5, which under normal conditions reside predominantly in intracellular membranes, give rise to outwardly rectifying currents that resemble voltage-gated Cl− channels when expressed in Xenopus oocytes (2). However, the currents are the product of electrogenic exchange of two Cl− for one H+ (3–5), a property shared with the voltage-independent CLC-ec1 from Escherichia coli (6).
Despite these differences in ion conduction and voltage dependence, CLC proteins share a common structural architecture, which was clarified by crystallographic studies of the prokaryotic CLC-ec1 from E. coli and a homologue from Salmonella typhimurium (7). Unlike most ion channels that have varying degrees of rotational symmetry around a central aqueous pore, CLC proteins are dimers, with each subunit containing a complete conduction pathway, a protopore (7). Toward the cytoplasmic face of the subunit, the pore diverges into independent Cl− and H+ conducting routes (8). Two glutamate residues, E148 and E203, play key roles in CLC-ec1; the former is found at the extracellular end of the pore, and in the “closed state,” it occupies the putative external anion binding site (9). In this position, it occludes the pore, but it is thought to move in order for ion conduction to occur, and in this sense, it acts as a gate: the “gating glutamate” (9). E203, the “proton glutamate” is found toward the cytoplasmic face and acts as a proton acceptor (8). Neutralization of either of these glutamate residues uncouples H+ exchange and turns CLC-ec1 into a passive Cl− conductance (6, 8).
Structural studies have aided our understanding of ion binding and permeation of CLC Cl− channels and Cl−/H+ transporters, but the molecular basis of voltage-dependent gating is less well understood. Unfortunately, exploring this further with structure-function studies of prokaryotic CLCs is somewhat limited since wild-type and mutant CLC-ec1 appear constitutively active at all voltages and give linear current-voltage relationships (10). Several explanations for voltage dependence in CLC Cl− channels have been proposed, including the permeating Cl− (11) or H+ (12), acting as gating charges or inducing structural changes in the voltage field (13), or that the protein possesses an intrinsic voltage sensor (14). On the other hand, the S4 superfamily of voltage-gated cation channels possess charged transmembrane segments, the S4 in particular, which move in the voltage field and couple open probability to the membrane potential (15). Domains structurally homologous to the S4 voltage sensor are absent in voltage-gated CLC channels (16).
Endogenous CLC-5 is found in endosomal membranes of renal epithelia (17), where it contributes to the processes of endocytosis and endosomal acidification (18, 19). Dent's disease is caused by loss-of-function mutations in the human CLCN5 gene, which result in truncated CLC-5 protein, or CLC-5 with defective function or trafficking (20–22); symptoms include proteinuria, caused by defective endocytosis in the proximal tubule, kidney stones, and renal failure (20). The defects are recapitulated in the CLC-5 knockout mouse, and studies on the isolated tissue have demonstrated impaired endocytosis and endosomal acidification (18, 19). Functionally, CLC-5 is an outwardly rectifying 2Cl−/1H+ exchanger, so shares features of both the CLC-ec1 2Cl−/H+ exchanger and the voltage-gated Cl− channels, CLC-0 and CLC-1. However, the activation properties differ somewhat to the CLC Cl− channels: activation is strongly outwardly rectifying with no detectable currents at negative potentials (2, 22) and lowering extracellular pH reduces CLC-5 currents (2). Neutralization of the CLC-5 gating glutamate, E211, uncouples H+ transport and removes voltage dependence, resulting in a passive nonrectifying Cl− conductance (3, 4). E268 in CLC-5 is the proton-accepting glutamate, which is conserved among the Cl−/H+ exchanger subclass of CLC proteins (8), and neutralization of this residue prevents ion flow through CLC-5 (23). This led to the hypothesis that voltage-dependent movement of H+ through CLC-5 from the proton to gating glutamates underlies voltage-dependent gating (23); in many respects, this is harmonious with the proton-dependent gating of the CLC-0 Cl− channel (12).
In our studies of the permeation-deficient E268A CLC-5, we observed voltage-dependent transient currents that were reminiscent of the gating currents of voltage-gated cation channels. We hypothesized that these arose from the charge-movement that underlies activation of CLC-5 and used them to explore the voltage dependence and the relationship with permeating ions.
MATERIALS AND METHODS
Molecular biology and cell culture
A plasmid encoding CLC-5 with EYFP fused to the N terminus was mutated using the QuikChange site directed mutagenesis kit (Stratagene, La Jolla, CA, USA) to introduce the E268A mutation. The sequence was confirmed by automated DNA sequencing (Faculty of Biological Sciences facility, University of Leeds, Leeds, UK). HEK293 cells were transiently transfected with wild-type (WT) and mutant CLC-5-EYFP using Fugene 6 (Roche Diagnostics, Burgess Hill, West Sussex, UK), and transfected cells were identified by EYFP epifluorescence.
Electrophysiology
Patch pipettes were pulled from thin-walled borosilicate glass (Harvard Apparatus, Edenbridge, Kent, UK) and were polished, giving resistances of 1.5 to 2.5 MΩ in the experimental solutions. Currents were recorded using the whole-cell patch clamp configuration using an EPC-10 amplifier (Heka Electronics, Lambrecht, Germany), employing capacitance cancellation and >65% series resistance compensation. For measurements of time dependence, the fast 2-μs series resistance setting was used, while for measurement of total charge movement, this was slowed to 100 μs to avoid loss of fast components. Junction potentials were calculated using the calculator supplied with pClamp 9.0 software (Molecular Devices, Sunnyvale, CA, USA), and the voltage offset was corrected prior to seal formation. Currents were filtered at 10 kHz and digitized at 50 kHz using PatchMaster software (Heka Electronics). Cells were held at −30 mV, and 10-ms pulses from −100 to + 200 mV at 10-mV increments were applied at 1-s intervals. A P/−4 leak and residual capacitance current subtraction protocol was used. The standard bath solution contained (in mM): 136 CsCl, 1 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4 with CsOH. The standard pipette solution contained 42 CsCl, 98 aspartic acid, 10 EGTA, and 10 HEPES, pH 7.4 with CsOH. For experiments where intracellular pH was altered, the pipette solution was adjusted so as to contain 5 mM of both HEPES and MES to increase the buffering range. In extracellular anion substitution experiments, all of the CsCl in the bath solution was replaced by corresponding cesium salts while MgCl2 and CaCl2 were replaced by gluconate salts. When intracellular [Cl−] was altered, aspartate was the substituting anion, and a holding potential of −80 mV was used. For the ion-depletion experiment, a solution containing 300 mM mannitol was applied using a local microperfusion pipette. All salts were from Sigma-Aldrich Co. (Poole, Dorset, UK) and were of the highest purity available. Recordings were made at room temperature (22–23°C), except where the temperature of the recording bath was lowered by adding ice-cold extracellular solution to the bath prior to seal formation; the temperature was taken immediately after the recording using a digital thermometer (17–18°C).
Analysis
Data were analyzed using Fitmaster (Heka Electronics), Microsoft Excel, and OriginPro 7.5 (OriginLab Corporation, Northampton, MA, USA). Conductance (G) was calculated by dividing CLC-5 currents by V-Erev, using the predicted reversal potential, Erev, calculated from the equations in Accardi and Miller (6) and with a transport ratio of 2Cl−:1H+ in CLC-5 (5). Charge-voltage (Q-V) relationships were fitted with the Boltzmann functions of the form Q = QMAX/{1 + exp[(V1/2 − V)/k]}, where Q is the charge in coulombs measured by integrating the transient currents, QMAX the maximum charge displaced, V1/2 the voltage that gives a half-maximal charge movement, and k is the slope factor. Conductance-voltage (G-V) relationships were similarly analyzed, replacing G for Q. For comparison, data were normalized to QMAX or GMAX. Gating charges, zg, were estimated from the slope of the ln G-V or ln Q-V plots at minimal activation, zgF/RT, where F, R, and T have their usual thermodynamic meanings. The time course of the decay phase of the transient currents was fitted with a single exponential in Fitmaster to give the time constant τ. Data series were fitted with kinetic schemes in Microsoft Excel using the Solver function to perform least-squares fit of parameters. Mean data represent the mean measurement from ≥5 different cells, and the values are given as means ± se. Student's t test was used to compare pairs of data sets, but for multiple comparisons, ANOVA with post hoc Bonferroni correction was used.
RESULTS
E268A mutation reveals the CLC-5 gating currents
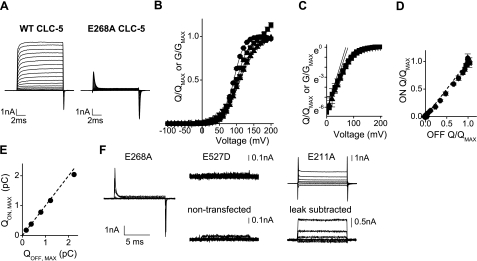
Wild-type (WT) and mutant (E268A) CLC-5 were transiently expressed in HEK293 cells and studied by whole-cell patch clamp (Fig. 1A). Depolarizing cells expressing WT CLC-5 to potentials above +30 mV resulted in rapidly activating and sustained outward currents. The G-V plot was described by a Boltzmann function with a half-maximal activation voltage of 116.0 ± 2.8 mV and a slope factor of 23.4 ± 0.9 mV. On repolarization to −30 mV, brief inward currents were observed. While they appeared to be tail currents arising from the movement of ions through CLC-5 prior to deactivation, they were much larger than one would expect at a holding potential close to the predicted reversal potential (−20.1 mV), and reverse at 53.3 ± 2.3 mV (n=6; see Supplemental Data and Supplemental Fig. 2), which does not relate to the equilibrium potential of any of the permeant ions: ECl was −30 mV, and EH was 0 mV. As observed previously (23), the E268A mutant does not conduct any significant sustained current, but we observed transient outward and inward currents when the membrane was depolarized to the same potentials that activate WT CLC-5 and then repolarized, respectively (Fig. 1A). Whole-cell capacitance cancellation and leak subtraction are illustrated in Supplemental Fig. 1. These transient currents were absent in nontransfected HEK293 cells, cells transfected with the constitutively inactive Dent's disease mutant E527D (22), and the constitutively active Cl− conductance formed by the E211A mutation (Fig. 1F). The E211A mutation results in sustained nonrectifying Cl− currents that can be removed from the recordings by leak subtraction (Fig. 1F). Since the size, direction, and time dependence of the transient currents did not appear to correspond to ion permeation, we hypothesized that they represented the charge movement associated with voltage-dependent gating, analogous to the gating charges of the S4 superfamily of voltage-gated cation channels. The charge displaced on depolarization (QON) to potentials between −100 and + 200 mV and repolarization (QOFF) to −30 mV were measured and the charge-voltage plots were fitted by Boltzmann functions (Fig. 1B). At the most strongly depolarizing potentials, an additional linear component was observed in the QON charge-voltage relationship (Fig. 1B), which was due to a small sustained endogenous current from HEK293 cells. To remove this from the subsequent analysis, a section of the current toward the end of the pulse was integrated and subtracted from a segment of the same duration measured from the start of the pulse. The QON had a V1/2 of 85.8 ± 2.2 mV and a slope of 17.0 ± 1.2 mV, and the corresponding values for QOFF were 80.3 ± 0.8 mV and 15.4 ± 0.3 mV. Thinking that the brief tail current recorded from WT CLC-5 might be a QOFF gating current, we integrated and analyzed these further. The charge displaced during the tail current also followed a Boltzmann distribution (Fig. 1B) with a V1/2 of 103.0 ± 6.1 mV and a slope of 23.7 ± 3.0 mV, which are very close to the values obtained from the WT CLC-5 conductance-voltage relationships (Fig. 1B). To see whether there was a correlation between the underlying gating charge responsible for the voltage dependence of both WT conductance and E268A charge displacement, the data were plotted on a semilogarithmic plot (Fig. 1C). The steepest part of both plots was found at lowest points of detectable activity and was linear between 10 and 70 mV. Straight lines were fitted to data in this region to give the slope zgF/RT, where zg is the gating charge, which is considered here to be the product of the valence of the gating particle and the fraction of the electrical field across the membrane acting on the charge. The zg values were 1.15 ± 0.19 and 1.25 ± 0.15 for the WT CLC-5 conductance and E268A QON currents, respectively, and were not significantly different. In each sweep to different voltages, the QOFF closely matched the QON charge (Fig. 1D) and in each cell, the maximum QON and QOFF were closely related (Fig. 1E).
Figure 1.
Voltage-dependent charge-displacement observed with a transport-deficient CLC-5 mutant. A) Representative whole-cell current families recorded from HEK cells transiently transfected with wild-type (WT) and E268A CLC-5. Cells were held at −30 mV, and 10-ms pulses were applied to voltages between −100 and +200 mV at 10-mV intervals. B) Relationship between the charge displaced and voltage for the transient currents at the onset of (QON, ■) and immediately after (QOFF, ●) the voltage pulse. Data are expressed as means ± se of ≥5 different cells. QON currents fail to plateau due to the activation of an endogenous conductance with large depolarization, which was subsequently subtracted as described in the main text. Also shown is the WT CLC-5 conductance-voltage relationship (▴) and the charge displaced during the WT CLC-5 tail current (▾). Fitted curves are all Boltzmann functions. C) Same data for WT CLC-5 conductance (▴) and E268A CLC-5 QON (■) on a semilogarithmic plot to measure the limiting logarithmic slope, zgF/RT, fitted to the steepest part of the plot, indicated by the solid lines. D) Relative E268A QON and QOFF data from the graph in B plotted against each other to show the correlation in each pulse to different potentials. Dotted line is QON = QOFF. E) Correlation between the maximum QON and QOFF measured from 5 different cells expressing E268A CLC-5. Data points are individual cells; dotted line is QON = QOFF. F) Current sweeps from voltage pulses to −100 to +200 mV in 50-mV intervals from a cell expressing E268A, E527D, a nontransfected cell, and E211A before (E211A), and after leak subtraction (leak subtracted).
Gating currents can be described by a 2-state kinetic scheme
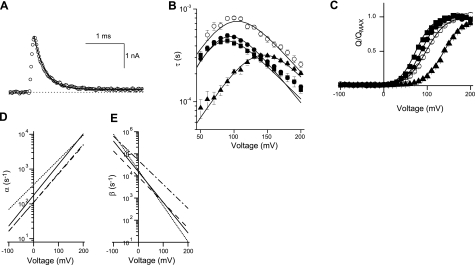
The decay phase of the transient gating currents were fitted by single exponential curves (Fig. 2A). Unlike membrane capacitance, which has a uniform time constant, the τ-voltage plot was nonlinear (Fig. 2B). Furthermore, the τ-voltage relationship could be fitted by parameters describing a 2-state kinetic scheme involving voltage-dependent rates: a forward (inactive to active) rate α and the reverse rate β. The model and rates are described in Fig. 2, and the parameters given in Table 1. To increase the reliability of the fitting procedure, the parameters were simultaneously fit to the charge-voltage relationship from the same cells (Fig. 2C). The effects of lowering the temperature of the recording bath, mutating CLC-5, and removing extracellular [Cl−] are shown in Fig. 2 and Table 1. At equivalent voltages, lowering the temperature by ∼5°C slowed time constants and also reduced α0 and β0. In contrast the τ-voltage relationship of the double mutant E268A/S520T were not markedly different to E268A (Fig. 2B), but α0 and β0 were both increased (Table 1). The S520T mutation slows activation of currents through CLC-5 expressed in Xenopus oocytes (2) and in HEK293 cells (Supplemental Fig. 3). Fitting exponential rise times to ionic currents through WT and S520T CLC-5 were hindered by the presence of the transient current immediately on depolarization but were best described as the sum of two exponential processes (Supplemental Fig. 3). Eliminating extracellular [Cl−] by using a methanesulfonate-based solution caused a large change in the τ-voltage relationship, decreased α0, and increased β0 (Table 1). Under these conditions, the charge-voltage relationship was also shifted to more positive potentials (Fig. 2C).
Figure 2.
Kinetic analysis of E268A gating currents. A) Transient gating currents, represented as digitized points (○), fitted by a single exponential (solid line), It = I0 e−t/τ + c to yield the time constant, τ. B) Values are expressed as means ± se; n=8–10 cells. τ-Voltage plots for the decay phase of the gating currents from E268A (●), E268A cooled to 5 below ambient (○), E268A/S520T (■), and E268A with Cl−-free, methanesulfonate-based extracellular solution (▴). Solid lines are the scheme τ = (α + β)−1, where α and β are forward and reverse voltage-dependent rate constants α0 ezα(FV/RT) and β0 e−zβ(FV/RT), respectively, using the mean values of α0, zα, β0, and zβ fitted to the individual data sets; α0 and β0 are the rates of α and β at 0 mV and zα and zβ their respective voltage dependence. C) Values are expressed as means ± se; charge-voltage plots from the same cells in B superimposed with the function Q/QMAX = α/(α + β) with the same values of α and β as in B. D, E) Representation of the voltage dependence of the rates α (D) and β (E) using mean values of α0, zα, β0, and zβ for E268A (solid line), E268A cooled to 5 below ambient (dashed line), E268A/S520T (dotted line), and E268A with the Cl−-free, MeSO4−-based extracellular solution (dash-dot-dash line).
Table 1.
Parameters describing the voltage-dependent transition rates in the 2-state kinetic model, fitted to data in Fig. 2
| Parameter | n | α0 (s−1) | zα | β0 (s−1) | zβ |
|---|---|---|---|---|---|
| E268A | 10 | 177 ± 15 | 0.51 ± 0.01 | 15458 ± 1315 | 0.80 ± 0.03 |
| E268A, cooled | 9 | 110 ± 14 | 0.48 ± 0.02 | 9178 ± 1383 | 0.67 ± 0.06 |
| E268A/S520T | 8 | 357 ± 31 | 0.41 ± 0.01 | 18304 ± 1935 | 0.93 ± 0.03 |
| E268A, MeSO4− | 10 | 112 ± 12 | 0.48 ± 0.02 | 50647 ± 1857 | 0.63 ± 0.01 |
Values are means ± se; n is the number of cells; α0 is the inactive-to-active rate at 0 mV; β0 is the reverse rate at 0 mV; and zα and zβ are their respective voltage dependence. Data represent, from top to bottom, recordings from cells expressing E268A CLC-5, recordings at 5°C below room temperature, cells expressing the double E268A/S520T CLC-5 mutant, and recordings of E268A CLC-5 in the Cl−-free, methanesulfonate-based extracellular solution.
Voltage-dependence of the gating charges is modulated by extracellular anions
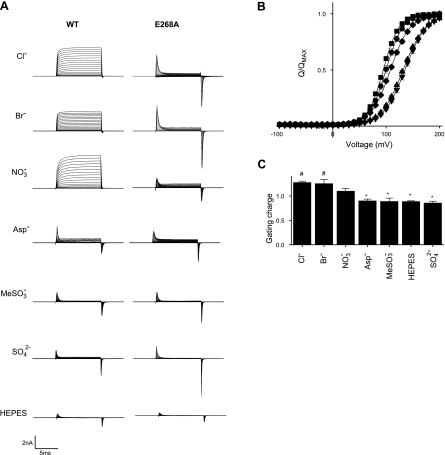
In CLC channels, voltage-dependent gating is strongly influenced by permeating anions and pH (11–13, 24). Knowing that CLC-5 is not stimulated by lowering extracellular pH and that removing [Cl−] affected the E268A CLC-5 currents (Fig. 2), we explored further how extracellular anions influenced the transient gating currents. The WT and E268A CLC-5 currents recorded in a Br−-based extracellular solution were similar to those in the standard Cl− solution (Fig. 3A). The gating charges zg, measured by the limiting logarithmic slope of the E268A CLC-5 QOFF currents were 1.28 ± 0.03 and 1.25 ± 0.10 in Cl− and Br−, respectively. The QOFF currents were used for this and all subsequent analysis as they are not contaminated by the residual sustained outward currents. E268A CLC-5 gating currents persisted in extracellular solutions, where Cl− was replaced by the impermeant ions aspartate, methanesulfonate, sulfate, or HEPES (Fig. 3A), but with significantly reduced QOFF zg values of 0.90 ± 0.5, 0.89 ± 0.08, 0.86 ± 0.04, and 0.90 ± 0.05, respectively (Fig. 3C). There was no significant difference in the total charge displaced (QMAX) between cells bathed in the different anion solutions (ANOVA, data not shown). Transient gating currents with similar kinetics were also recorded from cells expressing WT CLC-5 when extracellular solutions containing in each of these impermeant ions were used (Fig. 3A). Nitrate, which permeates CLC-5, but with reduced H+ coupling (5, 23), gave an intermediate zg of 1.10 ± 0.06 (Fig. 3C).
Figure 3.
Different E268A CLC-5 charge-displacement kinetics with permeant and impearmeant anions. A) Representative whole-cell currents recorded from both WT and E268A CLC-5 in different bath solutions as indicated. B) Charge-voltage relationships for the E268A CLC-5 QOFF currents recorded in bath solutions containing Cl− (■), Br− (●), NO3− (♦), aspartate (Asp−, ▴), or methanesulfonate (MeSO3−, ▾). Fitted curves are Boltzmann functions. C) Gating charge zg of the E268A QOFF current in each of the bath solutions, measured using the limiting logarithmic slope method. *P < 0.05 vs. Cl− solution; #P < 0.05 vs. aspartate solution. Data are expressed as means ± se of ≥5 different cells.
Gating currents are unchanged by lowering internal pH but are modulated by internal Cl−
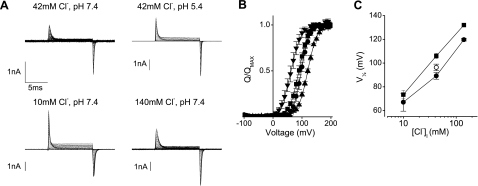
We also considered whether the E268A CLC-5 gating currents were influenced by the concentration of permeating ions in the internal solution. The availability of protons was increased by lowering the internal pH from 7.4 to 5.4 and E268A CLC-5 gating currents recorded using the standard extracellular solution; these were qualitatively similar to those obtained in pH 7.4 (Fig. 4A), and there was no significant difference between the V1/2 values of 89.3 ± 2.8 mV in control pH 7.4 solution and 96.4 ± 2.7 in pH 5.4 (Fig. 4B, C). Ionic currents through WT CLC-5 were of similar amplitude when intracellular solutions at pH 7.4 and 5.4 were used, but lowering pHI shifted the activation (conductance-voltage plots) to more positive potentials (see Supplemental Data and Supplemental Fig. 5). In contrast, intracellular Cl− significantly modulated the voltage dependence of the charge displacement. Reducing [Cl−]I from 42 mM to 10 mM shifted the V1/2 to the more negative 67.1 ± 7.6 mV, while increasing it to 140 mM shifted V1/2 to the more positive 119.7 ± 1.1 mV (Fig. 4B, C). This mirrored the effects on the voltage-dependent activation of ionic currents conducted by CLC-5 when [Cl−]I was altered (Fig. 4C; see Supplemental Data and Supplemental Fig. 4). None of these intracellular pH or [Cl−] changes significantly altered the maximum charge movement of the gating currents recorded from E268A CLC-5.
Figure 4.
Effects of altering internal [Cl−] and pH. A) Representative currents from cells expressing E268A CLC-5 using different intracellular solutions as indicated. B) Charge-voltage relationships of the QOFF currents in control solutions (■), when internal pH was lowered from 7.4 to 5.4 (●), or when internal [Cl−] was lowered and raised from 42 to 10 mM (▾) and 140 mM (▴), respectively. Fitted curves are Boltzmann functions. C) Plot of the fitted half-maximal V1/2 values vs. intracellular [Cl−] at pH 7.4 (♦) plus the value obtained with 42 mM Cl− when pH was lowered to 5.4 (○). V1/2 values obtained from the conductance-voltage plots of wild-type CLC-5 currents (see Supplemental Fig. 4) with the same solutions are indicated (■). Data are expressed as means ± se of ≥5 different cells.
Effects of extracellular ion depletion on the gating currents
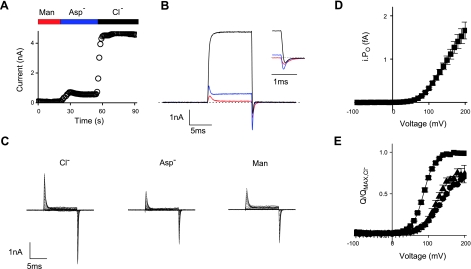
Whether voltage-gated CLC proteins contain an intrinsic voltage sensor or are gated by Cl− or H+ moving in and out of the voltage field has been the subject of debate (11, 14, 25). None of our extracellular anion substitution experiments reduced the gating charge lower than ≈0.9 (Fig. 3C), measured with all the impermeant anions tested. To exclude any other type of extracellular ion-dependence, we conducted an experiment that depleted all of the available ions close to the cell surface. After establishing a WT CLC-5 recording in standard Cl− solution, the cell was perfused with a solution containing only 300 mM mannitol, followed by aspartate solution, and then returning to the initial Cl− solution. The change in the steady-state current from one experiment is shown in Fig. 5A, where at 200 mV, there was no appreciable outward current in mannitol, a small outward current in aspartate, and a large CLC-5 current in Cl−, demonstrating effective solution change and that CLC-5 remained functional after these maneuvers. Gating currents were observed in both the mannitol and the aspartate solutions (Fig. 5B). The QOFF currents were analyzed further in the aspartate solution to measure both zg and the total charge displacement, QMAX, thereby estimating the number of active CLC-5 monomers, N, in the membrane, where n = QMAX/(zgqe), where qe is the elementary charge. The whole-cell CLC-5 current in Cl− solution, using ion channel phraseology, is the product of the number of pores (N), the current passing through an open pore (i) and the proportion of the time that the pore is open (PO). The macroscopic WT CLC-5 current-voltage relationship was divided by N to give the unitary iPO-voltage relationship shown in Fig. 5C. The concept of PO of voltage-gated Cl−/H+ exchanger is not understood but may correspond to transitions between active and inactive states (23) or rectification of the unitary current (27). Cells expressing E268A CLC-5 were also similarly perfused by the three solutions (Fig. 5D), and the Q-V relationships were normalized to the QMAX obtained in the Cl− solution (Fig. 5E). The currents obtained in aspartate and mannitol solutions were qualitatively identical and gave similar Q-V relationships. The relative QMAX was 0.71 ± 0.02 and 0.74 ± 0.1 in aspartate and mannitol, respectively.
Figure 5.
Gating currents persist following depletion of extracellular ions. A) Time course of the steady-state amplitude of WT CLC-5 currents evoked by pulses to +200 mV when the cell was locally perfused with 300 mM mannitol (Man), aspartate (Asp−), and then Cl− extracellular solution as indicated. B) Whole-cell currents evoked by 10-ms voltage pulses to +200 mV in the 3 solutions (red trace, mannitol; blue trace, aspartate; black trace, Cl−). Inset: QOFF currents on an expanded timescale. C) Representative E268A CLC-5 currents from the same cell perfused with Cl−, aspartate (Asp−), and mannitol extracellular solutions. D) WT CLC-5 unitary current-voltage relationship obtained by dividing the macroscopic WT CLC-5 current-voltage relationship in Cl− solution by the number of active pores. Latter was calculated by recording QOFF currents from the same cell in aspartate solution and dividing the total charge (QMAX/qe) by the gating charge zg measured using the limiting logarithmic slope method. E) Mean charge-voltage relationships following normalization to the QMAX measures in the Cl− extracellular solution (■) and the relative values in aspartate (●) and mannitol (♦). Data are expressed as means ± se of ≥5 different cells.
DISCUSSION
In discussing our findings in context with other studies of CLC proteins, we largely reference the plethora of structural and functional studies of CLC-ec1, a voltage-independent 2Cl−/1H+ exchanger (6), and the voltage-gated Cl− channel CLC-0, which has gating that is sensitive to Cl− and H+ through the ancestral remnants of transport activity (12). CLC-5 is a voltage-gated 2Cl−/1H+ exchanger (3–5) and in contrast with CLC-0 activation is inhibited and not stimulated by lowering external pH (2), and voltage dependence is not appreciably altered by changing the permeant anion (2, 28) nor by disease-causing mutations (20, 22). Thus, we focus our discussion points on the similarities with other CLCs rather than the multitude differences. As was found previously, E268A CLC-5 is nonconducting (23). This internal proton glutamate is thought to be the intracellular proton acceptor providing the pore with protons for coupled transport (8). The equivalent E203A mutation in the constitutively active CLC-ec1 results in uncoupled transport and passive Cl− conduction through the pore, similar to when the gating glutamate E148 is neutralized. Since neutralizing the human CLC-5 gating glutamate E211 also results in an uncoupled and a nonvoltage-gated Cl− conductance (3, 4), one might have thought that the E268A mutation would have a similar effect. The nonconducting phenotype of E268A CLC-5 led to the hypothesis that the voltage dependence of CLC-5 was conferred by movement of protons in the voltage field from E268 to E211 (23). In the present study, WT and E268A CLC-5 were expressed by transient transfection of cultured mammalian cells and studied by whole-cell patch clamp, which has increased recording bandwidth and temporal voltage control compared with voltage-clamp of Xenopus oocytes used in many previous studies. We recorded transient currents from cells expressing E268A CLC-5 that were capacitative in appearance but exhibited a voltage dependence that mirrored the activation kinetics of WT CLC-5 currents. These currents were absent in nontransfected HEK293 and in cells expressing constitutively inactive (E527D) and active (E211A) CLC-5 mutants. In the standard solutions, the E268A CLC-5 charge-voltage and WT CLC-5 conductance voltage-relationships had similar limiting logarithmic slopes, yielding a gating charge of ≈1.3 (Fig. 1B, C). The charge displaced from the onset of each of the depolarizing pulses (QON) was similar in amplitude but opposite in polarity to the charge displaced on repolarization (Fig. 1D, E). This led us to believe that the charge movement that we recorded from E268A CLC-5 was that responsible for voltage-dependent activation of this exchanger. While protonation of E268 and presumably E211 are essential for activation of CLC-5 (23), the E268A mutation does not affect the voltage-sensing mechanism. Interestingly, the QOFF current appears as the tail current in WT CLC-5 recordings (Fig. 1). This implies that CLC-5 is very strongly rectifying under these conditions where it only conducts outward currents and the Cl−/H+ exchange mechanism cannot be driven in the opposite direction. Transient voltage-dependent currents with similar properties were also recorded from WT CLC-5 when extracellular Cl− was replaced by each of the impermeant anions (Fig. 3), providing additional evidence E268 is not involved in voltage sensing. Instead, our data demonstrate that E268 is critical for Cl−/H+ exchange in CLC-5, but only once the protein has been activated by a separate voltage-dependent step involving another part of the protein.
The time course of the gating currents could be described by a 2-state kinetic model in which the voltage dependence arises from voltage-dependent rate constants between what we considered to be an inactive and an activated state. This kinetic process resulted in the nonlinear τ-voltage relationship observed in Fig. 2B. The time constants of E268A CLC-5 current decay were increased (Fig. 2B) when the recording environment was cooled, which was caused by a reduction in the underlying transition rates (Table 1), providing further support that these currents arise from a kinetic process. We explored whether a mutation known to slow activation of ion transport through CLC-5, S520T (2; Supplemental Data and Supplemental Fig. 2) had an effect on the gating currents. Both the forward and reverse rates were increased (Table 1), but there was no appreciable increase in the time constants that reflected the slowing of CLC-5 activation. The time course of WT CLC-5 current activation was best fitted by the sum of two exponential processes indicating multiple closed or nonconducting states. The fast time constants appeared on a similar range, as those for E268A CLC-5 gating currents (100–200 μs), the slow component had τ values an order of magnitude larger, and both the fast and slow CLC-5 activation time constants were increased by the S520T mutation (Supplemental Fig. 2). It is conceivable that voltage dependence involves transitions between nonconducting states with ion transport occurring in bursts (23) from the activated state. The major effect of the S520T mutation may be on altering the transition rates between the nonconducting activated and the transporting states, but further investigation and evaluation of expanded kinetic models are required. Removing extracellular [Cl−] had the largest effect on the E268A CLC-5 τ-voltage relationship (Fig. 2B), as a result of reduced forward and increased reverse transition rates (Table 1), and indicate that voltage-dependent transitions are influenced by the presence of a permeant anion.
We initially explored the idea that the transient currents were analogous to the voltage-dependent pre-steady-state currents that are observed with ion-coupled transporters such as Na+-K+-ATPase (29) and GABA transporter (30). Here, transient capacitative currents arise from the binding and unbinding of substrate ions to transporter binding sites in the voltage field. Replacing extracellular Cl− with impermeant anions did not remove the E268A CLC-5 transient currents but reduced the gating charge from ≈1.3 to ≈0.9 (Fig. 2). This left us with several possible mechanisms; the first of which was that reduced occupancy by impermeant anions could account for the change in kinetics and the decrease in charge movement. Crystal structures of CLC-ec1 have been solved in the presence of Br−, which in CLC-5, is indistinguishable to Cl−, and in the presence of SeCN−, a polyatomic anion that is likely to uncouple proton transport (26). While Br− ions are able to occupy all three anion binding sites in the ion pathway, SeCN− was only found at the internal site (26). If the structures are similar, this would suggest that the impermeant anions used in our study would not be able to access the CLC-5 pore from the extracellular solution. To support this, we found that no anion substitution reduced the gating charge further from ≈0.9 (Fig. 3C). Furthermore, compared with the other impermeant anions, the divalent SO42− anion did not alter the charge displacement kinetics (Fig. 3C), where binding or occlusion of equimolar amounts would double the valence of the charge displaced. Extracellular NO3− ions, while able to permeate WT CLC-5, induced an intermediate zg of 1.1, which may reflect the partial uncoupling of proton exchange (5, 23).
Another possibility is that internal Cl− and H+ contribute to the voltage-dependent charge displacement. Increasing the availability of intracellular protons by lowering the pH from 7.4 to 5.4 had no significant effect on the amplitude and voltage dependence of the charge movement (Fig. 4). Increasing intracellular [Cl−] from 42 to 140 mM and so increasing the amount available to bind and unbind at intracellular sites had an inhibitory effect on charge displacement and shifted the voltage dependence to more positive potentials (Fig. 4). Lowering intracellular [Cl−] had the opposite effect; thus intracellular [Cl−] has a modulatory effect on the voltage dependence but does not contribute toward the gating charge movement. Intracellular [Cl−] had a similar effect on the activation of ion transport through WT CLC-5 (Supplemental Data and Supplemental Fig. 4). The evidence from CLC-ec1 studies that anions can occupy the internal anion binding site without having to overcome a gate (26) makes this a candidate for this modulatory site, where its occupancy by Cl− could result in a shift of the charge-voltage relationship to more positive potentials.
To exclude the possibility that any other extracellular ion contributes to the gating charge, we depleted all ions by local perfusion of the cell with a 300 mM mannitol solution. Cells expressing WT CLC-5 remained intact following this maneuver, as reapplication of Cl− resulted in sustained outwardly rectifying CLC-5 currents (Fig. 5A). Application of mannitol reduced the sustained outward current, presumably ion conduction through endogenous channels or residual uncoupled E268A CLC-5 current, but not the transient currents associated with gating (Fig. 5B). The charge-voltage kinetics with E268A CLC-5 were similar in both mannitol and aspartate extracellular solutions (Fig. 5E). Measuring the gating charge and the total charge displaced while cells expressing WT CLC-5 were perfused with aspartate solution permitted an estimate of the number of gated pores and yielded a femtoamp current-voltage relationship of a single CLC-5 pore (Fig. 5C). Using nonstationary noise analysis, Zdibik et al. (23) reported an estimate of the unitary current in the order of 60 fA at +140 mV. The plot in Fig. 5C accounts for this order of unitary activity multiplied by a voltage-dependent open probability or, in more correct terms for a Cl−/H+ exchanger, the proportion of time spent in activated transporter states. Alternatively, this plot represents the upper limit for ion transport and ion exchange activity in the CLC-5 pore itself is strongly voltage dependent, analogous to single-channel rectification. Such a property has been described for the closely related CLC-4 Cl−/H+ exchanger (27).
This only leaves to account for the source of the charge-displacement observed with E268A CLC-5 in our studies. Since the transient gating currents cannot be removed by ion replacement experiments and does not involve the intracellular proton acceptor E268, we conclude that voltage-dependent rearrangements of the CLC-5 protein are the most likely explanation. This raises the possibility that CLC-5 possesses what is functionally analogous but structurally unrelated to the voltage-sensor that is found in the S4 superfamily of voltage-gated cation channels. In these channels, movement of charged transmembrane helices in the membrane voltage field is coupled to the channel gating mechanism (see ref. 15 for a review). The gating charge for Shaker potassium channels is ∼12 (31) and other voltage-gated cation channels give a similar value, considerably larger than 1.3 obtained with CLC-5 in the present study. This reflects a relatively weak voltage-dependence and the need to stretch the voltage range to strongly depolarized potentials to appreciate the full range of CLC-5 activity (Fig. 1). Gating charges close to 1 have been proposed for other voltage-dependent members of the CLC family, including the Cl− channels, CLC-0 (32), and CLC-2 (33), which correlate with the proposals that the Cl− (11, 13), or H+ ion (12) moving through the pore acts as the voltage sensor and gating charge. The CLC protein structure (7) does not possess an S4-like domain, where single transmembrane segments contain charged residues at regular intervals. An intrinsic protein voltage-sensor has previously been proposed for CLC-1 (14) as a result of a mutation that reverses voltage dependence, but this was subsequently rebutted, on the basis of the evidence that a charge-conserving mutation at the same site and mutations in other parts of the protein also affect voltage dependence (25).
The voltage-dependent activation of CLC-5 ion exchange appears to be a complex process, requiring an interaction with the ionic substrates Cl− and H+ in addition to a depolarization-induced structural change. In the absence of permeating anions, there is a charge movement with a gating charge of ≈0.9. We propose that this is a result of a structural rearrangement of the protein whether it is a small movement of a charged region of the protein, a larger movement of a single residue, or the rearrangement of a helix dipole in the voltage field (15). With E268A CLC-5, extracellular Cl− increases the gating charge to 1.3. Either Cl− enters the pore in a voltage-dependent manner and adds to the intrinsic charge displacement or Cl− binding is voltage independent but promotes a different structural change with a larger charge displacement that ultimately results in ion transport. The fact that the charge-voltage data are fitted by a single Boltzmann function and that Cl− gives a steeper limiting logarithmic slope favors the latter idea, which is strongly reminiscent of the Cl− and voltage-dependent gating model for CLC-0 proposed by Chen and Miller (13). This model argued that Cl− binds to an extracellular site and induces a change in the structure of the pore when the membrane is depolarized. In CLC-5, this structural change may reveal sites in the pore that are important for Cl−/H+ exchange or even open one of the two gates expected to exist in the transporter subclass class of CLCs (34). To date, the only mutation that is able to abolish voltage dependence in CLC-5 is the neutralization of the external gate E211 (2), which also uncouples H+ transport (3, 4). How the conservative E527D mutation, which results in an inactive CLC-5 protein in the plasma membrane (22), prevents any measurable gating or ionic current is not clear. The questions that remain center on which part of CLC-5 might act as a voltage sensor and whether a similar mechanism can be found in other CLC proteins. It is not inconceivable that voltage-induced structural changes do take place in proteins, such as CLC-ec1 and CLC-0. Intrinsic voltage dependence with a gating charge close to unity could be masked by differential coupling with the gates of the pore and the relative influence of permeating ions on gating to give the appearance of ion-dependent gating. It is worth remembering that members of the CLC family, while functionally diverse, do share many common structural features and one does usually expect closely related proteins to behave in a similar manner.
In summary, we have recorded the charge movement that is associated with voltage-dependent activation of the CLC-5 2Cl−/1H+ exchanger and found that a structural change in the protein is the most likely explanation for its origin. CLC-5 Cl−/H+ exchange activity requires delivery of H+ from E268, but this does not appear to be the voltage-dependent step that underlies rectification in CLC-5. Instead, CLC-5 contains a separate voltage-sensing mechanism that is modulated by permeant anions, namely Cl−. The recording of gating currents from this permeation-deficient CLC-5 mutant will enable us to separate voltage sensing from permeation and will help us to understand the basis of voltage dependence in this enigmatic family of proteins.
Supplementary Material
Acknowledgments
This study is supported by the Wellcome Trust grant number 076545/Z/05/Z. We thank Dr. Malcolm Hunter (University of Leeds) for helpful discussions during the experimental work and for comments on the manuscript.
REFERENCES
- 1.Jentsch T. J., Neagoe I., Scheel O. (2005) CLC chloride channels and transporters. Curr. Opin. Neurobiol. 15, 319–325 [DOI] [PubMed] [Google Scholar]
- 2.Friedrich T., Breiderhoff T., Jentsch T. J. (1999) Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J. Biol. Chem. 274, 896–902 [DOI] [PubMed] [Google Scholar]
- 3.Picollo A., Pusch M. (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436, 420–423 [DOI] [PubMed] [Google Scholar]
- 4.Scheel O., Zdebik A. A., Lourdel S., Jentsch T. J. (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436, 424–427 [DOI] [PubMed] [Google Scholar]
- 5.Zifarelli G., Pusch M. (2009) Conversion of the 2 Cl(−)/1 H+ antiporter ClC-5 in a NO3(−)/H+ antiporter by a single point mutation. EMBO J. 28, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Accardi A., Miller C. (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427, 803–807 [DOI] [PubMed] [Google Scholar]
- 7.Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 [DOI] [PubMed] [Google Scholar]
- 8.Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., Miller C. (2005) Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 126, 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutzler R., Campbell E. B., MacKinnon R. (2003) Gating the selectivity filter in ClC chloride channels. Science 300, 108–112 [DOI] [PubMed] [Google Scholar]
- 10.Accardi A., Kolmakova-Partensky L., Williams C., Miller C. (2004) Ionic currents mediated by a prokaryotic homologue of CLC Cl- channels. J. Gen. Physiol. 123, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pusch M., Ludewig U., Rehfeldt A., Jentsch T. J. (1995) Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature 373, 527–531 [DOI] [PubMed] [Google Scholar]
- 12.Lisal J., Maduke M. (2008) The ClC-0 chloride channel is a ‘broken’ Cl−/H+ antiporter. Nat. Struct. Mol. Biol. 15, 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T. Y., Miller C. (1996) Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J. Gen. Physiol. 108, 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahlke C., Rudel R., Mitrovic N., Zhou M., George A. L., Jr. (1995) An aspartic acid residue important for voltage-dependent gating of human muscle chloride channels. Neuron 15, 463–472 [DOI] [PubMed] [Google Scholar]
- 15.Bezanilla F. (2000) The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 [DOI] [PubMed] [Google Scholar]
- 16.Jentsch T. J., Steinmeyer K., Schwarz G. (1990) Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 348, 510–514 [DOI] [PubMed] [Google Scholar]
- 17.Gunther W., Luchow A., Cluzeaud F., Vandewalle A., Jentsch T. J. (1998) ClC-5, the chloride channel mutated in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. U. S. A. 95, 8075–8080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piwon N., Gunther W., Schwake M., Bosl M. R., Jentsch T. J. (2000) ClC-5 Cl− channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408, 369–373 [DOI] [PubMed] [Google Scholar]
- 19.Gunther W., Piwon N., Jentsch T. J. (2003) The ClC-5 chloride channel knock-out mouse - an animal model for Dent's disease. Pflügers Arch. 445, 456–462 [DOI] [PubMed] [Google Scholar]
- 20.Lloyd S. E., Pearce S. H., Fisher S. E., Steinmeyer K., Schwappach B., Scheinman S. J., Harding B., Bolino A., Devoto M., Goodyer P., Rigden S. P., Wrong O., Jentsch T. J., Craig I. W., Thakker R. V. (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379, 445–449 [DOI] [PubMed] [Google Scholar]
- 21.Ludwig M., Doroszewicz J., Seyberth H. W., Bokenkamp A., Balluch B., Nuutinen M., Utsch B., Waldegger S. (2005) Functional evaluation of Dent's disease-causing mutations: implications for ClC-5 channel trafficking and internalization. Hum. Genet. 117, 228–237 [DOI] [PubMed] [Google Scholar]
- 22.Smith A. J., Reed A. A., Loh N. Y., Thakker R. V., Lippiat J. D. (2009) Characterization of Dent's disease mutations of CLC-5 reveals a correlation between functional and cell biological consequences and protein structure. Am. J. Physiol. Renal Physiol. 296, F390–F397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zdebik A. A., Zifarelli G., Bergsdorf E. Y., Soliani P., Scheel O., Jentsch T. J., Pusch M. (2008) Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 283, 4219–4227 [DOI] [PubMed] [Google Scholar]
- 24.Richard E. A., Miller C. (1990) Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science 247, 1208–1210 [DOI] [PubMed] [Google Scholar]
- 25.Ludewig U., Jentsch T. J., Pusch M. (1997) Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions. J. Gen. Physiol. 110, 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguitragool W., Miller C. (2006) Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J. Mol. Biol. 362, 682–690 [DOI] [PubMed] [Google Scholar]
- 27.Hebeisen S., Heidtmann H., Cosmelli D., Gonzalez C., Poser B., Latorre R., Alvarez O., Fahlke C. (2003) Anion permeation in human ClC-4 channels. Biophys. J. 84, 2306–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmeyer K., Schwappach B., Bens M., Vandewalle A., Jentsch T. J. (1995) Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J. Biol. Chem. 270, 31172–31177 [DOI] [PubMed] [Google Scholar]
- 29.De Weer P., Gadsby D. C., Rakowski R. F. (1988) Voltage dependence of the Na-K pump. Annu. Rev. Physiol. 50, 225–241 [DOI] [PubMed] [Google Scholar]
- 30.Peres A., Giovannardi S., Bossi E., Fesce R. (2004) Electrophysiological insights into the mechanism of ion-coupled cotransporters. News Physiol. Sci. 19, 80–84 [DOI] [PubMed] [Google Scholar]
- 31.Schoppa N. E., McCormack K., Tanouye M. A., Sigworth F. J. (1992) The size of gating charge in wild-type and mutant Shaker potassium channels. Science 255, 1712–1715 [DOI] [PubMed] [Google Scholar]
- 32.Miller C. (1982) Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B Biol. Sci. 299, 401–411 [DOI] [PubMed] [Google Scholar]
- 33.De Santiago J. A., Nehrke K., Arreola J. (2005) Quantitative analysis of the voltage-dependent gating of mouse parotid ClC-2 chloride channel. J. Gen. Physiol. 126, 591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadsby D. C. (2004) Ion transport: spot the difference. Nature 427, 795–797 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.