Abstract
Thiazolidinediones (TZDs) are insulin sensitizers used to treat patients with insulin resistance. To assess potential gene-drug interactions, mice expressing human apolipoprotein E3 or E4 (APOE3 or APOE4) were fed a Western-type high-fat diet for 12 wk, at which time they developed similarly impaired glucose tolerance. Supplementing the diet with rosiglitazone (1.5 mg/g body weight) for an additional 4 wk improved plasma lipid profiles in both APOE3 and APOE4 mice. However, glucose tolerance improved only in APOE3 mice. Induction of adipogenesis and lipogenesis was severely blunted in adipose tissues, but not in the livers, of APOE4 mice. Consequently, lipids were channeled to the liver, causing marked steatosis in these mice. Impaired glucose tolerance was not a prerequisite for this adverse effect, and long-term treatment with rosiglitazone altered liver enzymes and caused hepatic fibrosis in APOE4 mice. Finally, TZDs failed to stimulate PPARγ activation and adipocyte differentiation in preadipocytes and embryonic fibroblasts isolated from APOE4 mice compared to those from APOE3 mice. We conclude that the effects of TZDs are APOE isoform dependent, and that the metabolic damage observed in APOE4 mice is rooted in an impaired activation of the adipogenic program in the adipose tissues expressing APOE4.—Arbones-Mainar, J. M., Johnson, L. A., Altenburg, M. K., Kim, H.-S., Maeda, N. Impaired adipogenic response to thiazolidinediones in mice expressing human apolipoproteinE4.
Keywords: NAFLD, apoE, fatty liver, adipogenesis, Western-type diet, insulin resistance
Apolipoprotein e (APOE) mediates lipoprotein clearance and plays significant roles in controlling metabolic homeostasis (1–4). In humans, the APOE gene is polymorphic and has 3 alleles; APOE*2, APOE*3, and APOE*4, with population frequencies of 7, 77, and 15%, respectively (5). The APOE*4 allele is associated with an increased risk for coronary disease (6) and Alzheimer disease (7). More recently, associations of this allele with metabolic syndrome have also been suggested (8, 9). Interpretation of human studies is difficult because of the low presence of subjects homozygous for APOE*4. In this paper we have examined this relationship by investigating mice in which their endogenous Apoe gene has been replaced by the human APOE alleles and produces solely human apoE proteins at physiological levels.
We previously reported that when stressed for 2 mo by a Western-type diet (WD), homozygous APOE*4 mice (APOE4) gained less weight but, paradoxically, showed impaired glucose tolerance compared with homozygous APOE*3 carriers (APOE3). These phenomena were due to the failure of high-fat fed APOE4 mice to store lipids in the adipose tissue adequately (10). In the present paper we have evaluated the efficacy of rosiglitazone (ROSI) in these mice, and ciglitazone in vitro. Both drugs are thiazolidinediones (TZDs), ligands of the peroxisome proliferator-activated receptor γ (PPARγ), an essential regulator of adipocyte differentiation and fat formation (11). We found that treatment at 1.5 mg/kg body weight (BW)/d improved glucose tolerance in APOE3 mice. In contrast, ROSI not only failed to improve glucose tolerance but caused enhanced ectopic lipid deposition in the livers of the APOE4 mice. This differential effect can be explained by the fact that adipose tissues from APOE4 mice were resistant to the TZD action.
MATERIALS AND METHODS
Mice
Male mice homozygous for the human APOE*3 (APOE3) or APOE*4 (APOE4) allele in place of the mouse Apoe and with C57BL/6 genetic background (2) were fed ad libitum either chow containing by weight 5.3% fat and 0.019% cholesterol (Prolab-Isopro RMH 3000; Agway Inc., Syracuse, NY, USA) or a high-fat WD containing 21.2% fat, 34.1% sucrose, and 0.2% cholesterol (TD88137; Teklad, Madison, WI, USA) over the period indicated in each experiment. Avandia, rosiglitazone maleate tablets (GlaxoSmithKline, Research Triangle Park, NC, USA) were ground to a powder, added to the chow, and adjusted to administer 1.5 mg/kg BW/d. The animals were handled under protocols approved by the Institutional Animal Care and Use Committees of the University of North Carolina (UNC)–Chapel Hill.
Biochemical determinations
Plasma concentrations of nonesterified free fatty acids (NEFAs), ketone bodies, glucose, and cholesterol were determined by commercial kits from Wako (Richmond, VA, USA) according to the manufacturer's instructions. Triglyceride and insulin concentrations were determined using commercial kits from Stanbio (San Antonio, TX, USA) and Crystal Chem (Chicago, IL, USA), respectively. Plasma alanine aminotransferase (ALT) was measured using an automated chemical analyzer, Vitro350 (Ortho-Clinical Diagnostics Inc., Raritan, NJ, USA) through the Animal Clinical Laboratory Core facility at UNC–Chapel Hill. Pooled plasma samples (100 μl) were fractionated by fast protein liquid chromatography using a Superose 6 HR10/30 column (GE Healthcare, Piscataway, NJ, USA). Fractions 11–17 corresponded to VLDL, 17–25 to LDL and VLDL remnants, and 25–31 to HDL. Adiponectin and plasma APOE were measured using ELISA with antibodies specific for human apoE (Calbiochem, La Jolla, CA, USA) and murine adiponectin (Sigma, St. Louis, MO, USA) as described previously (12). Animals were deprived of food for 4 h, and oral glucose tolerance was performed with 2 g/kg BW glucose load by oral gavage. Approximately 30 μl of blood was collected at times indicated and assayed for glucose. Mice were denied access to food during the course of these tolerance tests.
Plasma VLDL clearance and VLDL uptake by hepatocytes
VLDL was isolated from mice deficient in APOE (Apoe−/−) by ultracentrifugation and labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI C18; Molecular Probes, Eugene, OR, USA). APOE3 and APOE4 mice fed WD for 8 wk were given a single 10 mg/kg BW dose of ROSI or PBS by gavage. Two hours later, DiI-labeled VLDL (100 μg protein) was injected via tail veins of mice, and DiI-labeled VLDL remaining in the plasma at 2, 10, 20, and 60 min was determined using microscopic fluorometry (Olympus FV500 with a SPOT 2 digital camera; Olympus, Tokyo, Japan) (13).
Primary hepatocytes were isolated from mice homozygous for APOE3 or APOE4 as described previously (13). The cells were plated onto 48-well plates coated with rat collagen IA (Sigma) at a density of 1 × 104 viable cells/well and cultured for 24 h in 10% fetal bovine serum (FBS) -F12:Dulbecco's modified Eagle's medium (F12:DMEM). Equal amounts of total DiI-labeled triglyceride were added in 0.25 ml serum-free, phenol red-free F12:DMEM supplemented with 0, 1, or 10 μM ciglitazone. After incubation at 37°C for 30 min, cellular lipid was extracted with isopropanol and measured using a fluorescent microscopy technique as described previously (14).
Adipocyte differentiation in vitro
Perigonadal preadipocytes from adult mice and fibroblasts from embryos were isolated as described previously (15, 16) and maintained in 10% F12:DMEM. Confluent preadipocytes and embryonic fibroblasts were transformed into mature adipocytes with 10% FBSF12:DMEM plus 500 μM 3-isobutyl-1-methylxanthine (IBMX), 1 μM dexamethasone, and 1.67 μM insulin, with or without DMSO-dissolved ciglitazone (Cayman Chemicals, Ann Arbor, MI, USA). The medium was replaced 3 d later with 10% FBSF12:DMEM containing 10 μg/ml insulin and 0, 1, or 10 μM ciglitazone. Medium was changed every other day for an additional 6 d. DMSO (vehicle) was added to achieve the same concentration in all samples. Adipocytes were lysed with RIPA (50 mM Tris, 150 mM Nacl, 0.1% SDS, 0.5% Na.Deoxycholate, and 1% Nonidet P-40) buffer to determine triglyceride accumulation as described above or with Nucleic Acid Purification Solution (Applied Biosystems, Foster City, CA, USA) to obtain RNA suitable for gene expression analysis.
Liver assays and histology
Livers were homogenized, and triglyceride concentration in homogenates was determined as described above. Fatty acid synthase activity was spectrophotemetrically measured as described previously (17). Liver samples were fixed in 10% formalin, embedded in paraffin, sectioned (10 μm thickness), and stained with hematoxylin and eosin. Liver fibrosis was estimated by Masson's trichrome staining and blindly quantified by 3 observers.
Gene expression
mRNA was purified using an Automated Nucleic Acid Workstation ABI 6700, and real-time PCR was performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems). β-Actin mRNA was used for normalization. Sequences for primers and probes are shown in Supplemental Table 1.
Statistical analysis
Results are expressed as means ± se. Two-way analysis of variance with genotype and ROSI treatment as factors were used unless otherwise stated. All statistical analyses were performed using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Body weight gain during ROSI treatment
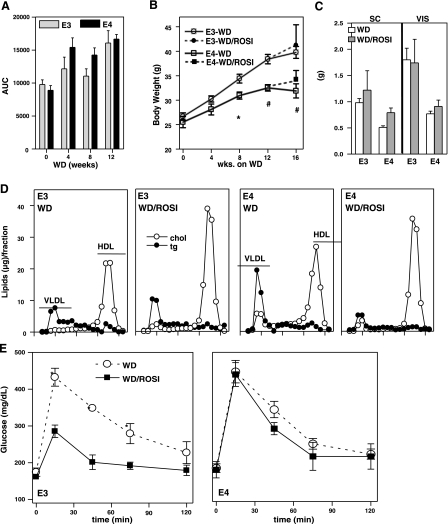
APOE4 mice develop glucose intolerance earlier than mice with APOE3, as we have reported previously (10). However, after consuming a WD for 12 wk, mice of both genotypes developed similar degrees of glucose intolerance, as judged by the oral glucose tolerance test (OGTT) (Fig. 1A). During the 12 wk, APOE3 mice gained an average of 14.0 g of body mass, whereas APOE4 mice gained an average of 7.5 g. This confirmed our previous observation of reduced diet-induced obesity in the APOE4 mice. We chose 12 wk of WD feeding as starting a point for the treatment with ROSI and divided mice of each genotype into 2 groups. The ROSI group was fed WD with ROSI at 1.5 mg/kg BW/d, and the control group was fed the same WD without the drug for another 4 wk. Although no additional weight gain was observed in the control groups, ROSI-APOE3 and ROSI-APOE4 mice gained 2.5 and 1.5 g, respectively (Fig. 1B). Despite the trend of increased body weights of ROSI-treated mice compared to the control mice, a 2-way ANOVA showed no ROSI effect on the mass of either subcutaneous (SC, inguinal) or visceral (epididymal) fat pads. However, fat tissues in APOE4 mice were significantly smaller than those in APOE3 mice after 16 wk of WD feeding (genotype effect, P=0.002 for visceral and P=0.02 for SC) (Fig. 1C). Thus, white adipose tissues of the ROSI-APOE4 mice appear to have a limited storage capacity compared to ROSI-APOE3 mice.
Figure 1.
Effects of WD and ROSI on mice expressing human APOE3 (○) or APOE4 (●). A) Area under the curve (AUC), defined as glucose plasma concentration plotted against time, was calculated using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA USA). B, C) Body mass (B) and adipose SC and VIS fat mass (C) after 12 wk of WD followed an extra 4 wk of WD without (WD) or with 1.5 mg/kg BW/d of ROSI (WD/ROSI). D) Distribution of cholesterol (○) and triglycerides (●) (μg lipids/fraction) in FPLC-fractionated plasma at the end of the experimental period. E) OGTT in APOE3 (○) or APOE4 mice (■) at the end of the experimental period. Data are means ± se, n = 5–6. Group comparisons were by t test for the difference between genotypes in each condition. *P ≤ 0.05, #P ≤ 0.01.
Plasma lipids and glucose tolerance
ROSI treatment reduced plasma triglycerides in the APOE4 mice without changing plasma cholesterol (Table 1). The plasma lipoprotein profile in ROSI-APOE4 mice showed a reduction in VLDL particles, accounting for the decreased triglycerides. However, plasma cholesterol remained unchanged because of the increased HDL cholesterol (Fig. 1D). Similarly, plasma cholesterol levels of ROSI-APOE3 mice were also significantly increased due to an increase of HDL cholesterol (Fig. 1D). ROSI treatment also raised plasma APOE, and this increase was more marked in APOE4 mice than in APOE3 mice. No difference in the distribution of APOE among lipoprotein particles was seen between the genotypes (Supplemental Fig. 1). These data demonstrate that ROSI treatment improved the plasma lipoprotein profiles in both APOE3 and APOE4 mice.
Table 1.
Effects of WD supplemented with ROSI on plasma biochemistry
| Parameter | E3 |
E4 |
2-way ANOVA |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 mg/kg | 0 | 1.5 mg/kg | GT | ROSI | GT * ROSI | |
| Triglycerides (mg/dl) | 41 ± 3 | 51 ± 4 | 56 ± 8 | 27 ± 4 | NS | 0.04 | <0.01 |
| Cholesterol (mg/dl) | 73 ± 7 | 114 ± 27 | 104 ± 9 | 102 ± 8 | NS | NS | NS |
| ApoE (AU) | 2.28 ± 0.17 | 2.41 ± 0.24 | 3.14 ± 0.23 | 4.14 ± 0.21 | <0.01 | 0.01 | 0.05 |
| NEFA (meq/L) | 0.80 ± 0.05 | 0.95 ± 0.13 | 0.76 ± 0.77 | 0.77 ± 0.19 | NS | NS | NS |
| Insulin (nM) | 1.70 ± 0.03 | 2.60 ± 1.50 | 1.22 ± 0.16 | 1.20 ± 0.12 | NS | NS | NS |
| Glucose (mg/dl) | 137 ± 9 | 143 ± 13 | 120 ± 13 | 183 ± 11 | NS | 0.01 | 0.03 |
| Ketone bodies (μm) | 480 ± 35 | 600 ± 87 | 575 ± 50 | 827 ± 41 | 0.01 | 0.01 | NS |
| Adiponectin (μg/ml) | 24.7 ± 1.0 | 33.9 ± 2.7 | 29.4 ± 2.3 | 34.5 ± 1.3 | NS | NS | NS |
Values are means ± se of 5–6 animals/group. Statistical analyses were done by 2-way ANOVA with genotype (GT) and ROSI treatment as factors. AU, arbitrary unit; NS, not significant.
ROSI did not alter the NEFA and insulin levels in the plasma of either APOE3 or APOE4 mice. ROSI increased plasma glucose only in APOE4 mice. Ketone bodies increased in both genotypes, but values were significantly higher in APOE4 mice than in APOE3 mice, suggesting impaired hypoglycemic and hypoketotic effects of ROSI in APOE4 mice. No genotype or treatment differences were observed in total plasma adiponectin, an adipocyte-specific protein that enhances insulin sensitivity (18, 19) (Table 1).
OGTTs revealed that the APOE3 and APOE4 mice fed WD for 16 wk exhibited similar glucose clearance, indicating that they developed equal degrees of impaired glucose tolerance as a consequence of a long-term WD feeding (Fig. 1E). After ROSI treatment during the last 4 wk of WD, ROSI-APOE3 mice displayed reduced plasma glucose at 15, 45, and 75 min postchallenge compared to the untreated APOE3 mice. In contrast, no improvement of glucose tolerance was observed in ROSI-APOE4 mice.
Taken together, ROSI has beneficial effects on plasma lipoprotein profiles in both WD-fed APOE3 and APOE4 mice, but beneficial effects on glucose metabolism were present only in APOE3 mice.
Effects of ROSI on adipogenic gene expression in adipose tissues
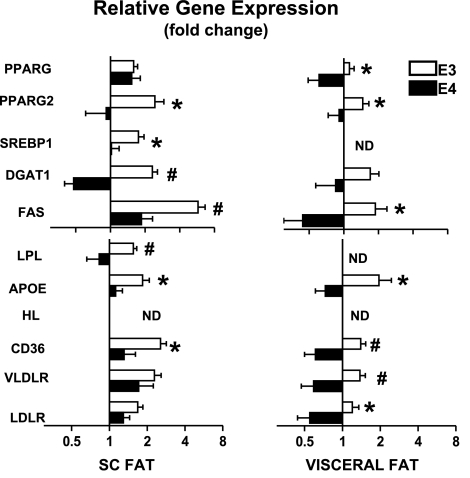
PPARγ ligands promote lipogenesis in adipose tissue during lipid overload by enhancing storage (20). We therefore examined how gene expression levels in SC and visceral fat pads of APOE3 and APOE4 mice responded to ROSI treatment. Figure 2 shows the ROSI effects for each APOE-genotype as fold differences in the mRNA levels between ROSI-treated and nontreated mice. Both received the same WD for 16 wk. ROSI treatment increased the expression of Srebp1, Pparg (total), and Pparg2 as well as their downstream target genes (Dgat1 and Fas) in both subcutaneous and visceral adipose tissues of APOE3 mice. These ROSI effects were blunted in the subcutaneous adipose tissues of APOE4 mice and even reduced in visceral depots (Fig. 2, top panels). Similarly, mRNA levels for the lipid-transport related Lpl, Apoe, Cd36, and Vldlr genes increased in both subcutaneous and visceral fat of ROSI-APOE3 mice, but were significantly blunted in the subcutaneous fat of ROSI-treated APOE4 mice and completely failed to activate the expression of those genes in APOE4 visceral fat (Fig. 2, bottom panels). These data indicate that ROSI-stimulated expression of the lipogenic program is intact in APOE3 adipocytes but is impaired in APOE4 adipocytes.
Figure 2.
Fold difference in mRNA levels of selected genes in white adipose tissues. Subcutaneous inguinal fat (left panels) and epididymal visceral fat (right panels) of APOE3 (open bars) and APOE4 mice (solid bars) after 12 wk of WD followed an extra 4 wk of WD with or without 1.5 mg/kg BW/d of ROSI (WD/ROSI). Values represent amount of mRNA in ROSI-treated tisues relative to that in nontreated tissues, which is arbitrarily defined as 1. Data are means ± se, n = 5–6. Values were log-transformed; genotype comparisons were by t test. *P ≤ 0.05, #P ≤ 0.01.
ROSI induces severe fatty liver in WD-fed APOE4 mice
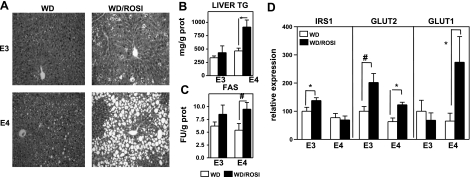
After 4 mo of WD, some fat deposition was observed in livers of both genotypes (Fig. 3A). However, hepatosteatosis became evident on ROSI treatment (Fig. 3A). ROSI caused a 110% increase in liver triglyceride concentration in the APOE4 mice compared to a 38% increase in APOE3 mice, over their respective nontreated counterparts (Fig. 3B). Increased activity of fatty acid synthase (FAS) paralleled this fat deposition in the ROSI treated livers (Fig. 3C).
Figure 3.
Steatotic livers after 12 wk of WD followed an extra 4 wk of WD without (WD) or with 1.5 mg/kg BW/d of ROSI (WD/ROSI). A) Liver stained with hematoxylin and eosin. B) Liver triglyceride content. C) Fatty acid synthase enzymatic activity. D) Effects of ROSI treatment on expression of IRS1, GLUT2, and GLUT1 in the liver from WD-fed APOE3 and APOE4 mice. Data are means ± se, n = 5–6, t test for effects of ROSI in each genotype. *P ≤ 0.05.
To investigate hepatic glucose handling, we quantified mRNAs encoding glucose transport-related genes in the liver. ROSI increased Irs1 expression levels only in APOE3 livers (Fig. 3D). Irs2 mRNA levels were not altered in either APOE3 or APOE4 livers (data not shown). ROSI doubled the expression of Glut2 in both APOE3 and APOE4 livers. However, expression of this PPARγ-dependent glucose transporter (21) was lower in the APOE4 livers and remained significantly lower in APOE4 livers even after ROSI treatment (P=0.02 for genotype effect). In contrast, Glut1 was up-regulated by ROSI only in APOE4 livers.
ROSI-induced fatty liver is associated with up-regulated lipid uptake genes
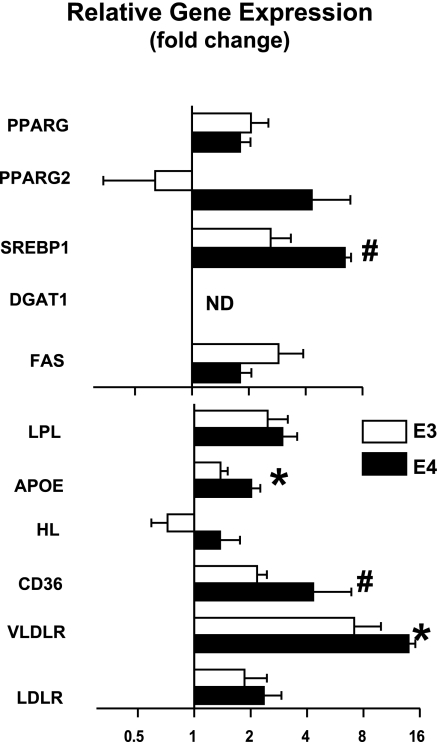
We next investigated the effects of ROSI on genes for lipogenesis in the liver. The levels of the total Pparg mRNA doubled after ROSI treatment in both APOE3 and APOE4 compared with their respective controls (Fig. 4). Pparg2 mRNA levels were low in the livers but were induced by ROSI treatment only in the APOE4 mice. Drug-treated APOE4 mice had a 6.4× increase in Srebp1 expression compared to 2.6× increase in ROSI-APOE3 mice with a significant ROSI-genotype interaction (P=0.01). Likewise, ROSI increased liver expression of genes related to lipid uptake such as hepatic lipase, Apoe, Cd36, and Vldlr, but the magnitude of the increases was larger in ROSI-APOE4 livers than in ROSI-APOE3 livers. No changes were observed in the mRNA levels of Srebp2, apolipoprotein AI or C3, and ATP-binding cassette transporter A1 (Abca1) on treatment (data not shown). Overall, the data show that ROSI altered gene expression patterns in the liver function to take up and store more lipids than nontreated mice.
Figure 4.
Changes in mRNA levels of selected genes in the liver. APOE3 (open bars) and APOE4 mice (solid bars) after 12 wk of WD followed by an additional 4 wk of WD with or without 1.5 mg/kg BW/d of ROSI (WD/ROSI). Values represent the amount of mRNA in ROSI-treated livers relative to that in nontreated livers, which is arbitrarily defined as 1. Data are means ± se, n = 5–6. Values were log-transformed; genotype comparisons were by t test. *P ≤ 0.05, #P ≤ 0.01.
To determine whether hepatic mRNA changes and ROSI-induced fatty liver are WD-dependent phenomena, APOE3 and APOE4 mice were fed a standard low-fat chow for 4 wk with or without ROSI (1.5 mg/kg BW/d). Regardless of APOE genotype, ROSI decreased plasma cholesterol, increased adiposity in subcutaneous and visceral adipose depots, and augmented hepatic lipid content (Supplemental Table 2). No lipid deposition was apparent in the hepatic micrographies (Supplemental Fig. 2). Nevertheless, changes in hepatic gene expression were apparent on ROSI treatment. Similar to the WD-fed mice, ROSI up-regulated lipid metabolism-related genes for uptake (Vldlr and Cd36), oxidation (Ppara), and storage (Pparg, Pparg2, and Fas) in both genotypes (Table 2). Thus ROSI induces adipogenic changes in the liver of mice expressing human APOE even when fed a low-fat chow. Interestingly, the levels of Pparg2 and Cd36 mRNA in APOE4 livers were higher than in APOE3 livers, even in the absence of ROSI. Significant interactions between APOE4 genotype and ROSI were observed in the expression of Pparg2, Srebp1, Vldlr, and Cd36 mRNA, with APOE4 mice demonstrating a larger up-regulation of these genes by ROSI compared to mice with APOE3. Therefore, the overall hepatic gene expression profile indicates that APOE4 mice are predisposed to accumulate fat in the liver.
Table 2.
Effects of low-fat diet supplemented with ROSI (1.5 mg/kg) on hepatic gene expression
| Gene | E3 |
E4 |
2-way ANOVA |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 mg/kg | 0 | 1.5 mg/kg | GT | ROSI | GT * ROSI | |
| PPARG1 | 100 ± 19 | 149 ± 36 | 115 ± 12 | 206 ± 33 | NS | 0.02 | NS |
| PPARG2 | 100 ± 32 | 389 ± 142 | 949 ± 261 | 1223 ± 226 | <0.001 | 0.02 | 0.06 |
| SREBP1 | 100 ± 30 | 81 ± 18 | 104 ± 20 | 297 ± 126 | 0.02 | NS | 0.06 |
| PPARA | 100 ± 23 | 239 ± 33 | 89 ± 4 | 172 ± 38 | NS | <0.001 | NS |
| APOE | 100 ± 8 | 102 ± 11 | 102 ± 10 | 118 ± 26 | NS | NS | NS |
| ADIPOR1 | 100 ± 16 | 99 ± 12 | 109 ± 11 | 141 ± 16 | NS | NS | NS |
| ADIPOR2 | 100 ± 17 | 102 ± 9 | 98 ± 10 | 154 ± 33 | NS | NS | NS |
| LDLR | 100 ± 20 | 109 ± 11 | 93 ± 14 | 145 ± 78 | NS | NS | NS |
| VLDLR | 100 ± 12 | 762 ± 118 | 150 ± 22 | 2956 ± 664 | <0.001 | <0.001 | 0.02 |
| CD36 | 100 ± 32 | 330 ± 55 | 123 ± 15 | 823 ± 158 | <0.01 | <0.001 | NS |
| FAS | 100 ± 18 | 295 ± 59 | 248 ± 115 | 187 ± 48 | NS | 0.07 | NS |
| DGAT1 | 100 ± 23 | 164 ± 12 | 127 ± 21 | 135 ± 29 | NS | NS | NS |
Values are means ± se of ≥5–6 animals/group. Statistics were done using 2-way ANOVA with genotype (GT) and ROSI treatment as 2 factors. mRNA expressed relative to the mean expression in untreated APOE3 mice as 100. NS, not significant.
ROSI fails to prevent the development of diet-induced glucose intolerance and fatty liver in WD-fed APOE4 mice
APOE4 mice develop impairment of glucose tolerance earlier than APOE3 mice (10). To examine whether the longer exposure to metabolic impairments caused the blunted response to ROSI and liver damage in APOE4 mice, we treated mice with ROSI in WD without the 12 wk WD preconditioning. After 8 wk of WD with or without 1.5 mg/kg BW/d ROSI, body weight as well as the mass of visceral fat, subcutaneous fat, and liver increased after the WD (not shown). OGTTs revealed that after 8 wk, ROSI-APOE3 mice displayed faster postchallenge glucose clearance compared to the untreated APOE3 mice. In contrast, the glucose clearance of ROSI-APOE4 mice was equally impaired as in untreated APOE4 mice (Supplemental Fig. 3A).
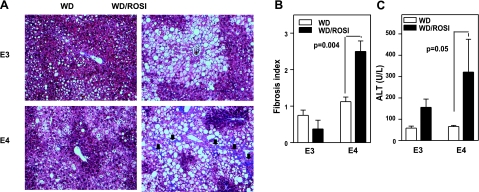
Hepatic lipid deposition positively correlated with the duration of the WD feeding. Again, the amount of liver triglycerides was significantly increased in APOE4 mice on ROSI treatment, while the increase was not significant in APOE3 mice (Supplemental Fig. 3B). The interaction between genotype and drug was evident as early as 4 wk of ROSI treatment (P=0.01). A long-term ROSI treatment with WD for 6 mo produced severe liver steatosis in both APOE3 and APOE4 mice. However, fibrosis was detected only in ROSI-treated APOE4 mice (Fig. 5A, B). A substantial increase in plasma ALT indicates liver damage (Fig. 5C). Thus, the ROSI-induced steatosis in APOE4 mice is not an innocuous lipid deposition but instead leads to a more harmful steatohepatitis.
Figure 5.
Hepatic damage. Mice were fed 6 mo of WD without (WD) or with 1.5 mg/kg BW/d of ROSI (WD/ROSI). A) Histological section of the liver stained with Masson's Trichrome. Scar tissue stained blue (black arrows). B) Average fibrotic index, graded 1–4. C) Circulating alanine transaminase activity. Data are means ± se, n = 5–6/group.
Blunted PPARγ activation of APOE4 adipocytes is the primary cause of liver steatosis
The noxious effects of ROSI treatment observed in APOE4 mice could be due to an isoform-specific increase of TG-rich lipoprotein uptake in the liver or could be secondary to reduced ROSI responsiveness in adipocytes. To investigate these issues, we determined whether PPARγ stimulation differently affects hepatocytes and/or adipocytes expressing 2 different human APOE isoforms.
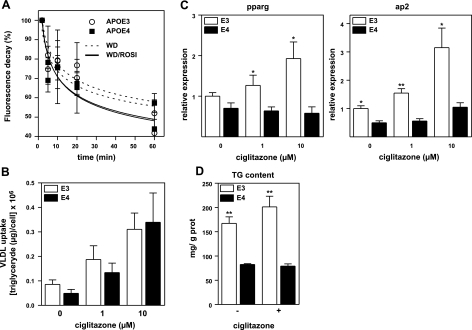
We first examined how a single 10 mg/kg BW dose of ROSI modifies plasma VLDL clearance in WD-fed APOE3 and APOE4 mice. Decay of DiI-labeled Apoe−/− VLDL particles from plasma showed that the ROSI-treated mice had the fastest clearance of VLDL (P=0.05 for treatment effect at 60 min time point). However, differences in clearance between genotypes were not detectable at any time points (Fig. 6A). We next measured how a PPARγ agonist, ciglitazone, affected the uptake of DiI-Apoe−/− VLDL in primary cultures of APOE3 and APOE4 hepatocytes. Thirty minutes after VLDL was added to the medium, uptake was significantly increased in cells treated with ciglitazone in a dose-dependent manner (Fig. 6B). Again, the VLDL uptake did not differ between cells expressing APOE3 or APOE4. These data demonstrate that the ROSI-induced fat accumulation in the APOE4 liver is not due to an increase in hepatic uptake of TG-rich lipoproteins in comparison to APOE3 mice.
Figure 6.
A) Plasma VLDL clearance. WD-fed APOE3 and APOE4 mice were given a single dosage of 10 mg/kg BW ROSI or PBS by gavage, and DiI-labeled Apoe−/− VLDL was injected 2 h later. Fluorescent signals in plasma were measured at 2, 10, 20, and 60 min; 2 min was taken as 100% (n=4). B) Uptake of DiI-labeled VLDL by hepatocytes from APOE3 (open bars) and APOE4 mice (solid bars). Cells were treated with 0, 1, or 10 μM ciglitazone for 24 h, washed, and incubated with DiI-labeled VLDL for 30 min at 37°C. Cells were lysed, and fluorescence was assayed as described in Methods and Materials (n=8/condition). C) Differentiation of preadipocytes isolated from APOE3 (open bars) and APOE4 (solid bars) mice in culture. Cells were differentiated for 3 d adipocytes with IBMX, dexamethasone, and insulin. Ciglitazone was added to the mixture in the indicated concentrations and cultured for an additional 6 d. Cells were lysed, and mRNA levels for PPARγ and ap2 were assayed and expressed relative to the mean expression in APOE3 cells as 100 (n=6 each). D) Triglyceride content in adipocytes differentiated from embryonic fibroblasts of APOE3 (open bars) and APOE4 embryos (solid bars). Cells were differentiated for 3 d into mature adipocytes with IBMX, dexamethasone, and insulin, followed by culturing in an insulin medium for an additional 6 d. Ciglitazone was added to the medium at 0 (−) and 10μM (+) throughout the 9 d. Lipids were extracted by isopropanol, and triglyceride content was assayed using enzymatic method (n=6/condition). Genotype comparisons were by t test. *P ≤ 0.05, **P ≤ 0.01.
In marked contrast, conversion of APOE4 preadipocytes into adipocytes was significantly reduced. When visceral fat preadipocytes isolated from APOE3 and APOE4 mice were induced to differentiate in a cocktail containing a PPARγ agonist (Zen-Bio proprietary medium), the time-dependent increase in the mRNA levels of adipocyte markers PPARγ and ap2 were significantly lower in APOE4 cells than in APOE3 cells (Supplemental Fig. 4). In a separate experiment, preadipocytes were differentiated in a medium without PPARγ agonists for 3 d and then allowed to become mature adipocytes for an additional 6 d in the presence of varying doses of ciglitazone. mRNA levels of adipocyte markers PPARγ2 and ap2 increased in a dose-dependent manner in the APOE3 adipocytes, but failed to increase their expression even at the highest doses in the APOE4 adipocytes (Fig. 6C). To examine how the impaired differentiation translated into adipocyte functionality, embryonic fibroblasts isolated from APOE3 and APOE4 embryos (MEF) were differentiated into mature adipocytes in a media with or without 10 μM of ciglitazone for 9 d. Ciglitazone did not increase triglyceride content in APOE4 MEF-derived adipocytes, whereas there was a 20% increase in APOE3 MEF-derived adipocytes (Fig. 6D). Interestingly, triglyceride content was higher in APOE3 MEF-derived adipocytes even in the absence of ciglitazone.
Taken together, these experiments demonstrate that ROSI increases VLDL uptake in hepatocytes independent of APOE genotype. In contrast, the effects of ROSI on adipocyte differentiation and maturation are APOE genotype dependent. Thus, impaired ROSI-stimulated adipogenesis of APOE4 adipocytes likely translates into a lesser ability to store lipids in adipocytes and explains the ectopic fat deposition in the mice with this genotype.
DISCUSSION
Our results show that ROSI improved the plasma lipid profile of both APOE3 and APOE4 mice. However, ROSI treatment improved glycemic control in APOE3 mice, but failed to improve the impaired glucose tolerance provoked by WD in mice expressing human APOE4. Gene expression analysis of APOE4 mice showed that ROSI induced adipogenic programs in the liver, but its effects on adipose tissues were severely blunted. The inability of adipose tissues to accommodate surplus lipids transformed the liver into a secondary lipid store and caused severe fatty liver disease in APOE4 mice.
Increased adipogenesis by ROSI has been shown to correlate with insulin sensitivity in humans (22). PPARγ, the target of ROSI, has 2 isoforms: the ubiquitous PPARγ1 and adipocyte-specific PPARγ2. Both regulate lipogenic enzymes such as acyl-coenzyme A:diacylglycerol transferase, and regulators of lipid transport into the cell (LPL, APOE, CD36, and VLDLR) (23). In addition to PPARγ, the overall lipogenic program is also controlled by the transcription factor SREBP1, which activates transcription of LDL receptor, FAS, and other enzymes required for synthesis of fatty acids and triglycerides (24). Activation of adipogenic programs by 1.5 mg/kg/d ROSI in adipose tissues and the improvement of glucose tolerance in WD-fed APOE3 mice are consistent with these observations in humans. In contrast, ROSI had either no effect or even reduced the expression of Pparg2 as well as Dgat1 and Fas in the white adipose tissues of APOE4 mice. We note the possibility that a higher dose of ROSI might result in more favorable outcomes in APOE4 mice. However, the adipocytes of APOE4 mice fed a WD diet are clearly resistant to the ROSI action at the administered dose of 1.5 mg/kg BW, and cultured APOE4 adipocytes treated in vitro with ciglitazone at 10 μM failed to increase lipid accumulation as well as Pparg and ap2 expression in a broad range of doses tested. A lack of improvement in glucose tolerance by ROSI in APOE4 mice, despite beneficial effects on plasma lipoprotein profiles, agrees with previous observations that functional adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of TZD (25).
Increased hepatic PPARγ expression, specifically PPARγ2, and augmented SREBP-1 expression, has been associated with hepatic steatosis (26–29). PPARγ stimulation by ROSI increased hepatic total transcripts for Pparg and Pparg2, as well as for Vldlr and Cd36, PPARγ downstream target genes, in both APOE3 and APOE4 mice. This lipogenic profile was reflected in a similarly increased plasma-VLDL clearance in both genotypes, even with a single dose of ROSI. We also found that the induction of the genes for lipid uptake and storage in the liver by TZD is independent of dietary fat intake and APOE genotype, because the effects of ROSI on the pattern of gene expression also occurred in the livers of mice fed normal chow, and ciglitazone equally augmented VLDL uptake in vitro by hepatocytes of both genotypes. However, Pparg2, Srebp1, and Vldlr transcripts show greater increases in APOE4 mice than in APOE3 mice even on normal chow, indicating there may be an APOE4-dependent predisposition to accumulate fat in the liver during ROSI treatment. We note, however, that activation of the lipogenic program in the liver by TZDs has no apparent adverse effects as long as the adipose tissues maintain their normal functionality, as mice fed a low-fat chow diet. Impaired adipose tissues, combined with long-term WD feeding, on the other hand, contribute to the accumulation of lipids in the liver of ROSI-treated APOE4 mice. Ultimately, fatty changes induced liver damage as evidenced by a significant elevation of plasma ALT and fibrosis observed after long-term treatment.
In this study, we traced the primary cause of the metabolic problems of ROSI treatment in APOE4 mice to the inability of their adipose tissues to respond. Our results strongly point to the existence of complex interactions between APOE and PPARγ during the adipocyte differentiation process. Recent studies support our conclusion, showing that the apoE gene is a target of the major metabolic regulations of nuclear receptors such as liver X receptor and PPARγ in adipocytes (30). Moreover, PPARγ agonists increased APOE mRNA in human adipose tissue and mouse 3T3-L1 cells via a downstream enhancer (31), and a ROSI-induced increase in triglyceride mass was suppressed in adipocytes lacking endogenous APOE expression (16). In this regard, we must consider why the presence of APOE4 mirrors results observed when APOE is absent. APOE plays an important role in cholesterol metabolism and flux, and we previously showed that APOE4 mice accumulate cholesterol-poor VLDL remnants similar to APOE-null mice when they were overexpressing LDLR and were on WD (32). Our evidence suggests that this is because APOE4 is trapped on the surface of hepatocyte to a greater extent than APOE3 and is prevented from participating in the uptake of triglyceride-rich VLDL particles (13). A similar interaction between APOE4 and cell surface receptors could alter trafficking of lipids to and from adipocytes. Adipocyte differentiation and maturation is orchestrated by the activation of transcription factors including PPARγ, C/EBPs, SREBP1, and their cofactors that together costimulate the process, and any alteration in cellular composition of sterols and fatty acids due to APOE4 could influence the effectiveness of PPARγ activation by TZD. Further studies are required to elucidate the precise mechanism underlying this interference.
The interaction between APOE genotypes and PPARG genotype in the context of fatty liver and diabetes has not been investigated in humans. However, further evidence supporting the existence of interactions between apoE isoforms and PPARγ activation in lipid metabolism and atherosclerosis stem from the observations of Cardona et al. (33), who found that among patients with metabolic syndrome 65% of those having the Ala12 allele of PPARG showed postprandial hypertriglyceridemia compared to only 20% of those having the Pro12 allele. This postprandial hypertriglyceridemia was observed at a higher frequency (85%) in patients with Ala12 who are not Apoe*3/3 (33). Independently, Peng et al. (34) have shown an interaction between APOE4 and the PPARG-C161T variant in the risk for coronary heart disease. Interestingly, recent reports suggest that the Ala12 PPARG allele is associated with early manifestations of late-onset Alzheimer disease as is Apoe*4 (34). Impaired glucose metabolism in the brain and reduced levels of IRS1 are hallmarks of Alzheimer disease (35). Apoe*4 is strongly associated with an increased risk of early onset of this disease (7) and is associated with reduced neuronal glucose utilization even in young subjects with no signs of dementia (36). Although the mechanisms underlying these association are not yet understood, Risner et al. (37) found that when patients with mild-to-moderate Alzheimer disease were treated with ROSI, cognitive improvements were observed only in patients who do not carry the Apoe*4 allele. Thus, differences in the crosstalk between PPARγ and the different APOE isoforms are not likely limited to lipid metabolism and adipocyte differentiation, but instead are important universal phenomena that contribute to varying susceptibility to various disease states, particularly when combined with insulin resistance or diabetes.
In conclusion, our data demonstrate that TZDs fail to improve lipid storage in cultured adipocytes as well as in adipose tissues of WD-fed APOE4 mice and consequently exacerbates hepatic steatosis. Because our experimental mice were fed a high-fat diet during the ROSI treatment, our results may not directly translate to humans with APOE*4, who are likely on strict dietary restrictions during ROSI treatment. Nevertheless, APOE4 isoform-dependent adverse effects with ROSI treatment warrant further investigations for potential gene-drug interactions.
Supplementary Material
Acknowledgments
This study was supported by U.S. National Institute of Health grants HL42630 and HL87946. J.M.A.-M. was partially supported by a postdoctoral fellowship from the Ministerio de Educacion y Ciencia (Spain). The authors thank Marcus McNair, Anna Garcia, and Jennifer Wilder for their assistance and Prof. Jesus Osada for his critical reading of this work.
REFERENCES
- 1.Mahley R. W., Innerarity T. L., Weisgraber K. H., Oh S. Y. (1979) Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J. Clin. Invest. 64, 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knouff C., Hinsdale M. E., Mezdour H., Altenburg M. K., Watanabe M., Quarfordt S. H., Sullivan P. M., Maeda N. (1999) Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Invest. 103, 1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z. H., Gu D., Mazzone T. (2009) Role of adipocyte-derived apoE in modulating adipocyte size, lipid metabolism, and gene expression in vivo. Am. J. Physiol. Endocrinol. Metab. 296, E1110–E1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendse A. A., Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Maeda N. (2009) Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J. Lipid Res. 50, S178–S182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davignon J., Gregg R., Sing C. (1988) Apolipoprotein E polymorphism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 8, 1–21 [DOI] [PubMed] [Google Scholar]
- 6.Bennet A. M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A., Keavney B., Collins R., Wiman B., de Faire U., Danesh J. (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311 [DOI] [PubMed] [Google Scholar]
- 7.Corder E., Saunders A., Strittmatter W., Schmechel D., Gaskell P., Small G., Roses A., Haines J., Pericak-Vance M. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 8.Elosua R., Demissie S., Cupples L. A., Meigs J. B., Wilson P. W. F., Schaefer E. J., Corella D., Ordovas J. M. (2003) Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obes. Res. 11, 1502–1508 [DOI] [PubMed] [Google Scholar]
- 9.Sima A., Iordan A., Stancu C. (2007) Apolipoprotein E polymorphism: a risk factor for metabolic syndrome. Clin. Chem. Lab. Med. 45, 1149–1153 [DOI] [PubMed] [Google Scholar]
- 10.Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Maeda N. (2008) Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int. J. Obes. 32, 1595–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yki-Jarvinen H. (2004) Thiazolidinediones. N. Engl. J. Med. 351, 1106–1118 [DOI] [PubMed] [Google Scholar]
- 12.Arbones-Mainar J. M., Navarro M. A., Acin S., Guzman M. A., Arnal C., Surra J. C., Carnicer R., Roche H. M., Osada J. (2006) Trans-10, cis-12-and cis-9, trans-11-conjugated linoleic acid isomers selectively modify HDL-apolipoprotein composition in apolipoprotein E knockout mice. J. Nutr. 136, 353–359 [DOI] [PubMed] [Google Scholar]
- 13.Altenburg M., Arbones-Mainar J., Johnson L., Wilder J., Maeda N. (2008) Human LDL receptor enhances sequestration of ApoE4 and VLDL remnants on the surface of hepatocytes but not their internalization in mice. Arterioscler. Thromb. Vasc. Biol. 28, 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson L. A., Altenburg M. K., Walzem R. L., Scanga L. T., Maeda N. (2008) Absence of hyperlipidemia in LDL receptor-deficient mice having apolipoprotein B100 without the putative receptor-binding sequences. Arterioscler. Thromb. Vasc. Biol. 28, 1745–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conner D. (2001) Mouse embryo fibroblast (MEF) feeder cell preparation. Curr. Protoc. Mol. Biol. 23, 23.2.1–23.2.7 [DOI] [PubMed] [Google Scholar]
- 16.Huang Z. H., Reardon C. A., Mazzone T. (2006) Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 55, 3394–3402 [DOI] [PubMed] [Google Scholar]
- 17.Bazin R., Ferre P. (2001) Assays of lipogenic enzymes. In Methods in Molecular Biology–Adipose Tissue Protocols, Vol. 155 (Ailhaud G. ed), pp. 121–127, Humana Press, Towota, NJ, USA: [DOI] [PubMed] [Google Scholar]
- 18.Xu A., Wang Y., Keshaw H., Xu L. Y., Lam K. S., Cooper G. J. (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 112, 91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagano C., Soardo G., Esposito W., Fallo F., Basan L., Donnini D., Federspil G., Sechi L. A., Vettor R. (2005) Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur. J. Endocrinol. 152, 113–118 [DOI] [PubMed] [Google Scholar]
- 20.Ye J.-M., Dzamko N., Cleasby M. E., Hegarty B. D., Furler S. M., Cooney G. J., Kraegen E. W. (2004) Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 47, 1306–1313 [DOI] [PubMed] [Google Scholar]
- 21.Im S., Kang S., Kim S., Kim H., Kim J., Kim K., Ahn Y. (2005) Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes 54, 1684–1689 [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan G., Unal R., Pokrovskaya I., Yao-Borengasser A., Phanavanh B., Lecka-Czernik B., Rasouli N., Kern P. A. (2006) The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 47, 2444–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogacka I., Xie H., Bray G. A., Smith S. R. (2004) The effect of pioglitazone on peroxisome proliferator-activated receptor-γ target genes related to lipid storage in vivo. Diabetes Care 27, 1660–1667 [DOI] [PubMed] [Google Scholar]
- 24.Horton J. D., Goldstein J. L., Brown M. S. (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao L., Marcus-Samuels B., Mason M. M., Moitra J., Vinson C., Arioglu E., Gavrilova O., Reitman M. L. (2000) Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 106, 1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimomura I., Bashmakov Y., Horton J. D. (1999) Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 274, 30028–30032 [DOI] [PubMed] [Google Scholar]
- 27.Gavrilova O., Haluzik M., Matsusue K., Cutson J. J., Johnson L., Dietz K. R., Nicol C. J., Vinson C., Gonzalez F. J., Reitman M. L. (2003) Liver peroxisome proliferator-activated receptor {gamma} contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 278, 34268–34276 [DOI] [PubMed] [Google Scholar]
- 28.Westerbacka J., Kolak M., Kiviluoto T., Arkkila P., Siren J., Hamsten A., Fisher R. M., Yki-Jarvinen H. (2007) Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes 56, 2759–2765 [DOI] [PubMed] [Google Scholar]
- 29.García-Ruiz I., Rodríguez-Juan C., Díaz-Sanjuán T., Ángel Martínez M., Muñoz-Yagüe T., Solís-Herruzo J. (2007) Effects of rosiglitazone on the liver histology and mitochondrial function in ob/ob mice. Hepatology 46, 414–423 [DOI] [PubMed] [Google Scholar]
- 30.Yue L., Mazzone T. (2009) Peroxisome proliferator-activated receptor γ stimulation of adipocyte ApoE gene transcription mediated by the liver receptor X pathway. J. Biol. Chem. 284, 10453–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue L., Rasouli N., Ranganathan G., Kern P. A., Mazzone T. (2004) Divergent effects of peroxisome proliferator-activated receptor {gamma} agonists and tumor necrosis factor α on adipocyte ApoE expression. J. Biol. Chem. 279, 47626–47632 [DOI] [PubMed] [Google Scholar]
- 32.Malloy S. I., Altenburg M. K., Knouff C., Lanningham-Foster L., Parks J. S., Maeda N. (2004) Harmful effects of increased LDLR expression in mice with human APOE*4 but not APOE*3. Arterioscler. Thromb. Vasc. Biol. 24, 91–97 [DOI] [PubMed] [Google Scholar]
- 33.Cardona F., Morcillo S., Gonzalo-Marin M., Garrido-Sanchez L., Macias-Gonzalez M., Tinahones F. J. (2006) Pro12Ala sequence variant of the PPARG gene is associated with postprandial hypertriglyceridemia in non-E3/E3 patients with the metabolic syndrome. Clin. Chem. 52, 1920–1925 [DOI] [PubMed] [Google Scholar]
- 34.Peng D. Q., Zhao S. P., Nie S., Li J. (2003) Gene-gene interaction of PPARγ and ApoE affects coronary heart disease risk. Int. J. Cardiol. 92, 257–263 [DOI] [PubMed] [Google Scholar]
- 35.Smith G. S., de Leon M. J., George A. E., Kluger A., Volkow N. D., McRae T., Golomb J., Ferris S. H., Reisberg B., Ciaravino J., La Regina M. E. (1992) Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer's disease: pathophysiologic implications. Arch. Neurol. 49, 1142–1150 [DOI] [PubMed] [Google Scholar]
- 36.Small G. W., Mazziotta J. C., Collins M. T., Baxter L. R., Phelps M. E., Mandelkern M. A., Kaplan A., La Rue A., Adamson C. F., Chang L., Guze B. H., Corder E. H., Saunders A. M., Haines J. L., Pericak-Vance M. A., Roses A. D. (1995) Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 273, 942–947 [PubMed] [Google Scholar]
- 37.Risner M. E., Saunders A. M., Altman J. F., Ormandy G. C., Craft S., Foley I. M., Zvartau-Hind M. E., Hosford D. A., Roses A. D. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 6, 246–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.