Abstract
Coughing protects and clears the airways and lungs of inhaled irritants, particulates, pathogens, and accumulated secretions. An initial urge to cough, and an almost binary output suggests gating mechanisms that encode and modulate this defensive reflex. Whether this “gate” has a physical location for the physiological barrier it poses to cough is unknown. Here we describe a critical component to cough gating, the central terminations of the cough receptors. A novel microinjection strategy defined coordinates for microinjection of glutamate receptor antagonists that nearly abolished cough evoked from the trachea and larynx in anesthetized guinea pigs while having no effect on basal respiratory rate and little or no effect on reflexes attributed to activating other afferent nerve subtypes. Comparable microinjections in adjacent brainstem locations (0.5–2 mm distal) were without effect on coughing. Subsequent transganglionic and dual tracing studies confirmed that the central terminations of the cough receptors and their primary relay neurons are found bilaterally within nucleus tractus solitarius (nTS), lateral to the commissural subnucleus and perhaps in the medial subnuclei. These synapses possess the physiological characteristics of a cough gate. Their localization should facilitate more mechanistic studies of the encoding and gating of cough.—Canning, B. J., Nanako Mori. An essential component to brainstem cough gating identified in anesthetized guinea pigs.
Keywords: solitary tract, capsaicin, respiration, C fiber, bradykinin
The cough reflex protects and clears the airways and lungs from aspirate, inhaled particulates, pathogens, irritants, and accumulated secretions. A compromised or inadequate cough reflex can have dire, acute consequences. But cough can also be problematic and even detrimental. Indeed, it is the most common presenting symptom among patients seeking medical advice from primary care providers. Acute and chronic cough have varied and often differing etiologies, but all of their presentations adversely affect quality of life. Surprisingly, however, existing therapeutics are minimally effective and can have unwanted side effects that limit their safety and utility (1, 2).
Cough is one of only several visceral reflexes that manifests in a discontinuous, threshold-dependent manner. These peculiar attributes imply the existence of gating mechanisms that regulate the encoding of cough, the forcefulness of each cough effort, and the number of explosive, almost epileptiform changes in respiratory drive. It is unclear, however, whether cough gating has a physical location for producing these physiological barriers (3, 4).
We have previously described the physiological and morphological features of the “cough receptors,” which play an essential role in regulating cough in anesthetized guinea pigs (5, 6). These afferents have their cell bodies in the nodose ganglia and are differentiated from C fibers by their conduction velocity and insensitivity to capsaicin. The cough receptors can also be differentiated from intrapulmonary rapidly adapting stretch receptors (RARs) and slowly adapting stretch receptors (SARs) by their insensitivity to smooth muscle contractions, changes in airway luminal pressure, as well as by conduction velocity. The afferent nerves regulating cough in anesthetized cats have similar attributes (7, 8). It seems likely that in all species possessing a cough reflex, cough receptors, similar to those described in cats and guinea pigs and distinct from C fibers and intrapulmonary RARs, play an essential role in regulating this defensive reflex of the airways and lungs.
The cough receptors may subserve primarily, if not exclusively, the initiation of cough. Suboptimal stimulation evokes a slight increase in respiratory rate or augmented breaths, and occasionally, after cough is evoked, a brief apnea may occur. But it is difficult to attribute these respiratory pattern changes to cough receptor activation alone (5, 9). This suggests the existence of medullary cell groupings likely within the nucleus of the solitary tract (nTS) that play an essential and specific role in the regulation of cough. It is reasonable to speculate that the primary relay neurons of the cough receptors comprise a physical location for cough gating. Studies using c-fos expression and brainstem electrical stimulation to define nTS cell groupings involved in cough have been carried out in several species (10–12). But none of these studies have specifically defined the central terminations of the cough receptors.
Only limited information is available regarding central terminations of airway vagal afferent nerve subtypes or their mode of encoding reflexes (13–26). In all of the functional studies, airway vagal afferents utilize glutamate primarily acting on nonNMDA receptors (25, 27–36). In this study, we describe novel stereotactic methods for studying cough in guinea pigs and identify the primary locations of cough receptor termination in nTS. These central terminations and the relay neurons involved have the physiological attributes of a cough-gating mechanism.
MATERIALS AND METHODS
All of the experiments described in this study were carried out using anesthetized male Hartley guinea pigs (200–400 g, pathogen-free; Harlan, Indianapolis, IN, USA) and were first approved by the Johns Hopkins Animal Care and Use Committee. Guinea pigs were anesthetized to effect with ∼1.5 g/kg urethane (i.p.). This anesthetic produces a deep anesthesia lasting far longer than the duration of a typical experiment (<2 h). We confirmed adequate anesthesia throughout the experiment by monitoring withdrawal responses to a sharp pinch of a hind limb. When the experiments were completed, animals were killed by asphyxiation in a vessel filled with carbon dioxide followed by exsanguination.
To study the cough reflex, guinea pigs were secured supine on a warming pad. The trachea was cannulated at its caudal most end with a bent leur stub adaptor. The cannula was attached to a length of tubing that terminated inside a chamber that warmed and humidified inspired room air. Care was taken to preserve the innervation and vasculature of the trachea. A pressure transducer attached to a side port in the tracheal cannula monitored respiratory efforts, which were recorded digitally (Biopac data acquisition system; Biopac Systems, Santa Barbara, CA, USA). The remaining segment of the extrathoracic trachea was opened lengthwise along its ventral-most aspect and retracted bilaterally. Polyethylene (PE) tubing was threaded through the larynx and nasal cavity and out a nostril. Warmed, oxygenated Krebs buffer (118 mM NaCl, 5.4 mM KCl, 1 mM NaHPO4, 1.2 mM MgSO4, 19 mM CaCl2, 25 mM NaHCO3, and 11.1 mM dextrose, pH 7.4) was superfused (3 ml/min) over the tracheal mucosa, introduced into the tracheal lumen at the caudal-most exposed segment of the trachea, and removed at the rostral end of the trachea by attaching the PE tubing threaded through the upper airways to a gentle suction source. The buffer contained 3 μM indomethacin to block formation of neuromodulatory prostanoids.
When the surgery was complete, animals were allowed to breathe spontaneously and without any further manipulations for 10 min. Thereafter, we evoked cough by applying citric acid (0.001–2 M) topically to the tracheal mucosa, delivered in 100-μl aliquots at 1-min intervals. Concentration-response curves were constructed in an ascending fashion. Cough was defined on the basis of visual confirmation of a cough-like respiratory effort, with associated signature pressures, including an ≥500% increase in peak expiratory pressure preceded by an enhanced inspiratory effort, all occurring in <1 s. In some experiments, cough was evoked by mechanically probing the laryngeal mucosa with a von Frey filament or by electrically stimulating (16 Hz, 1-ms pulse duration, 10-s train, 8 V) the tracheal mucosa using a custom-made platinum electrode. In other experiments, using previously described methods (37), we evoked respiratory reflexes by topical application of capsaicin (10 μM, 100 μl) to the laryngeal mucosa or by intravenous administration (via a cannulated jugular vein) of histamine (1–10 μg/kg) or bradykinin (2 nmol/kg). Vehicle control experiments for the laryngeal capsaicin challenge were carried out in parallel in an unpaired experimental design.
We attempted to modulate citric acid-induced coughing and the other respiratory reflex effects by microinjection of either codeine or combinations of glutamate NMDA and non-NMDA receptor antagonists (CNQX and AP-5 or NBQX and SDZ 220581). Guinea pigs were prepared surgically for cough as described above and then transferred to a Kopf stereotactic frame. A midline incision on the skull exposed the underlying sutures. Lambda was located and used as a stereotactic reference point. Various locations within nTS in relation to lambda were located in preliminary experiments (see Results). Microinjections were delivered in 100-nl volumes (in 1 min using a Razel syringe pump; Razel Scientific Instruments, St. Albans, VT, USA) through borosilicate micropipettes pulled sharp using a Flaming Brown micropipette puller (P-87; Sutter Instruments, Novato, CA, USA). The micropipettes were advanced through the skull into the brainstem via drilled access points over the positions of interest. Microinjections were made bilaterally in lateral locations within and adjacent to nTS, or as a single microinjection along midline. Microinjection sites were evaluated at autopsy. Once the microinjections were completed (the whole procedure with bilateral injection takes <10 min), animals were quickly repositioned on the heating pad and prepared for cough studies as described above.
In separate experiments we attempted to evoke respiratory reflexes (including cough) by electrically stimulating various locations within nTS using a concentric ring electrode (11). Stimulation voltage (0.5–5 V) and stimulation frequency (4–16 Hz) were varied to generate voltage and frequency response relationships for the various reflex effects evoked. Only one position within nTS was studied in any animal, and stimuli were delivered unilaterally.
Neuronal tracing
Neuronal tracing was carried out using tracer injections into the trachea or nTS. Guinea pigs were anesthetized with ketamine and xylazine (60 and 6 mg/kg i.m.). DiI (2%) was injected into the dorsal wall of the trachea (injections of 3, 3, and 4 μl in 3 locations) or microinjected (100 nl) into locations within nTS. Animals quickly recovered from these minor surgeries and were maintained thereafter for 60–70 d. Following a lethal overdose with pentobarbital (150 mg/kg i.p.), the trachea and brainstem were removed. Labeled afferent nerve terminals were visualized intravitally within the trachea using epifluorescence microscopy. Brainstems were fixed overnight in 4% paraformaldehyde, frozen in OCT and sectioned (20 μm).
Dual tracing was also carried out (38). DiI was injected into the brainstem as described above, followed immediately by fast blue injection into the trachea. Two weeks later, guinea pigs were killed by lethal overdose with 150 mg/kg pentobarbital and perfusion fixed with 4% paraformaldehyde. The trachea, brainstem, and right and left nodose ganglia were removed. The trachea and brainstem were inspected following section for the appropriateness of the tracer injections. The nodose ganglia were sectioned and the total number of neurons labeled by DiI and/or fast blue was counted.
Statistical analyses
A nonpaired experimental design had to be employed. Results are presented as a means ± se of n experiments, where n is a single animal. Differences among group means were assessed by 1-way ANOVA and Sheffé's F test for unplanned comparisons. Values of P < 0.05 were considered statistically significant. Rarely (<10% of animals), guinea pigs did not cough during surgery or had basal respiratory rates of ≤40 breaths/min not attributable to an intervention. These animals were excluded from subsequent analyses. Also, in the microinjection studies, if the targeted brainstem locations were missed with microinjection, these animals were excluded from further analysis.
Reagents
Bradykinin, capsaicin, CNQX, histamine, indomethacin, NBQX, and SDZ 220581 were purchased from either Sigma (St. Louis, MO, USA) or Tocris (Ellisville, MO, USA). RH421 was purchased from Molecular Probes (Eugene, OR, USA). All drugs were prepared as stock solutions in saline and diluted further on the day of experimentation in saline, except indomethacin (30 mM) and capsaicin (0.1 M), which were dissolved in ethanol prior to dilution in Krebs buffer or saline.
RESULTS
Establishing stereotactic coordinates for nTS in guinea pigs
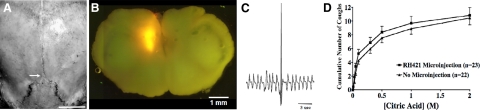
Stereotactic coordinates for microinjections into nTS were developed using the intact body and head of freshly killed male guinea pigs with body weights of 250–275 g (∼5 wk of age). We used lambda as a reference point on the skull (Fig. 1). Once lambda had been located stereotaxically on our reference specimens, parts of the skull and the entire cerebellum were carefully removed to expose the underlying brainstem. The coordinates for several locations in and adjacent to nTS were determined and are presented in Table 1. Adding the fluorescent styryl dye RH 421 (10 μM) to our microinjectate helped localize injections sites. RH 421 could be visualized in 1-mm-thick sections of freshly harvested brainstems and had no effect on the cough reflex (Fig. 1).
Figure 1.
Microinjection strategy used to identify the central terminations of the cough receptors in guinea pigs. A) Microinjections were delivered using lambda as a stereotactic reference point (arrow; see Table 1). Scale bar = 1 cm. B) Microinjection sites were localized by adding the fluorescent styryl dye RH421 (10 μM) to the microinjection fluid, brilliantly visible in 1-mm-thick sections of freshly harvested brainstems. C) Coughing was evoked by applying citric acid directly to the tracheal mucosa and was defined by the characteristic airway pressures and by visual confirmation of a cough-like effort. D) Microinjecting RH421 was without effect on citric acid-induced coughing.
Table 1.
Stereotaxic coordinates for microinjection in guinea-pig brainstem
| Brainstem location targeted | Relative to obexb | Injection location relative to lambdaa |
||
|---|---|---|---|---|
| Lateral (mm) | Caudal (mm) | Ventral (mm) | ||
| Intermediate nTS (SolIM) | 2.0 mm rostral | 1.6 | 4.5 | 10.5 |
| Intermediate nTS (SolIM) | 1.4 mm rostral | 1.6 | 5.0 | 11.0 |
| Commissural nTS (SolC) | 0.8 mm rostral | 0.0 | 6.0 | 12.0 |
| Medial nTS (SolM)c | 0.8 mm rostral | 0.8 | 6.0 | 12.0 |
| Ventrolateral nTS (SolVL) | 0.8 mm rostral | 1.6 | 6.0 | 12.0 |
| Commissural nTS (SolC) | at obex | 0.0 | 6.5 | 13.0 |
| Reticular nucleus (RN) | at obex | 1.6 | 6.5 | 13.0 |
| Cervical spinal cord (CSC) | 1 mm caudal | 0.0 | 7.0 | 14.0 |
Coordinates for microinjection relative to lambda were determined on the intact body and head of male Hartley guinea pigs, ∼5 wk of age, body weight ∼ 250 g. See text for further details.
These approximate coordinates describe the position of the microinjection sites in the rostral/caudal plane relative to obex.
This location for microinjection was found to be most effective for the selective inhibition of coughing.
Microinjections were delivered bilaterally to lateral nTS locations or as a single injection targeting medial nTS locations. Points of access were created with a drill at specific rostral/caudal and medial/lateral coordinates, with the micropipettes advanced through the skull surface to the appropriate dorsal/ventral location. In our first 56 consecutive experiments with bilateral injection, we found (by RH421 fluorescence) microinjection sites in 92 of 112 injections (82%). Both injections were located in the brainstems of 38 of 56 animals (68%), and in 35 of 56 animals, these injection sites were seen in the same rostral-caudal plane of the brainstem (63%). In 2 animals (3.6%), no injection sites were located, while in 16 animals (29%), only one injection site was found at autopsy. Injections reached the targeted dorsal aspects of the brainstem in 40/56 animals (71%), with the remaining injections found in more ventral locations. Coordinates in the medial/lateral plane were reached with almost perfect fidelity (>90%).
In an attempt to improve our accuracy, particularly in the rostral/caudal (63%) and dorsal/ventral (71%) planes, it seemed most likely that our success rates could be limited more by the size of the animals used than by experimental error, poor microinjection techniques or variance in brainstem or skull structure. Indeed, although the animals used were all between 5 and 8 wk of age, body weights varied considerably (mean 271±6 g; range 207–392 g). Success rates were optimal (>80%) in guinea pigs with body weights of 225–290 g. All experiments described below were thus restricted to animals of this size.
Selective modulation of the cough reflex by glutamate receptor antagonist microinjection
Respiratory rate averaged 65 ± 5 breaths/min in control animals (n=23). Nearly all control animals coughed during surgical preparation of the trachea. Coughing was evoked experimentally by citric acid (0.001–2 M) applied in 100-μl aliquots to the tracheal mucosa, mechanical stimulation with a von Frey filament, or by electrical stimulation of the tracheal mucosa (16 Hz, 8 V, 1-ms pulse duration, 10-s train). Coughs were differentiated from enhanced breaths (sighs) by the magnitude of the peak expiratory pressures, the pronounced inspiratory efforts preceding the expiration, the timing of the respiratory efforts (roughly half the duration of a tidal respiratory effort), as well as by a visual confirmation of a cough-like respiratory effort. Coughs evoked by the stimuli used in this study were never paroxysmal but frequently occurred in pairs.
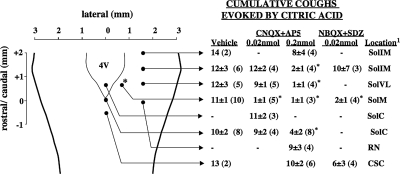
Citric acid applied topically to the tracheal mucosa in ascending concentrations evoked 11 ± 1 coughs cumulatively in control preparations. We attempted to prevent citric acid evoked coughing by microinjecting codeine into various locations in nTS. Bilateral microinjection of codeine rostral and lateral to obex did significantly reduce citric acid-induced coughing (5±2 coughs; n=8), but the effects were variable and difficult to differentiate from the effects of codeine microinjection into adjacent brain stem locations (data not shown; n=8–10). We then studied the effects of the combination of glutamate receptor antagonists CNQX and AP-5, and this yielded superior results (Fig. 2). All subsequent experiments designed to localize the nTS termination sites of the cough receptors were carried out using a combination of glutamate receptor antagonists microinjected into various positions in nTS on the rostral/caudal axis.
Figure 2.
The effects of combinations of NMDA and nonNMDA receptor antagonists on citric acid-induced coughing in anesthetized guinea pigs on microinjection into several nTS and brainstem locations (see Table 1 for stereotactic coordinates utilized). Data are presented as means ± se of n (in parentheses) experiments. Injection sites are marked only unilaterally, but unless delivered along midline, bilateral injections were made. Asterisks indicate microinjections that resulted in a statistically significant inhibition of citric acid coughing relative to that seen in vehicle control animals. Location of microinjection indicated is the location targeted based on the stereotactic coordinates described in Table 1 and confirmed postmortem. With diffusion, however, adjacent brainstem structures and nTS subnuclei were targeted.
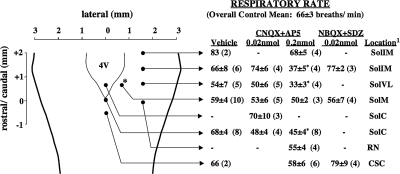
Microinjection of 0.2 nmol each of AP-5 and CNQX bilaterally (0.1 nmol/side) in several nTS locations and a single microinjection of the antagonists medially at obex markedly reduced or abolished citric acid-induced coughing (Fig. 2). These effects of 0.2 nmol AP-5 and CNQX were accompanied by respiratory slowing in several locations, including obex and locations in more rostral areas of nTS (Fig. 3). Reducing the concentration of AP-5 and CNQX to 0.02 nmol each eliminated their effects on respiration, except with microinjection at obex, where respiratory rate was again reduced. This reduction in concentration also largely limited the effects of AP-5 and CNQX on cough except for microinjections bilaterally targeting nTS locations 0.8 mm lateral and ∼0.8 mm rostral to obex. Comparable results were obtained when a different combination of glutamate receptor antagonists (NBQX and SDZ 220581) was used (Figs. 2 and 3). As with AP-5 and CNQX, the combination of NBQX and SDZ 220581 prevented citric acid-evoked coughing, while having little or no effects on basal respiratory rate. NBQX and SDZ 220581 microinjected into sites distal to the location targeted were without effect on cough. Vehicle (saline) microinjections were also without effect on either cough or respiration, regardless of stereotactic coordinates.
Figure 3.
Effects of combinations of NMDA and nonNMDA receptor antagonists on basal respiratory rate in anesthetized guinea pigs on microinjection into several nTS and brainstem locations (see Table 1 for stereotactic coordinates utilized). Data are presented as means ± se of n (in parentheses) experiments. Injection sites are marked only unilaterally, but unless delivered along midline, bilateral injections were made. Asterisks indicate microinjections that resulted in a statistically significant reduction in respiratory rate relative to that seen in vehicle control animals. Location of microinjection is the location targeted based on the sterotaxic coordinates described in Table 1 and confirmed postmortem. With diffusion, however, adjacent brainstem structures and nTS subnuclei were targeted.
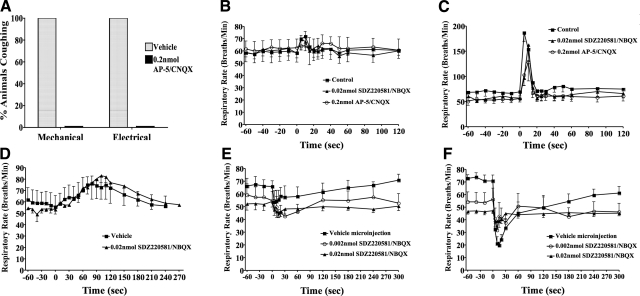
The effects of bilateral glutamate receptor antagonist microinjection targeting nTS locations ∼1 mm rostral and lateral to obex, perhaps within and/or adjacent to the medial subnuclei, appeared to be selective for cough. As described above, these microinjections inhibited citric acid-evoked coughing without altering basal respiratory rate. Identical microinjections of glutamate receptor antagonist combinations also prevented coughing evoked by mechanically probing the laryngeal mucosa or electrically stimulating the tracheal mucosa (Fig. 4A). In contrast, the increases in respiratory rate evoked by intravenous histamine (an RAR-dependent reflex) or intravenous bradykinin (a pulmonary C-fiber dependent reflex) were largely unaffected by these same microinjections (Fig. 4B–D). The respiratory slowing and apneas evoked by laryngeal capsaicin were reduced but not abolished by microinjecting the glutamate receptor antagonists into the nTS location targeted for cough (Fig. 4E). But it is possible that a component of this response to laryngeal capsaicin involves the physical stimulus of applying capsaicin to the laryngeal mucosa and perhaps activation of the cough receptors, as saline challenge alone evoked a pronounced slowing of respiratory rate (Fig. 4F).
Figure 4.
Microinjecting glutamate receptor antagonists in an nTS location rostral and lateral to obex selectively prevents the cough reflex in anesthetized guinea pigs. A) Percentage of 5 animals coughing in response to mechanical (von Frey filament) or electrical (16 Hz, 10-s train, 8 V) stimulation of the tracheal mucosa. B, C) Tachypnea was evoked by 2 μg/kg i.v. (B) and 10 μg/kg i.v. histamine (C). D) Tachypnea was also evoked by the C-fiber stimulant bradykinin (2 nmol/kg i.v.). E, F) Respiratory slowing was evoked by topical laryngeal challenges with saline (E) or capsaicin (F). Consistent with previous studies (5, 6, 9, 37), histamine, bradykinin, and capsaicin all failed to evoke coughing in these experiments. Data in panels B–F are presented as means ± se of 3–6 experiments.
Electrical stimulation of nTS and neuronal tracing of the cough receptors
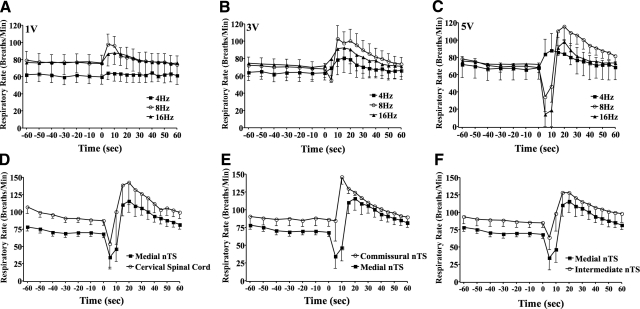
The results summarized above suggest that an nTS location rostral and lateral to obex may be a primary site of termination of the extrathoracic tracheal and laryngeal afferent nerves regulating cough. We set out to further substantiate this assertion with electrical stimulation studies and with neuronal tracing (11, 38, 39). Respiratory effects evoked by electrical stimulation in nTS were both voltage and frequency dependent. At threshold voltages (≤1 V) modest, frequency dependent (4–16 Hz) increases in respiratory rate were evoked from the targeted nTS location (Fig. 5A). Increasing the stimulation intensity (≥3 V) augmented the magnitude of the respiratory rate increases at all stimulation frequencies and uncovered a respiratory slowing with stimulation at 16 Hz (Fig. 5B). Stimulation at 5 V produced pronounced biphasic effects on respiration with stimulation frequencies of 8 and 16 Hz. Respiration nearly stopped during 5-V stimulation at 8 or 16 Hz, and when stimulation ceased, a pronounced tachypnea occurred lasting nearly a minute beyond the end of stimulation (Fig. 5C). Even at 5 V, however, stimulation at 4 Hz still only evoked respiratory rate increases. These effects of electrical stimulation at this nTS location were nearly duplicated, albeit with varying magnitudes, voltage, and frequency-response relationships when electrical stimuli were delivered to other nTS locations (Fig. 5D–F). It thus seemed that this approach would not reliably differentiate nTS locations in terms of regulating distinct respiratory reflexes. Indeed, cough was evoked from the nTS site of interest by electrical stimulation but also by electrical stimulation at obex.
Figure 5.
Respiratory reflexes evoked by unilateral electrical stimulation (1-ms pulse duration, 10-s train) within nTS. A–C) Stereotaxic coordinates targeting the medial nTS were used. Stimulation voltage was varied from 1 V (A), 3 V (B), and 5 V (C), as was stimulation frequency (4–16 Hz), while respiration was monitored. D–F) Effects of stimulating (5 V, 8 Hz) the medial nTS (location implicated in cough in Figs. 2–4) on respiration were compared to effects of stimulation in the cervical spinal cord (D), commissural nTS (E), and intermediate nTS (F). Coughing occurred on stimulation whithin the medial nTS, but also with stimulation at the obex. Data are presented as means ± se of 3–5 experiments.
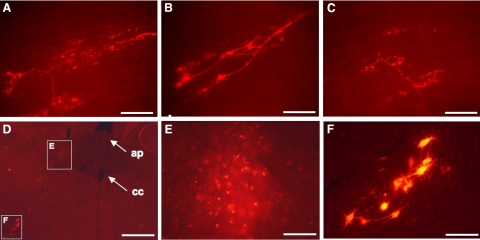
In subsequent studies, we attempted to directly demonstrate that tracheal/laryngeal cough receptors terminate in the nTS location identified above using tracing techniques (38, 39). The hydrophobic retrograde tracer DiI was injected into the trachea or microinjected into nTS to label the central and peripheral terminals of the cough receptors, respectively. The methods of Doyle et al. (39) were utilized, with 60 d of survival following injection to ensure terminal labeling and to avoid false-positive results of through fiber labeling. DiI injection into the nTS location described above resulted in labeling of cough receptor terminals in living whole mounts of the guinea-pig trachea (Fig. 6A–C). Conversely, tracheal injection of DiI resulted in patchy but clear evidence for DiI accumulation in nTS in areas surrounding our targeted injection sites (Fig. 6D–F). The DiI accumulation in nTS was apparent in brain stem sections that also included retrogradely labeled neurons in nucleus ambiguus, likely preganglionic parasympathetic neurons innervating tracheal parasympathetic/cholinergic ganglia, or perhaps laryngeal motor neurons. Brainstem DiI injections into sites adjacent but lateral to our target area resulted in no labeling of cough receptor terminals in the trachea. Preliminary results analyzing c-fos labeling following tracheal citric acid challenge are also consistent with the involvement of the medial subnuclei in the initiation of cough (unpublished results).
Figure 6.
Retrograde tracing from the nTS and trachea selectively labels the peripheral and central terminals of cough receptors, respectively. The hydrophobic tracer DiI was microinjected into the brainstem location implicated in cough. On tissue harvest ≥60 d later, living whole mounts of the trachea were viewed using epifluorescence microscopy. A–C) Representative images of labeled cough receptor terminals from 3 guinea pigs. Structure and orientation of these terminals match exactly with those previously identified as the peripheral terminals of the cough receptors (6). Microinjecting DiI into an adjacent brainstem location resulted in no afferent nerve terminal labeling in the trachea (not shown; n=2). DiI injection into the tracheal mucosa resulted in DiI accumulations, visible in fixed sections within the region of nTS implicated in cough. D, E) Location of the accumulated DiI [dorsal and lateral to central canal (cc), ventral and lateral to area postrema (ap)] and appearance are depicted at low (D) and high magnification (E). F) DiI found in nTS was seen within a brain stem plane that also included retrogradely labeled perikarya of neurons in nucleus ambiguous, likely preganglionic parasympathetic neurons innervating intrinsic ganglia of the trachea, or perhaps laryngeal motor neurons. These neurons are visible at low magnification in panel D. Scale bars = 50 μm (A–C, E, F); 500 μm (D).
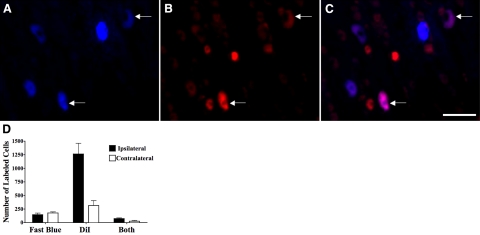
A subsequent dual tracing study further substantiated the assertion that nTS locations just rostral and lateral to obex are primary sites of tracheal/laryngeal cough receptor termination (Fig. 7). DiI injected unilaterally into this location resulted in labeling of ∼1000 nodose ganglia neurons in the ganglion ipsilateral to the injection. Considerably fewer neurons in the nodose ganglia contralateral to the DiI injections were labeled. In these same animals, fast blue was simultaneously injected into the trachea. Fast blue labeled ∼100 neurons in both the right and left nodose ganglia. Notably, however, whereas DiI labeled only ∼5% of all neurons in the nodose ganglia (40, 41), DiI labeled nearly 50% of the fast blue-labeled neurons in the ganglia ipsilateral to the DiI injections. DiI also labeled some of the fast blue-labeled neurons in the ganglia contralateral to the DiI injection.
Figure 7.
A–C) Representative photomicrographs showing nodose ganglia neurons retrogradely labeled from both the nTS location implicated in cough and from the trachea in guinea pigs. DiI was microinjected unilaterally into the nTS location implicated in cough (A). Simultaneously, fast blue was microinjected into the tracheal mucosa (B). Two weeks later, the nodose ganglia were removed following fixation, sectioned, and visualized by epifluorescence microscopy. Dually labeled neurons (arrows) appear purple in merged image (C). D) Numbers of fast blue and/or DiI-labeled neurons in the nodose ganglia ipsilateral and contralateral to the nTS microinjection site for DiI. Results are means ± se of 3 experiments.
DISCUSSION
The cough reflex is unique among respiratory and airway reflexes in that it does not occur in a continuous, stimulus-dependent manner. Rather, cough is defined by thresholds and an urge to cough, with extraordinarily powerful cortical controls that can produce coughs in the absence of stimulation, enhance coughing based on inappropriate perception of an urge to cough, or suppress cough entirely depending on existing social and environmental conditions. The well-defined existence of a preceding urge to cough and the established evidence of a reconfiguration of respiratory motor outputs during cough implies the existence of a cough-gating mechanism that controls the intensity and repetitions of cough efforts. Many brainstem and midbrain structures involved in cough have been described. It is unclear, however, whether cough gating comprises a physical structure, with relay neurons filtering and then encoding the epileptiform changes in motor output, or whether gating is the physiological sum of multiple regulatory elements that influence both breathing and cough (3, 4).
Whatever form cough gating ultimately takes, it is certain that a critical component of this mechanism includes the primary central synapses of the vagal afferent nerves that regulate cough. Previous studies have used c-fos and multichannel recordings to define cell groupings within nTS that are activated when coughing occurs (3, 10–12). But it is unclear whether these cell groupings are the primary cough receptor relay neurons or modulatory circuits recruited during the physical forces associated with this explosive expiratory effort. Indeed, one of these studies was carried out in rats, and it is controversial as to whether rats even possess a cough reflex (6). Also problematic in these studies in anesthetized animals has been the false premise that the cough reflex could be attributed to the activation of RARs and/ or C fibers, both of which having reasonably well-defined central sites of termination. It is now clear that neither C fibers nor RARs regulate cough in anesthetized animals (42).
The cough receptors are a distinct subtype of airway vagal sensory nerve, anatomically and physiologically differentiated from bronchopulmonary C fibers and intrapulmonary stretch receptors and subserving an essential role in the initiation of cough evoked from the larynx, trachea, and mainstem bronchi (5). Their unique physiological attributes and sites of termination in the airways suggest that they would be ideally suited to studies aimed at identifying their central terminals. But there are relatively few cough receptors innervating the airways (<300 roughly equally distributed in the right and left vagus nerves of guinea pigs), and they are likely quiescent at eupnea, being insensitive to changes in airway luminal pressure. Although several immunohistochemical markers, including the glutamate transporters vGlut1 and vGlut2 can label cough receptor terminals in the airways and/or cell bodies in the nodose ganglia (43), no defining neurochemical marker has yet been identified for the central terminals of cough receptors. The cough reflex also quickly desensitizes to repetitive challenge, and while the cough receptors are exquisitely sensitive to both acid and mechanical stimuli, laryngeal and tracheal C fibers are also activated by these stimuli (9, 44, 45). Without some guidance, then, localizing the central terminals of the cough receptors using electrophysiological approaches or neuronal tracing would be a very difficult endeavor.
In designing these experiments, we reasoned that we could use the sparseness of cough receptor innervation to our advantage in microinjection studies aimed at defining the central terminals of the cough receptors. Such an approach would probably fail in attempts at identifying the central terminals of RARs, SARs, or C fibers, given that far greater parts of the airways and lungs are innervated by these airway sensory nerve subtypes, and because there are likely far more of these subtypes involved when initiating reflexes typically associated with their activation (e.g., Hering-Breuer reflex, deflation reflex, pulmonary chemoreflex). A single microinjection would probably fail against the stimuli initiating these reflexes, as these units have been shown in several studies to terminate over a large rostral/caudal axis of nTS (17–23, 26). But with coughing evoked selectively from the extrapulmonary segment of the trachea, only a fraction of the cough receptors would be stimulated, and thus a smaller region of nTS would need to be targeted to achieve blockade of cough, while minimally if at all affecting other visceral reflexes. The results of our physiological analyses largely substantiate our reasoning, while our tracing studies further support our assertions about central termination sites of the cough receptors.
Our use of RH421 in the microinjectate to localize the injection sites is novel and could be used to monitor diffusion rates in nTS. The microinjection volumes used (100 nl) would without any diffusion and with a symmetric pattern of deposition occupy a spherical volume with a diameter of 580 μm. Diffusion from the injection site to occupy a sphere with a diameter of 1.2 mm would reduce the concentration of drug by ≥10-fold along the perimeter of the diffusing volume. On the basis of these idealized calculations, our approach to identifying the central terminations of the cough receptors could be accurate to a distance of ∼1 mm. Consistent with these theoretical predictions, we found that bilateral microinjection of a low concentration of glutamate receptor antagonists at the locations implicated in cough was as effective at preventing cough as similar microinjections with 10-fold higher concentrations of antagonists in adjacent nTS locations ∼0.8 mm distal to our targeted location.
There is limited information available to aid demarcation of nTS subnuclei in guinea pigs (46, 47). Accordingly, and also because of the broad-brush nature of our experiments, we cannot argue strongly for or against a termination pattern, including or excluding any nTS subnuclei. But assuming an nTS structure comparable to that in rats, the data indicate that the tracheal cough receptors may terminate anywhere from the medial and intermediate subnuclei, the interstitial and ventrolateral subnuclei, and possibly the central and dorsomedial subnuclei. These subnuclei have been implicated as sites of termination for other vagal afferent nerves, notably SARs, cardiac afferents, arterial chemoreceptors and baroreceptors, and esophageal afferent nerves (18, 22, 26, 48–54). The data are, however, not supportive of our previous assertions that the cough receptors terminate in the commissural subnucleus (55). This conclusion is supported by studies in cats and in guinea pigs (11, 12) but is inconsistent with the results of studies in rabbits by Mutolo and colleagues (34–36), which implicate more caudal portions of nTS. This may be explained by the different airways targeted in our studies (extrathoracic trachea) and those targeted by Mutolo and colleagues (mainstem bronchi). That we were able to evoke coughing with electrical stimulation of the commissural subnucleus at the obex is consistent with the notion that bronchial cough receptors may terminate in the caudal nTS.
While the commissural subnucleus may not be essential to the cough reflexes studied here, it is thought to be a primary site of RAR and C-fiber termination (15–17, 20, 21, 23, 24, 26, 38). We found that microinjecting glutamate receptor antagonists at the obex consistently slowed respiratory rate, while electrical stimulation at the obex evoked only tachypnea. We speculate that these effects on respiration reflect the actions of RARs and/or C fibers on respiration at eupnea (56–58). These observations are also consistent with our notion that in anesthetized guinea pigs, C fibers and intrapulmonary RARs play a nonessential role in cough (5, 37).
Given their exquisite mechanical sensitivity and responsiveness to acid, it seems likely that cough receptors regulate coughing in both health and disease. As such, we speculate that the results of the present study have relevance to cough gating, even in conscious animals. But a limitation of our approach and of all studies of cough in anesthetized animals is that anesthesia prevents entirely coughing evoked by C-fiber activation. C fibers may be especially relevant to the cough associated with airway inflammation and inhalation of environmental irritants (e.g., cigarette smoke, ozone, toluene diisocyanate). Anesthesia does not prevent C-fiber activation in any animals and does not prevent other C-fiber-dependent reflexes and yet cough evoked by C-fiber-selective stimuli, such as bradykinin and capsaicin, is consistently absent following anesthesia (5, 6, 9, 37, 38, 42, 55). Implicit in these observations is that cough gating through C-fiber activation differs from cough gating through cough receptor pathways. Precisely how these pathways differ awaits a systematic evaluation of cough gating following C-fiber activation.
The relative role of NMDA and nonNMDA glutamate receptors in the regulation of cough also awaits further analysis. The drugs used in the present study (CNQX, NBQX, AP-5, SDZ220581) are highly selective (≤100-fold) for NMDA (AP-5, SDZ220581) and nonNMDA (CNQX, NBQX) receptor subtypes but were administered in combination and at doses known to block synaptic transmission in nTS (25, 27–36). NMDA receptor antagonists, including DM, are known to prevent evoked coughing in multiple species, including humans (4). Accordingly, we speculate that NMDA receptors play a primary role in cough gating.
CONCLUSIONS
We have described the stereotactic coordinates for microinjection into various locations in nTS of the guinea pig. Mostly because of the rarity with which guinea pigs have been used to study central nervous system (CNS) terminations of vagal afferent nerves and/or nTS synaptic physiology (46, 47, 59–61), this may be the most comprehensive stereotactic mapping of the guinea-pig nTS. The utility of this initial mapping awaits a more thorough physiological, morphological and neurochemical analyses. We also describe a novel and useful method for localizing microinjection sites with the addition of the fluorescent styryl dye RH421 into the microinjection fluid. We found the dye to be essentially inert against the endpoints we measured and it provides an accurate account of the location and spread of injectate in brainstem.
Unlike rats or mice, guinea pigs produce a cough reflex comparable to that described in larger animals (dogs, cats, humans) and cough to the same stimuli that initiate coughing in human subjects (62). Guinea pigs are also readily and routinely manipulated in experimental studies with interventions, such as cigarette smoke, allergen, environmental irritant exposures, or infections in studies designed to evaluate changes in cough reflex sensitivity (63). Guinea-pig cough can also be studied in the conscious state, rarely attempted in larger species such as rabbits, cats, or dogs, and the most desirable approach to pharmacological studies. With respect to studies of CNS synaptic physiology, the guinea pig has some disadvantages, including the paucity of published results of guinea-pig brain stem physiology and their extraordinary growth rates (∼100 g at 1 wk, ∼500 g at 12 wk, and as much as 1.2 kg at 1 yr). But the many advantages offered in studies of cough in guinea pigs outweigh these considerable disadvantages.
We described a location within nTS that we believe contains the primary terminations of the cough receptors innervating the extrathoracic trachea and larynx and their primary relay neurons. We found that we could largely prevent coughing evoked from the trachea and larynx by microinjecting glutamate receptor antagonists into this location, without affecting respiratory rate or reflexes that we attribute to the activation of intrapulmonary RARs and C fibers. These results further support our notion that the cough receptors are an anatomically and physiologically distinct subset of airway vagal afferent nerves. The data also show that this population of synapses has the physiological attributes of a cough-gating mechanism. The results will facilitate further characterization of the encoding of cough in CNS and potential interactions among the airway afferent nerve subtypes implicated in cough.
Acknowledgments
The research described in this manuscript was funded by a grant (HL083192) from the National Institutes of Health (Bethesda, MD, USA).
REFERENCES
- 1.Irwin R. S., Baumann M. H., Bolser D. C., Boulet L. P., Braman S. S., Brightling C. E., Brown K. K., Canning B. J., Chang A. B., Dicpinigaitis P.V., Eccles R., Glomb W. B., Goldstein L. B., Graham L. M., Hargreave F. E., Kvale P. A., Lewis S. Z., McCool F. D., McCrory D. C., Prakash U. B., Pratter M. R., Rosen M. J., Schulman E., Shannon J. J., Smith Hammond C., Tarlo S. M. American College of Chest Physicians (ACCP) (2006) Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 129(Suppl.), 1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morice A. H., Fontana G. A., Sovijarvi A. R., Pistolesi M., Chung K. F., Widdicombe J., O'Connell F., Geppetti P., Gronke L., De Jongste J., Belvisi M., Dicpinigaitis P., Fischer A., McGarvey L., Fokkens W. J., Kastelik J. ERS Task Force. (2004) The diagnosis and management of chronic cough. Eur. Respir. J. 24, 481–492 [DOI] [PubMed] [Google Scholar]
- 3.Bolser D. C., Poliacek I., Jakus J., Fuller D. D., Davenport P. W. (2006) Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir. Physiol. Neurobiol. 152, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canning B. J. (2009) Central regulation of the cough reflex: therapeutic implications. Pulm. Pharmacol. Ther. 22, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canning B. J., Mazzone S. B., Meeker S. N., Mori N., Reynolds S. M., Undem B. J. (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J. Physiol. 557, 543–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzone S. B., Reynolds S. R., Mori N., Kollarik M Farmer D. G., Myers A. C., Canning B. J. (2009) Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J. Neurosci. 29, 13662–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widdicombe J. G. (1954) Receptors in the trachea and bronchi of the cat. J. Physiol. 123, 71–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widdicombe J. G. (1954) Respiratory reflexes from the trachea and bronchi of the cat. J. Physiol. 123, 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canning B. J., Farmer D. G., Mori N. (2006) Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R454–R463 [DOI] [PubMed] [Google Scholar]
- 10.Gestreau C., Bianchi A. L., Grelot L. (1997) Differential brainstem Fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J. Neurosci. 17, 9340–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohi Y., Yamazaki H., Takeda R., Haji A. (2005) Functional and morphological organization of the nucleus tractus solitarius in the fictive cough reflex of guinea pigs. Neurosci. Res. 53, 201–209 [DOI] [PubMed] [Google Scholar]
- 12.Jakus J., Poliacek I., Halasova E., Murin P., Knocikova J., Tomori Z., Bolser D. C. (2008) Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir. Physiol. Neurobiol. 160, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia M., Richter D. (1985) Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. I. A light microscopic analysis. J. Comp. Neurol. 241, 503–520 [DOI] [PubMed] [Google Scholar]
- 14.Kalia M., Richter D. (1985) Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. II. An ultrastructural analysis. J. Comp. Neurol. 241, 521–535 [DOI] [PubMed] [Google Scholar]
- 15.Kalia M., Richter D. (1988) Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J. Comp. Neurol. 274, 560–573 [DOI] [PubMed] [Google Scholar]
- 16.Kalia M., Richter D. (1988) Rapidly adapting pulmonary receptor afferents: II. Fine structure and synaptic organization of central terminal processes in the nucleus of the tractus solitarius. J. Comp. Neurol. 274, 574–594 [DOI] [PubMed] [Google Scholar]
- 17.Davies R. O., Kubin L. (1986) Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J. Physiol. 373, 63–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies R. O., Kubin L., Pack A. I. (1987) Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J. Physiol. 383, 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezure K., Otake K., Lipski J., She R. B. (1991) Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Res. 564, 268–278 [DOI] [PubMed] [Google Scholar]
- 20.Lipski J., Ezure K., Wong She R. B. (1991) Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J. Physiol. 443, 55–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubin L., Kimura H., Davies R. O. (1991) The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J. Physiol. 435, 207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonham A. C., McCrimmon D. R. (1990) Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J. Physiol. 427, 261–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonham A. C., Joad J. P. (1991) Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J. Physiol. 441, 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otake K., Ezure K., Lipski J., Wong She R. B. (1992) Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J. Comp. Neurol. 324, 365–378 [DOI] [PubMed] [Google Scholar]
- 25.Ezure K., Tanaka I., Miyazaki M. (1999) Electrophysiological and pharmacological analysis of synaptic inputs to pulmonary rapidly adapting receptor relay neurons in the rat. Exp. Brain Res. 128, 471–480 [DOI] [PubMed] [Google Scholar]
- 26.Kubin L., Alheid G. F., Zuperku E. J., McCrimmon D. R. (2006) Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 101, 618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonham A. C., Coles S. K., McCrimmon D. R. (1993) Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J. Physiol. 464, 725–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vardhan A., Kachroo A., Sapru H. N. (1993) Excitatory amino acid receptors in the nucleus tractus solitarius mediate the responses to the stimulation of cardio-pulmonary vagal afferent C fiber endings. Brain Res. 618, 23–31 [DOI] [PubMed] [Google Scholar]
- 29.Karius D. R., Ling L., Speck D. F. (1994) Nucleus tractus solitarius and excitatory amino acids in afferent-evoked inspiratory termination. J. Appl. Physiol. 76, 1293–1301 [DOI] [PubMed] [Google Scholar]
- 30.Chianca D. A., Jr., Machado B. H. (1996) Microinjection of NMDA antagonist into the NTS of conscious rats blocks the Bezold-Jarisch reflex. Brain Res. 718, 185–188 [DOI] [PubMed] [Google Scholar]
- 31.Wilson C. G., Zhang Z., Bonham A. C. (1996) Non-NMDA receptors transmit cardiopulmonary C fibre input in nucleus tractus solitarii in rats. J Physiol. 496, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aylwin M. L., Horowitz J. M., Bonham A. C. (1997) NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J. Neurophysiol. 77, 2539–2548 [DOI] [PubMed] [Google Scholar]
- 33.Haxhiu M. A., Yamamoto B., Dreshaj I. A., Bedol D., Ferguson D. G. (2000) Involvement of glutamate in transmission of afferent constrictive inputs from the airways to the nucleus tractus solitarius in ferrets. J. Auton. Nerv. Syst. 80, 22–30 [DOI] [PubMed] [Google Scholar]
- 34.Mutolo D., Bongianni F., Fontana G. A., Pantaleo T. (2007) The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res. Bull. 74, 284–293 [DOI] [PubMed] [Google Scholar]
- 35.Mutolo D., Bongianni F., Cinelli E., Fontana G. A., Pantaleo T. (2008) Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am. J. Physiol. 295, R243–R251 [DOI] [PubMed] [Google Scholar]
- 36.Mutolo D., Bongiani F., Cinelli E., Pantaleo T. (2009) Role of excitatory amino acids in the mediation of tracheobronchial cough induced by citric acid inhalation in the rabbit. Brain Res. Bull. 80, 22–29 [DOI] [PubMed] [Google Scholar]
- 37.Chou Y. L., Scarupa M. D., Mori N., Canning B. J. (2008) Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am. J. Physiol. 295, R1572–R1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzone S. B., Canning B. J. (2002) Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R86–R98 [DOI] [PubMed] [Google Scholar]
- 39.Doyle M. W., Bailey T. W., Jin Y. H., Appleyard S. M., Low M. J., Andresen M. C. (2004) Strategies for cellular identification in nucleus tractus solitarius slices. J. Neurosci. Methods 137, 37–48 [DOI] [PubMed] [Google Scholar]
- 40.Johnson EM, Jr., Gorin P. D., Brandeis L. D., Pearson J. (1980) Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science 210, 916–918 [DOI] [PubMed] [Google Scholar]
- 41.Christian E. P., Togo J. A., Naper K. E., Koschorke G., Taylor G. A., Weinreich D. (1993) A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. J. Neurosci. Methods 47, 147–160 [DOI] [PubMed] [Google Scholar]
- 42.Canning B. J., Mori N., Mazzone S. B. (2006) Vagal afferent nerves regulating the cough reflex. Respir. Physiol. Neurobiol. 152, 223–242 [DOI] [PubMed] [Google Scholar]
- 43.Mazzone S. B., McGovern A. E. (2008) Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs. Cough 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricco M. M., Kummer W., Biglari B., Myers A. C., Undem B. J. (1996) Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J. Physiol. 496, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kollarik M., Undem B. J. (2002) Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J. Physiol. 543, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen W. F. (1923) Origin and distribution of the tractus solitarius in the guinea pig. J. Comp. Neurol. 35, 171–204 [Google Scholar]
- 47.Allen W. F. (1923) Origin and destination of the secondary visceral fibers in the guinea pig. J. Comp. Neurol. 35, 275–311 [Google Scholar]
- 48.Mifflin S. W., Spyer K. M., Withington-Wray D. J. (1988) Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J. Physiol. 399, 349–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altschuler S. M., Bao X. M., Bieger D., Hopkins D. A., Miselis R. R. (1989) Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol. 283, 248–268 [DOI] [PubMed] [Google Scholar]
- 50.Lu W. Y., Bieger D. (1998) Vagal afferent transmission in the NTS mediating reflex responses of the rat esophagus. Am. J. Physiol. 274, R1436–R1445 [DOI] [PubMed] [Google Scholar]
- 51.Paton J. F. (1998) Convergence properties of solitary tract neurones driven synaptically by cardiac vagal afferents in the mouse. J. Physiol. 508, 237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seagard J. L., Dean C., Hopp F. A. (1999) Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol. 520, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva-Carvalho L., Paton J. F., Rocha I., Goldsmith G. E., Spyer K. M. (1998) Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J. Neurophysiol. 79, 2374–2382 [DOI] [PubMed] [Google Scholar]
- 54.Wank M., Neuhuber W. L. (2001) Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J. Comp. Neurol. 435, 41–59 [DOI] [PubMed] [Google Scholar]
- 55.Mazzone S. B., Mori N., Canning B. J. (2005) Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J. Physiol. 56, 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies A., Sant'Ambrogio F., Sant'Ambrogio G. (1981) Onset of inspiration in rabbit during artificial ventilation. J. Physiol. 318, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisarri T. E., Yu J., Coleridge H. M., Coleridge J. C. (1986) Background activity in pulmonary vagal C-fibers and its effects on breathing. Respir. Physiol. 64, 29–43 [DOI] [PubMed] [Google Scholar]
- 58.Green J. F., Kaufman M. P. (1990) Pulmonary afferent control of breathing as end-expiratory lung volume decreases. J. Appl. Physiol. 68, 2186–2194 [DOI] [PubMed] [Google Scholar]
- 59.Dekin M. S., Getting P. A., Johnson S. M. (1987) In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. I. Identification of neuronal types and repetitive firing properties. J. Neurophysiol. 58, 195–214 [DOI] [PubMed] [Google Scholar]
- 60.Ohi Y., Kato F., Haji A. (2007) Codeine presynaptically inhibits the glutamatergic synaptic transmission in the nucleus tractus solitarius of the guinea pig. Neuroscience 146, 1425–1433 [DOI] [PubMed] [Google Scholar]
- 61.Sekizawa S., Chen C. Y., Bechtold A. G., Tabor J. M., Bric J. M., Pinkerton K. E., Joad J. P., Bonham A. C. (2008) Extended secondhand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs. Eur. J. Neurosci. 28, 771–781 [DOI] [PubMed] [Google Scholar]
- 62.Canning B. J. (2008) The cough reflex in animals: relevance to human cough research. Lung 186(Suppl. 1), S23–S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canning B. J., Chou Y. (2008) Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 21, 702–720 [DOI] [PMC free article] [PubMed] [Google Scholar]