Abstract
Gastric and small intestinal circular smooth muscle layers have a transwall resting membrane potential (RMP) gradient that is dependent on release of carbon monoxide (CO) from interstitial cells of Cajal (ICCs). Our aim was to determine whether a RMP gradient exists in the mouse colon and whether the gradient is CO dependent. Microelectrodes were used to record RMPs from muscle cells at different depths of the circular muscle layer from wild-type and heme oxygenase-2-knockout (HO-2-KO) mice. A transwall RMP gradient was present in wild-type mice. The CO scavenger oxyhemoglobin (20 μM) and the heme oxygenase inhibitor chromium mesoporphyrin IX (CrMP, 5 μM) abolished the transwall gradient. The gradient was absent in HO-2-KO mice. Tetrodotoxin (1 μM) caused a significant depolarization in circular smooth muscle cells throughout the circular muscle layer and abolished the transwall gradient. Removal of the submucosal neurons abolished the gradient. The majority of submucosal neurons contained HO-2 immunoreactivity (HO-2-IR), while ICCs did not. These data show for the first time that a transwall gradient exists across the circular smooth muscle layer of the mouse colon, that the gradient is due to CO, and that the source of CO is the submucosal neurons.—Sha, L., Farrugia, G., Linden, D. R., Szurszewski, J. H. The transwall gradient across the mouse colonic circular muscle layer is carbon monoxide dependent.
Keywords: heme oxygenase-2, interstitial cells of Cajal, smooth muscle, submucosal ganglion neurons
A transwall gradient in resting membrane potential (RMP) exists across the circular muscle layer of the gastrointestinal tract of dogs (1–9), cats (10), mice (11, 12), and humans (3, 7). In the stomach and small intestine, the RMP of circular smooth muscle cells in the myenteric region in the different species is more hyperpolarized by 9–23 mV compared with RMP of circular muscle cells adjacent to the submucosa (1–3, 6, 10–12), whereas in the dog colon, the transwall gradient is reversed, with circular smooth muscle cells adjacent to the submucosal plexus 23–36 mV more hyperpolarized compared with muscle cells adjacent to the myenteric plexus (4–6, 8, 9). The transwall gradients enable the circular muscle layer to produce weak contractions that only involve a portion of the circular muscle layer to strong, propulsive contractions that involve the entire muscle layer to gradations in strength between these two extremes (7).
The transwall gradient in the stomach and small intestine depends on the generation and focal release of carbon monoxide (CO) by interstitial cells of Cajal (ICCs) located in the myenteric region (11, 12). Heme oxygenase-2 (HO-2) activity and endogenous production and release of CO mirror the transwall gradient in the stomach and small intestine (11), and neither NG-nitro-l-arginine (l-NNA), an inhibitor of nitric oxide synthase, nor the neural toxin tetrodotoxin (TTX) affect the gradient. In mice with targeted knockout of HO-2 (HO-2-KO mice) and in W/WV mutant mice lacking small intestinal myenteric ICCs, the RMP of circular smooth muscle cells in the myenteric region is depolarized, and the transwall gradient is abolished (12, 13).
Although a gradient was not looked for, the RMP of circular smooth muscle cells of the mouse colon is sensitive to TTX (14–16), suggesting that, unlike in the stomach and small intestine (12), nerve activity in the mouse colon might modulate the RMP of colonic circular smooth muscle cells. There is no information as to whether a transwall gradient exists across the circular muscle layer of the mouse colon and, if there is one, whether it is dependent on the release of gaseous molecules such as CO from ICCs or CO from enteric neurons. In this study, we provide evidence for a transwall gradient in murine colon, and that, similarly to the dog colon, it is reversed compared to the gradient across the circular muscle layer of the stomach and small intestine. However, in sharp contrast to the stomach and small intestine, we found that the gradient was due to the release of CO from submucosal ganglion neurons and not due to release of CO from ICCs. Parts of this study were communicated in abstract form (17).
MATERIALS AND METHODS
Adult (6 wk or older) male wild-type (WT; SJL/J) and HO-2-KO mice were used. WT mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The HO-2-KO mice we used, provided to us by Dr. Solomon Snyder (Johns Hopkins University, Baltimore, MD, USA), were previously characterized (18) and were back-crossed with C57BL/6J mice for >10 generations. All procedures using animals were approved by the Mayo Institutional Animal Care and Use Committee. Immediately after the animals were sacrificed with CO2 gas, a piece of colon (4×8 mm), 2–3 cm distal to the cecum, was removed and placed in a Petri dish containing normal Krebs solution (NKS) at room temperature. The mucosa was removed by cutting the tissue with spring-form scissors at about the level of the muscularis mucosa. This dissection left the submucosal neural plexus intact. In some preparations, both the submucosa and mucosa were removed by peeling. Preparations were stretched to no more than 5% greater than the resting length in any direction and pinned serosal side down to the floor of a Sylgard-coated recording chamber for intracellular recordings. The chamber had a volume of 2 ml. The recording chamber was perfused with oxygenated NKS at 37°C with a flow rate of 2 ml/min. The composition of NKS was (in mM) 137.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg2+, 124 Cl−, 15.5 HCO3−, 1.2 H2PO4−, and 11.5 glucose, and the solution was bubbled continuously with 97% O2 and 3% CO2. For preparations with electrical field stimulation (EFS) of nerves, two platinum wires were placed parallel to the long axis of the preparation. Repetitive square-wave pulses (0.3 ms, 100 V, 0.1 Hz) were through a stimulator (Grass 588; Grass Instruments, Quincy, MA, USA) and a stimulus isolation unit (Grass SIU 5A).
Sharp glass microelectrodes filled with 3 M KCl (with input resistance of 40–70 MΩ) were used to record intracellularly the RMPs of circular smooth muscle cells. Thirty minutes before recording, nifedipine (1 μM) was added to the superfusate to inhibit contractions. Recorded signals were amplified through an amplifier (Intra 767, World Precision Instruments, Sarasota, FL, USA), digitized (Digidata 1322A; Axon Instruments, Sunnyvale, CA, USA), analyzed and stored in a computer. A microelectrode manipulator (LSS-8000; EXFO Burleigh, Mississauga, ON, Canada) was used to advance the microelectrode. The readout meter of the Burleigh manipulator was set at 0 when the tip of recording microelectrode touched the tissue surface. The distance of a recorded cell from the surface of the tissue was obtained by the read-out from the meter. The distance of the recorded cell from the surface of the tissue was verified, in some experiments, by injecting Lucifer yellow into the recorded cell and comparing the distance on the read-out from the meter to the distance measured microscopically, as described previously (12).
Values for RMPs at different depths of the circular muscle layer are reported in the Results section in two ways. In the first, RMP values were placed in 3 groups based on whether the recorded cell's location was in the inner region, middle region, or outer region of the circular muscle layer. Each group represented one-third of the measured thickness of the circular muscle layer. The inner group of cells was closest to the submucosal border, whereas the outer group of cells was closest to the myenteric plexus. For each preparation (animal) and experimental condition, multiple impalements were made within each group. The mean ± se RMP for each of the 3 groups of cells from all experiments and the overall RMP by averaging recordings from all 3 groups were obtained. The RMPs among inner, middle, and outer groups were analyzed by ANOVA, and the RMPs between a particular group and the control group were analyzed by Student's t test. A P value less than 0.05 was considered significant. Using the second method of analysis, RMPs were plotted against the depth (μm), from which the recordings were made. A random effect model was used to model RMPs of recorded cells vs. the location of the cell in the circular muscle layer (19). This method of analysis accounts for the linear correlation of RMPs and their related locations. This analysis tested whether the slope of the mean regression line was significantly different than zero. Using this second analysis method, we plotted the regression lines (RMPs across the thickness of the circular muscle layer) without individual data points. The slopes of the mean regression lines are given as means ± se. A value of P < 0.05 was considered significant.
For immunohistochemical staining, immediately after the animal was euthanized with CO2 gas, a section of colon (9–11 mm long) 2–3 cm from the cecum and a section of jejunum (9–11 mm long) ∼6 cm from the pylorus were removed and cut open. After we carefully removed the mucosa, colonic preparations were fixed with 4% paraformaldehyde at 4°C for 3 h for double labeling for PGP 9.5 (to identify nerves) and HO-2, or fixed with acetone at 4°C for 15 min for double labeling for ACK2 (to identify ICCs) and HO-2. After removing the mucosa, jejunal preparations were fixed with acetone at 4°C for 15 min for double labeling for ACK2 and HO-2 as positive control for colonic preparations. After fixation, the fixative was washed out with PBS (5 washes in 1 h). Preparations were then blocked with PBS with 0.3% Triton X-100 and 10% serum overnight at 4°C. After blocking, we incubated the preparations with primary antibodies in PBS with 0.3% Triton X-100 and 5% serum for 36 h at 4°C. For double labeling of PGP 9.5 and HO-2, we used guinea pig anti-PGP 9.5 (Novus Biological, Littleton, CO, USA), diluted at 1:500; and rabbit anti-human HO-2, diluted at 1:5000 (gift from Dr. Solomon Snyder). For double labeling of ACK2 and HO-2, we used rat anti-mouse ACK2, diluted at 1:500, (Bioscience, San Diego, CA, USA) and rabbit anti-human HO-2 diluted at 1:5000. After washout of the primary antibodies with PBS (5 times for 1 h), the preparations were incubated with fluorescent secondary antibodies overnight at 4°C in PBS with 0.3% Triton X-100 and 2.5% serum. For double-labeling of PGP 9.5 and HO-2, goat anti-guinea pig CY3 and goat anti-rabbit FITC (Jackson ImmunoResearch Laboratory, West Grove, PA, USA) were used. For double labeling of ACK2 and HO-2, donkey anti-rabbit CY5 and donkey anti-rat CY3 (Chemicon, Temecula, CA, USA) were used. After incubation, the secondary antibodies were washed out with PBS (5 times for 1 h). The preparations were mounted onto slides with slowfade and examined under a fluorescent microscope.
Some of the colonic preparations that were prepared by peeling off the submucosa and mucosa were fixed with 4% paraformaldehyde and stained with PGP 9.5 to verify that the submucosal neuronal plexus was removed.
Chromium mesoporphyrin IX was purchased from Frontier Scientific (Logan, UT, USA). All other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
RESULTS
A transwall gradient exists in WT mouse colon
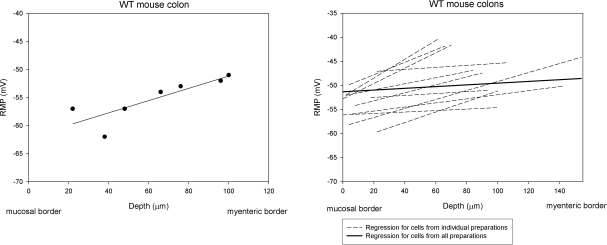
Intracellular recordings were made from 98 circular smooth muscle cells from 11 preparations. The RMP of cells close to the submucosal border, the inner group, was −53.6 ± 0.9 mV, whereas the RMP of cells close to the myenteric region, the outer group, was −47.5 ± 1.2 mV (P<0.01 compared to inner group; Table 1). The RMP for cells in the middle group was −51.5 ± 1.4 mV, which was more depolarized than the cells of the inner group and more hyperpolarized than those of the outer group (Table 1). The average RMP for all cells in all three groups was −50.7 ± 1.1 mV. When plotted by location of the cell in the circular muscle layer, the slope of the mean regression line was 0.6 ± 0.1 mV/10 μm, which was significantly (P<0.01) different from 0 slope, indicating that a transwall RMP gradient existed across the thickness of the circular muscle layer (Fig. 1).
Table 1.
RMPs of circular smooth muscle cells
| Preparation | RMP (mV) |
|||
|---|---|---|---|---|
| Inner circular | Middle circular | Outer circular | All groups | |
| WT control (n=11) | −53.6 ± 0.9 | −51.5 ± 1.4 | −47.5 ± 1.2## | −50.7 ± 1.1 |
| WT + oxyhemoglobin (n=4) | −43.6 ± 1.4** | −45.4 ± 1.7* | −45.5 ± 1.8 | −44.8 ± 1.4* |
| WT + l-NNA (n=4) | −52.8 ± 2.4 | −47.0 ± 2.7 | −45.1 ± 1.4# | −48.3 ± 1.8 |
| WT + CrMP (n=4) | −45.2 ± 1.5** | −46.0 ± 1.4* | −45.1 ± 1.6 | −45.5 ± 1.5* |
| WT + TTX (n=4) | −44.6 ± 2.0** | −44.8 ± 1.7* | −41.9 ± 1.8* | −43.7 ± 1.4** |
| HO-2 KO mouse (n=6) | −45.8 ± 1.7** | −45.7 ± 1.1* | −45.5 ± 0.8 | −45.6 ± 1.1** |
| WT w/o submucosa (n=9) | −44.6 ± 0.6** | −44.9 ± 0.7** | −44.4 ± 0.6* | −44.6 ± 0.6** |
| WT w/o submucosa with EFS (n=4) | −44.5 ± 0.7** | −44.0 ± 0.5** | −44.4 ± 0.9 | −44.3 ± 0.7** |
P < 0.05,
P < 0.01 vs. inner group; 1-way ANOVA with posttest.
P < 0.05,
P < 0.01 vs. WT control; Student t test.
Figure 1.
RMP gradient across the circular muscle layer of the colon of WT mice. Left panel: each dot represents the RMP from a recorded cell; line is the regression for all cells from the same preparation. The x axis is the location (μm) of the cell relative to the submucosa; the y axis is the RMP from that cell. Right panel: regression lines for cells from all (n=11) recorded WT mouse colon preparations. Each dashed line is the regression line for an individual preparation. Thick solid line is the mean regression line for all preparations. Slope of the mean regression line was 0.6 ± 0.1 mV/10 μm. Data points are not shown in right panel.
Effect of oxyhemoglobin on the transwall gradient
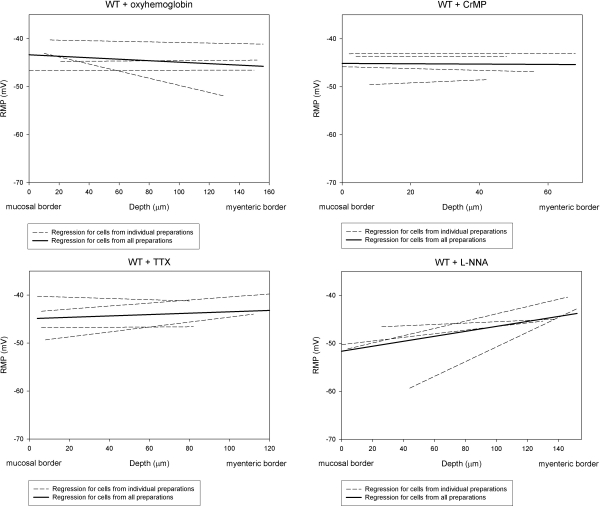
Oxyhemoglobin, a molecular trapping agent for CO (20), and NO (21) were used to determine whether the transwall gradient was CO and NO dependent. Recordings were made from 66 circular smooth muscle cells in 4 preparations with oxyhemoglobin. The mean RMP for cells in the inner, middle, and outer groups was −43.6 ± 1.4, −45.4 ± 1.7, and −45.5 ± 1.8 mV, respectively (Table 1). Thus, oxyhemoglobin caused significant depolarization of cells in the inner and middle groups of cells (Table 1). The lack of effect of oxyhemoglobin on RMPs in the outer group of cells suggested that little endogenously generated CO or NO reached smooth muscle in this area of the circular muscle layer. The mean RMP for all cells in all 3 groups was −44.8 ± 1.4 mV, which was significantly different (P<0.05) when compared with the RMP in NKS (control group) (Table 1). The slope of the mean regression line across the oxyhemoglobin-treated circular muscle layer was −0.2 ± 0.1 mV/10 μm. This slope was not significantly different when compared to 0 slope (P=0.256), indicating that the transwall gradient across the circular smooth muscle layer was abolished by oxyhemoglobin (Fig. 2). Since oxyhemoglobin will trap NO as well as CO, we tested the effect of l-NNA, a NO synthase inhibitor, to determine whether the effect of oxyhemoglobin on the RMP gradient was due solely to trapping of CO or whether it was also due to trapping NO.
Figure 2.
Transwall gradient across the circular muscle layer in WT mouse colon was abolished by oxyhemoglobin, CrMP, and TTX, while l-NNA had no significant effect on the transwall gradient. Slope of the mean regression line in WT mouse colon preparations in the presence of oxyhemoglobin was −0.2 ± 0.1 mV/10 μm. Slopes of the mean regression line in WT mouse colon preparations in the presence of CrMP and in the presence of TTX were −0.01 ± 0.4 and 0.2 ± 0.2 mV/10 μm, respectively. Slopes in the treated preparations were not significantly different from 0 slope (P>0.05). Slope of the mean regression line in WT mouse colon preparations in the presence of l-NNA (0.6±0.1 mV/10 μm) was significantly different from 0 slope (P<0.01).
Effect of l-NNA on the transwall gradient
NO is an inhibitory modulator/neurotransmitter in the gastrointestinal tract (22). Furthermore, it has been shown to be released from inhibitory neurons in enteric plexus to cause hyperpolarization of smooth muscle cells. Four preparations were treated with l-NNA (200 μM), and intracellular recordings were made from 33 circular smooth muscle cells 45 min after l-NNA was applied. The RMP was −52.8 ± 2.4 mV for the inner group of cells and −47.0 ± 2.7 mV for the middle group of cells (Table 1). Cells in the outer group had a mean RMP of −45.1 ± 1.4 mV (Table 1). The mean RMP for all cells recorded from was −48.3 ± 1.8 mV. None of these values for RMP were significantly different from RMPs in NKS (P>0.05) (Table 1). In the presence of l-NNA, the slope of mean regression line across the circular muscle layer was 0.6 ± 0.1 mV/10 μm (Fig. 2), which was not significantly different (P>0.05) compared to the slope of mean regression line observed in NKS without l-NNA. The slope in preparations treated with l-NNA was significantly different from 0 slope (P<0.01). These results indicated that the RMP gradient was not NO dependent.
Effect of CrMP on the transwall gradient
To further examine the role of CO in generating the transwall voltage gradient, we tested the effect of chromium mesoporphyrin IX (CrMP), an inhibitor of heme oxygenase activity (23). Intracellular recordings were made from 27 circular smooth muscle cells in 4 preparations treated with CrMP (5 μM). The RMP for cells in the inner, middle, and outer groups in the presence of CrMP was −45.2 ± 1.5, −46.0 ± 1.4, and −45.1 ± 1.6 mV, respectively (Table 1). Similar to the effect of oxyhemoglobin, CrMP significantly depolarized the RMP of cells in the most hyperpolarized regions (inner and middle groups) of the circular muscle layer. The lack of effect of CrMP on RMPs in cells in the outer group suggested that there was little endogenous generation of CO in this area of the circular muscle layer. The mean RMP for all recorded cells was −45.5 ± 1.5 mV, which was significantly (P<0.05) different compared to the RMP in NKS in WT mice (Table 1). The slope of the mean regression line in the presence of CrMP was −0.01 ± 0.4 mV/10 μm (Fig. 2). When the slope of mean regression line in CrMP-containing Krebs solution was compared to 0 slope, the value of P was 0.986. Thus, CrMP abolished the transwall gradient across the circular muscle layer, suggesting the transwall gradient was CO dependent.
Effect of TTX on the transwall gradient
Recordings were made from 42 cells from 4 preparations 30 min after TTX (1 μM) was applied. The RMP was −44.6 ± 2.0 mV for cells in the inner group, −44.8 ± 1.7 mV for cells in the middle group, and −41.9 ± 1.8 mV for cells in the outer group (Table 1). All were significantly (P<0.05) different when compared to RMPs recorded in the same groups in NKS. There was no significant difference (P>0.05) among inner, middle, and outer groups. The mean RMP for all cells recorded in the presence of TTX was −43.7 ± 1.4 mV, a value significantly different from control (P<0.01) (Table 1). The slope of the mean regression line for all 4 preparations treated with TTX was 0.2 ± 0.2 mV/10 μm, which was not significantly different from 0 slope (P>0.05). These results indicated that TTX abolished the transwall gradient.
Transwall gradient was absent in HO-2-KO mice
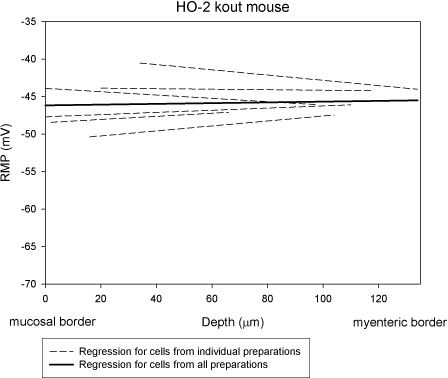
Ninety-six colon circular smooth muscle cells were recorded from 6 HO-2-KO mice. The mean RMP for all cells was −45.6 ± 1.1 mV, which was significantly (P<0.01) different when compared to the RMP from WT mice. Smooth muscle cells in the inner and middle groups from HO-2-KO mice were significantly depolarized compared to WT (inner group: −45.8± 1.7 vs. −53.6±0.9 mV, P<0.01; middle group: −45.7±1.1 vs. −51.5±1.4 mV, P<0.05; Table 1). There was no significant difference (P>0.05) in RMP between cells in the outer group of cells in HO-2-KO mice (−45.5±0.8 mV) and in WT (−47.5±1.2 mV). The slope of the mean regression line in HO-2-KO mice was 0.01 ± 0.1 mV/10 μm (Fig. 3), which was not significantly different from 0 slope (P>0.05).
Figure 3.
Transwall gradient was absent in HO-2-KO mice. Slope of the mean regression line in HO-2-KO mice (0.01±0.1 mV/10 μm) was not significantly different from 0 slope (P>0.01).
HO-2 immunoreactivity (HO-2-IR)
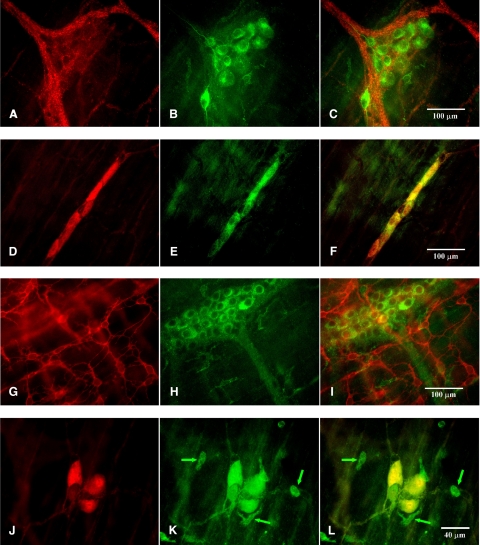
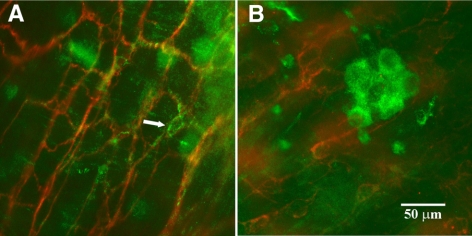
The majority of cells containing HO-2-IR were observed in the myenteric plexus and submucous plexus of colonic preparations, and these cells had an appearance consistent with enteric neurons (n=4 animals, Fig. 4B, E, H, K).
Figure 4.
HO-2-IR was found in myenteric ganglia (A–C) and submucous ganglia (D–F) but was not found in cells that contained ACK2-IR (G–I) in colonic preparations. A) PGP 9.5 IR in a myenteric ganglion. B) HO-2-IR in the same ganglion as in A. C) Superimposed images from A and B. D) Submucous ganglion with PGP 9.5 IR. E) Same region with HO-2-IR. F) Superimposed images from D and E. G) ACK2-IR in the myenteric plexus region. H) Same region with HO-2-IR. I) Superimposed images from G and H. J) Submucosal border region with a PGP 9.5 IR containing submucous ganglion. K) Same region with HO-2-IR. L) Superimposed images from J and K. There were some single cells (arrows in J, K) that contained HO-2-IR.
Colonic preparations with ACK2 labeling clearly showed ACK2 immunoreactivity (ACK2-IR) networks in the myenteric and submucosal regions and long spindle-shaped ACK2-IR-containing cells between these two networks consistent with the known distribution of ICCs in the mouse colon. In contrast to the small intestine, where HO-2 was colocalized with ACK2 (Fig. 5A), double labeling with HO-2 and ACK2 in colonic preparations showed that none of the ACK2-IR-containing cells and their processes contained HO-2-IR (Figs. 4G–I and 5B).
Figure 5.
Colocalization of HO-2-IR and ACK2-IR was found in jejunal preparations but not in colonic preparations. A) HO-2-IR (green) was colocalized with ACK2-IR (red) positive structures (arrow) in the myenteric plexus area of a jejunal preparation. B) ACK2-IR-positive ICCs of the submucosa and HO-2-IR-positive cells in submucosal area of a colonic preparation. There was no colocalization of HO-2-IR and ACK2-IR.
Double labeling with HO-2 and PGP 9.5 in colonic preparations showed that numerous double-labeled cells were present in the myenteric plexus (Fig. 4A–C) and in the submucous plexus (Fig. 4D–F, J–L). HO-2-IR-containing axons could be seen rising from HO-2-IR-containing cell bodies (Fig. 4B) that were also PGP 9.5 positive. There were a few single oval or irregularly shaped cells that were immunolabeled for HO-2-IR, but they were negative for PGP 9.5-IR (Fig. 4J–L). Most of these cells were found in the submucosal border region. It is likely that these cells were macrophages, as they were immunopositive for CD45 and F4/80 (unpublished results).
Effect of removing the submucosa
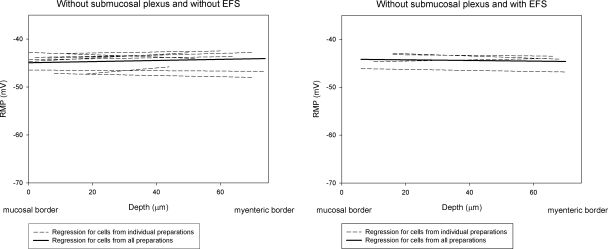
Because the inner group of smooth muscle cells adjacent to the submucosa was the most hyperpolarized region of the circular muscle layer, and since most neurons in submucous plexuses had HO-2-IR, we developed a dissection procedure to obtain strips of circular muscle without the mucosa and submucosa. Sections of tissue stained with PGP 9.5 and examined microscopically indicated that the submucosal plexus was removed. Eighty-eight cells were recorded from 9 preparations. The mean RMP for all recorded cells across the circular muscle layer was significantly depolarized compared to muscle cells in preparations in which the submucosa were left attached (−44.6±0.6 vs. −50.7±1.1 mV, P<0.01; Table 1). The mean RMP for smooth muscle cells in the inner group and middle group was −44.6 ± 0.6 and −44.9 ± 0.7 mV, respectively. Both were significantly (P<0.01) depolarized compared with RMPs observed in preparations with attached submucosa (Table 1). Removing the submucosa, including the submucosal plexus also depolarized on RMPs in cells in the outer group (P<0.05; Table 1). The slope of the mean regression line was 0.12 ± 0.08 mV/10 μm (Fig. 6), which was not significantly different from the 0 slope (P>0.05). Thus, removing the submucosa, including the submucosal plexus, abolished the transwall gradient.
Figure 6.
Removal of the submucosa abolished the transwall gradient in WT mice. Slope of mean regression line in WT mouse colon without the submucosa was 0.12 ± 0.08 mV/10 μm (n=9), which was not significantly different from 0 slope (P>0.05). Transwall gradient was still absent during repetitive EFS to enhance the activity of myenteric ganglion neurons. With repetitive EFS, slope of the mean regression line was −0.06 ± 0.07 mV/10 μm (n=4, P>0.05, compared to 0 slope).
The data above show that although HO-2-IR was found in myenteric ganglion neurons, there was no evidence to suggest that CO was released from HO-2 containing myenteric ganglion neurons to cause hyperpolarization of outer circular smooth muscle cells. To test whether enhancing the activity of myenteric ganglion neurons could induce CO release from HO-2 containing myenteric ganglion neurons, we performed a set of experiments using repetitive EFS to excite myenteric ganglion neurons. Membrane potentials of circular smooth muscle cells were recorded with repetitive EFS (0.3 ms, 100 V, 0.1 Hz, up to 2 h) in 4 WT mouse colonic preparations without the submucosa. In all recorded cells, an inhibitory junction potential was evoked with every pulse of EFS throughout the entire recording period, indicating that the parameters of EFS that we used were sufficient to evoke activity in myenteric ganglion neurons. The slope of the mean regression line was −0.06 ± 0.07 mV/10 μm with the EFS (Fig. 6). Thus, with repetitive EFS, the gradient was still absent (P>0.05, compared to 0 slope). With repetitive EFS, the mean RMP for smooth muscle cells in the outer circular muscle was −44.4 ± 0.9 mV, which was not significantly different from the mean RMP without EFS (−44.4±0.6 mV, P>0.05; Table 1). These results suggest that the gradient was not due to the absence of activity in myenteric ganglion neurons and that activation of myenteric ganglion neurons by EFS did not release CO from myenteric neurons to hyperpolarize the membrane potential in outer circular smooth muscle cells.
DISCUSSION
The results of this study show that a transwall gradient in RMP exists across the circular muscle layer of the murine colon, similar to the gradient that exists in the canine colon (4–6, 8, 9). In the murine colon, circular smooth muscle cells near the submucosa were 6 mV more hyperpolarized compared with RMPs of circular smooth muscle cells in the myenteric region, and the slope of the transwall gradient was 0.6 ± 0.1 mV/10 μm. In the dog colon in vitro, smooth muscle cells adjacent to the submucous plexus are 23 mV to 36 mV more hyperpolarized compared with RMPs of muscle cells in the myenteric region, and the slope of the transwall gradient ranges from 0.21 to 0.24 mV/10 μm (4–6, 9). Taking into account the difference in thickness of the circular muscle layer in the two species and the possibility that CO is generated in and released only from submucosal ganglion neurons in the dog colon as in the mouse colon suggest that the further away smooth muscles cells are from the site of CO production, the more depolarized their RMP.
Our results also show that, as in the stomach and small intestine, the transwall gradient in the mouse colon depends on the generation and release of CO within the muscle wall. A number of observations support this conclusion. First, oxyhemoglobin abolished the transwall gradient. Although oxyhemoglobin scavenges CO and NO, the failure of l-NNA to abolish the gradient suggests that the loss of the gradient in the presence of oxyhemoglobin was due to the removal of endogenously produced CO rather than of NO. In previous reports, it was found that inhibition of NO synthase activity causes a depolarization of the RMP of mouse colonic circular smooth (15, 24). However, in our study, we found that l-NNA depolarized the RMP by only 2 mV. This difference between our results and those in the other reports might be due to different experimental procedures. In our experiments, l-NNA was applied to the tissue 45 min before recordings were made and was maintained throughout the recording period eliminating any possible transient/short-term changes in RMP, whereas in the previous experiments, recordings of membrane potentials were made during application of l-NNA (15, 16). As NO and CO are cotransmitters (13, 25), the sudden and immediate loss of NO might affect release of CO in the short term. Second, CrMP, a selective inhibitor of HO activity (23), abolished the transwall gradient. Third, the gradient was abolished in HO-2-KO mice. Identical results were found in the stomach and jejunum of the mouse where CrMP and the genomic deletion of HO-2 abolished the gradient (11, 12).
Transwall gradients in RMPs also exist in the dog (1, 2), mouse (11), and human antrum (Anthony J. Bauer, University of Pittsburgh, Pittsburgh, PA, USA; personal communication, January 15, 2010), and in the cat (10), dog (3), human (3), and mouse (12) small intestine. However, the gradient in all of these regions, when compared to the one in the colon wall, is reversed where circular smooth muscle cells near the submucosa are more depolarized compared to muscle cells in the myenteric region. The mechanism by which the transwall gradient is established has been studied in the mouse small intestine (12). In the mouse small intestine, the generation and release of CO, which maintains the gradient, are due to the activity of HO-2 in myenteric ICCs, and the gradient is not dependent on nerve activity (12). Unlike in the small intestine, in the colon of WT mice, the results of the present study show that HO-2-IR and ACK2-IR were not colocalized, suggesting that ICCs were not the source of CO. Instead, numerous HO-2-IR-containing cells that were also positive for PGP 9.5 were found in submucosal ganglia. In a previous study, we measured the amount of CO produced in the dog colon and found that the region of the circular muscle layer where the submucosal plexus resides has the highest HO activity (11). Given these observations and the finding in the present study that the majority of submucous neurons had HO-2-IR, we suggest that submucosal ganglion neurons in the WT mouse colon were the main source of endogenously produced CO. This idea is supported by our results that showed that TTX depolarized the RMP in the inner and middle group of muscle cells in the mouse colon and abolished the transwall gradient indicating that, in the mouse colon, CO release was nerve activity dependent. The neural source of CO appeared to be submucosal ganglion neurons because removal of the submucosal plexus abolished the transwall gradient. Thus, unlike in the mouse stomach and small intestine, where the transwall gradient is due to the generation and focal release of CO from myenteric ICCs, the transwall gradient in the colon is due to the generation and focal release of CO from submucosal ganglion neurons.
Although HO-2-IR was found in PGP 9.5-positive cells in the myenteric plexus, there was no effect of oxyhemoglobin and no effect of CrMP on RMPs of cells in the outer group of muscle cells adjacent to the myenteric plexus. Thus, although neurons in both major plexi contain HO-2, it appears that the principle source of endogenously generated and released CO in the muscle wall of the colon is from ganglion neurons in submucous plexus. This supposition receives support from our previous studies in the dog colon (11). In the dog colon, where the polarity of the gradient is identical to that in the mouse colon, HO activity in the inner half of the circular muscle layer is significantly greater than it is in the outer half of the circular muscle layer. Why CO generated from the myenteric neurons does not reach the smooth muscle layer is not known. Whether myenteric neurons make but do not release CO or whether CO is released from myenteric neuronal cell bodies but not processes will require further investigation.
Studies of the human colon found that HO-2-IR and ACK2-IR are colocalized (26). We did not find in our studies colocalization of HO-2-IR and ACK2-IR in the mouse colon.
There remains the question of how CO hyperpolarizes the RMP of circular smooth muscle cells in the colon. It is known that like NO, CO increases soluble guanylyl cyclase activity to stimulate the formation of cGMP (25, 27, 28), which, in turn, can act on several ion channels. CO also can directly increase the open probability of KCa channels and increase the activity of other families of K+ channels (29–32). Our previous study in mouse small intestine showed that inhibition of soluble guanylyl cyclase activity by 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) had no effect on the transwall gradient, suggesting that the maintenance of the transwall gradient in the mouse small intestine is cGMP independent (12). In the dog small intestine, CO was found to significantly increase whole cell K+ current and to hyperpolarize the RMP (33). In the dog colon, it has been suggested that the voltage gradient may be related to the activity of a Na+, K+ ATPase electrogenic pump (34, 35) or to an inward rectifying K+ current (36). It has also been reported that the Na+, K+ ATPase pump is a site of CO action for long-term modulation for cellular activity (37). These data suggest that CO may act directly on K+ channels and may also act to stimulate the activity of the electrogenic Na+, K+ ATPase pump. The exact mechanism of action by which CO maintains the transwall gradient in the colon and the rest of the gastrointestinal tract remains to be determined.
Although we did not study the functional significance of the absence of the gradient in the colon of HO-2-KO mice, it is reasonable to suggest that colonic motility is adversely affected. In HO-2-KO mice, transit time is significantly longer than transit time in WT mice (25). In patients with diabetic gastroparesis, HO-2 immunoreactivity in the stomach wall is significantly reduced and gastric emptying is significantly slowed (38).
In summary, the results of this study show for the first time that a transwall gradient in RMP exists across the circular muscle layer of the murine colon and that endogenously generated CO establishes and maintains the voltage gradient. Thus, in all species examined, a CO-dependent voltage gradient exists across the circular muscle layer throughout the gastrointestinal tract. However, unlike in the stomach and small intestine, the source of CO in the colon does not appear to be ICCs. Rather, it appears that CO is generated and released primarily from submucosal ganglion neurons.
Acknowledgments
This work is supported by U.S. National Institutes of Health grant NIH DK17238. The authors thank Dr. Solomon Snyder (Johns Hopkins University, Baltimore, MD, USA) for providing HO-2-KO mice and the antiserum used in this work. The authors thank Mr. William Harmsen for statistical analysis and Jan Applequist for her help in preparing this manuscript.
REFERENCES
- 1.Bauer A. J., Reed J. B., Sanders K. M. (1985) Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J. Physiol. 366, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer A. J., Sanders K. M. (1985) Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J. Physiol. 369, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara Y., Kubota M., Szurszewski J. H. (1986) Electrophysiology of smooth muscle of the small intestine of some mammals. J. Physiol. 372, 501–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L. W., Huizinga J. D. (1993) Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J. Physiol. 470, 445–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith T. K., Reed J. B., Sanders K. M. (1987) Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am. J. Physiol. Cell Physiol. 252, C290–C299 [DOI] [PubMed] [Google Scholar]
- 6.Smith T. K., Reed J. B., Sanders K. M. (1987) Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am. J. Physiol. Cell Physiol. 252, C215–C224 [DOI] [PubMed] [Google Scholar]
- 7.Szurszewski J. H. (1987) Electrical basis for gastrointestinal motility. In Physiology of the Gastrointestinal Tract (Johnson L. R., Christensen J., Jackson M. J., Jacobson E. D., Walsh J. H. eds) pp. 383–422, Raven Press, New York [Google Scholar]
- 8.Serio R., Barajas-Lopez C., Daniel E. E., Berezin I., Huizinga J. D. (1991) Slow-wave activity in colon: role of network of submucosal interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Physiol. 260, G636–G645 [DOI] [PubMed] [Google Scholar]
- 9.Smith T. K., Reed J. B., Sanders K. M. (1988) Effects of membrane potential on electrical slow waves of canine proximal colon. Am. J. Physiol. Cell Physiol. 255, C828–C834 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki N., Prosser C. L., Dahms V. (1986) Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am. J. Physiol. Gastrointest. Physiol. 250, G287–G294 [DOI] [PubMed] [Google Scholar]
- 11.Farrugia G., Lei S., Lin X., Miller S. M., Nath K. A., Ferris C. D., Levitt M., Szurszewski J. H. (2003) A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 100, 8567–8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha L., Farrugia G., Harmsen W. S., Szurszewski J. H. (2007) Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am. J. Physiol. Gastrointest. Physiol. 293, G438–G445 [DOI] [PubMed] [Google Scholar]
- 13.Xue L., Farrugia G., Miller S. M., Ferris C. D., Snyder S. H., Szurszewski J. H. (2000) Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc. Natl. Acad. Sci. U. S. A. 97, 1851–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bywater R. A., Small R. C., Taylor G. S. (1989) Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J. Physiol. 413, 505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyster D. J., Bywater R. A., Taylor G. S. (1995) Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology 108, 1371–1378 [DOI] [PubMed] [Google Scholar]
- 16.Spencer N. J., Bywater R. A., Taylor G. S. (1998) Disinhibition during myoelectric complexes in the mouse colon. J. Auton. Nerv. Syst. 71, 37–47 [DOI] [PubMed] [Google Scholar]
- 17.Sha L., Farrugia G., Szurszewski J. H. (2005) Oxyhemoglobin abolishes the membrane potential gradient across the mouse intestine (abstract). Gastroenterology 128(Suppl. 2), A-629 [Google Scholar]
- 18.Poss K. D., Thomas M. J., Ebralidze A. K., O'Dell T. J., Tonegawa S. (1995) Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron 15, 867–873 [DOI] [PubMed] [Google Scholar]
- 19.Diggle P. J., Liang K. Y., Zeger S. L. (1994) Analysis of Longitudinal Data, Oxford University Press, New York [Google Scholar]
- 20.Piantadosi C. A. (2002) Carbon monoxide poisoning. N. Engl. J. Med. 347, 1054–1055 [DOI] [PubMed] [Google Scholar]
- 21.Doherty D. H., Doyle M. P., Curry S. R., Vali R. J., Fattor T. J., Olson J. S., Lemon D. D. (1998) Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 16, 672–676 [DOI] [PubMed] [Google Scholar]
- 22.Shah V., Lyford G., Gores G., Farrugia G. (2004) Nitric oxide in gastrointestinal health and disease. Gastroenterology 126, 903–913 [DOI] [PubMed] [Google Scholar]
- 23.Appleton S. D., Chretien M. L., McLaughlin B. E., Vreman H. J., Stevenson D. K., Brien J. F., Nakatsu K., Maurice D. H., Marks G. S. (1999) Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab. Dispos. 27, 1214–1219 [PubMed] [Google Scholar]
- 24.Spencer N. J., Bywater R. A., Taylor G. S. (1998) Evidence that myoelectric complexes in the isolated mouse colon may not be of myogenic origin. Neurosci. Lett. 250, 153–156 [DOI] [PubMed] [Google Scholar]
- 25.Zakhary R., Poss K. D., Jaffrey S. R., Ferris C. D., Tonegawa S., Snyder S. H. (1997) Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. U. S. A. 94, 14848–14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piotrowska A. P., Solari V., De C. D., Puri P. (2003) Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J. Pediatr. Surg. 38, 73–77 [DOI] [PubMed] [Google Scholar]
- 27.Ingi T., Cheng J., Ronnett G. V. (1996) Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron 16, 835–842 [DOI] [PubMed] [Google Scholar]
- 28.Kharitonov V. G., Sharma V. S., Pilz R. B., Magde D., Koesling D. (1995) Basis of guanylate cyclase activation by carbon monoxide. Proc. Natl. Acad. Sci. U. S. A. 92, 2568–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Mount D. B., Nasjletti A., Wang W. (1999) Carbon monoxide stimulates the apical 70-pS K+ channel of the rat thick ascending limb. J. Clin. Invest. 103, 963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X. D., Santarelli L. C., Heinemann S. H., Hoshi T. (2004) Metabolic regulation of potassium channels. Annu. Rev. Physiol. 66, 131–159 [DOI] [PubMed] [Google Scholar]
- 31.Wang R., Wu L., Wang Z. (1997) The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflügers Arch. 434, 285–291 [DOI] [PubMed] [Google Scholar]
- 32.Xi Q., Tcheranova D., Parfenova H., Horowitz B., Leffler C. W., Jaggar J. H. (2004) Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am. J. Physiol. Heart Circ. Physiol. 286, H610–H618 [DOI] [PubMed] [Google Scholar]
- 33.Farrugia G., Irons W. A., Rae J. L., Sarr M. G., Szurszewski J. H. (1993) Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am. J. Physiol. Gastrointest. Physiol. 264, G1184–G1189 [DOI] [PubMed] [Google Scholar]
- 34.Burke E. P., Reed J. B., Sanders K. M. (1988) Role of sodium pump in membrane potential gradient of canine proximal colon. Am. J. Physiol. Cell Physiol. 254, C475–C483 [DOI] [PubMed] [Google Scholar]
- 35.Burke E. P., Sanders K. M., Horowitz B. (1991) Sodium pump isozymes are differentially expressed in electrically dissimilar regions of colonic circular smooth muscle. Proc. Natl. Acad. Sci. U. S. A. 88, 2370–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn E. R., McManus C. A., Bradley K. K., Koh S. D., Hegarty T. M., Horowitz B., Sanders K. M. (1999) Inward rectifier potassium conductance regulates membrane potential of canine colonic smooth muscle. J. Physiol. 518, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathanson J. A., Scavone C., Scanlon C., McKee M. (1995) The cellular Na+ pump as a site of action for carbon monoxide and glutamate: a mechanism for long-term modulation of cellular activity. Neuron 14, 781–794 [DOI] [PubMed] [Google Scholar]
- 38.Pasricha P. J., Pehlivanov N. D., Gomez G., Vittal H., Lurken M. S., Farrugia G. (2008) Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]