Abstract
The purpose of this study was to investigate the role of the mutant CUGn RNA in the induction of stress in type 1 myotonic dystrophy (DM1) cells and in the stress-mediated inhibition of protein translation in DM1. To achieve our goals, we performed HPLC-based purification of stress granules (SGs), immunoanalysis of SGs with stress markers TIA-1, CUGBP1, and ph-eIF2, site-specific mutagenesis, and examinations of RNA-protein and protein-protein interactions in myoblasts from control and DM1 patients. The cause-and-effect relationships were addressed in stable cells expressing mutant CUG repeats. We found that the mutant CUGn RNA induces formation of SGs through the increase of the double-stranded RNA-dependent protein kinase (PKR) and following inactivation of eIF2α, one of the substrates of PKR. We show that SGs trap mRNA coding for the DNA repair and remodeling factor MRG15 (MORF4L1), translation of which is regulated by CUGBP1. As the result of the trapping, the levels of MRG15 are reduced in DM1 cells and in CUG-expressing cells. These data show that CUG repeats cause stress in DM1 through the PKR-ph-eIF2α pathway inhibiting translation of certain mRNAs, such as MRG15 mRNA. The repression of protein translation by stress might contribute to the progressive muscle loss in DM1.—Huichalaf, C., Sakai, K., Jin, B., Jones, K., Wang, G.-L., Schoser, B., Schneider-Gold, C., Sarkar, P., Pereira-Smith, O. M., Timchenko, N., Lubov, T. Expansion of CUG RNA repeats causes stress and inhibition of translation in myotonic dystrophy 1 (DM1) cells.
Keywords: PKR, CUG-binding protein, CUGBP1, MRG15/MORF
Type 1 myotonic dystrophy (DM1) is a multisystem disorder (1) caused by an expansion of CTG repeats in the 3′ UTR of the DMPK gene on chromosome 19q (2–7). CTG repeats cause the disease through accumulation of noncoding CUGn RNA (8–11). CUG repeats alter levels of RNA-CUG-binding proteins, CUGBP1 and MBNL1, misregulating translation and splicing (12–24). The elevation of CUGBP1 in DM1 mouse models delays muscle development and differentiation (20, 21) and causes muscular dystrophy (20, 22) and myotonia (22). Reduced levels of MBNL1 lead to muscular dystrophy, myotonia, cardiac abnormalities, and cataracts (19).
It was recently demonstrated that expression of the mutant 3′ UTR of DMPK in DM1 mouse models causes progressive muscle wasting (23); however, the mechanisms by which the mutant 3′ UTR causes muscle loss are not well understood. A number of publications have suggested that the muscle loss in DM1 patients might be associated with a reduction in translation due to stress caused by CUGn RNA (25–27). CUG-expressing cells increase proteins that are markers of oxidative and endoplasmic reticulum (ER) stresses (25–27). Because stress inactivates eIF2α, a major regulator of initiation of translation (reviewed in 28), it is possible that a stressful environment in DM1 cytoplasm inhibits translation of mRNAs leading to muscle weakness and wasting. In addition, it was found that CUGn RNA activates PKR kinase (29), which inactivates eIF2α by phosphorylation at Ser-51 (28). The ph-S51-eIF2α has been identified in DM1 atrophic fibers (25), suggesting that protein translation is reduced in DM1 atrophic fibers contributing to muscle atrophy.
Initiation of translation starts when eIF2 forms a complex with GTP and when eIF2-GTP forms a tertiary complex with Met-tRNA (28). It has been found that stress leads to the phosphorylation of eIF2α, inhibiting the GDP-GTP exchange by reducing the dissociation rate of eIF2B (28). As a result, the levels of active eIF2-GTP-tRNAMet complex are reduced, and the amounts of inactive translation initiation complexes are increased. It has been shown that the inactive translation initiation complexes form SGs (30). The formation of SGs is initiated by the phosphorylation of eIF2α (31). Cytoplasmic SGs are usually formed in cells subjected to a variety of stresses (heat shock, oxidative stress, UV irradiation, and osmotic shock) (30–32). The major components of SGs are RNA-binding protein, TIA-1 (T cell internal antigen-1), and its related protein, TIAR. TIA-1/TIAR proteins control translation during development and in stressed cells. In SGs, TIA proteins bind to and prevent mRNAs from being translated on polysomes (33). In addition to TIA proteins, SGs also contain small ribosomal subunits, eIF factors (eIF3, eIFE, and eIF4G), and RNA-binding proteins (HuR, HuD, Staufen, TTP, PABP) that regulate the stability of mRNAs (30).
A recent report has shown that CUGBP1 binds directly to TIA-1 translocating to SGs in stressed cells (34). The localization of CUGBP1 in cytoplasmic SGs suggested that CUGBP1 might inhibit translation of certain mRNAs through association with inactive translational complexes in SGs. CUGBP1 is a multifunctional RNA-binding protein that regulates translation (16, 17, 20, 24, 35) and RNA stability (36–38) in cytoplasm and splicing in nuclei (11, 18). The active form of CUGBP1 (ph-S302-CUGBP1) promotes translation via interactions with eIF2α and bringing the eIF2 complex to the 5′ regions of mRNAs (39, 40). CUGBP1 also activates translation through a cap-independent mechanism by recruiting the initiation translation complex to the internal ribosome entry site (35). The specificity of RNA binding of CUGBP1 is regulated by phosphorylation (24). Phosphorylation also controls interactions of CUGBP1 with eIF2 (24, 39, 40).
In this report, we have examined the hypothesis that the expansion of the mutant CUG RNA might induce stress and inhibit translation of proteins. We have identified cytoplasmic aggregates containing TIA-1 and CUGBP1 proteins in DM1 myoblasts and in CHO cells expressing the mutant CUG RNA (CUG914). Cytoplasmic aggregates in the CUG-expressing cells contain a significant amount of mRNA, encoding the DNA repair and remodeling factor MRG15 (MORF4L1) (41, 42), one of the translational targets of CUGBP1. We found that MRG15 protein levels are reduced in DM1 myoblasts and in stable clones expressing CUG repeats. These data suggest that CUG-mediated stress inhibits translation of mRNAs associated with cytoplasmic SGs.
MATERIALS AND METHODS
Plasmids
Wild-type GFP-CUGBP1 was generated by cloning of the PCR product produced with specific primers covering the full-length open reading frame of CUGBP1 (1.5 kb) into the HindIII site of pEGFP-C1. The GFP-CUGBP1-S302G and S28A mutants were generated by the cloning of the corresponding PCR products with S302G and S28A mutations (24) into the HindIII site of pEGFP-C1. The constructs were verified by sequencing.
To generate TRE-GFP-CUG914 plasmid, the long CTG/CAG duplexes were made using a modified protocol described previously (43). In brief, equimolar amounts of CTG15 and CAG15 oligos were mixed and amplified by PCR using Pfx platinum DNA polymerase (Invitrogen, Carlsbad, CA, USA). The CTG/CAG duplexes were fractionated by gel electrophoresis, and the DNA fragment located in the region of 1 kb was purified, phosphorylated with T4 PNK (New England Biolabs, Ipswich, MA, USA), and cloned into the EcoRV site of pcDNA3.1-Hygro (Invitrogen). The CTG sequence in the resulting plasmid was determined by digesting the plasmid with PmeI and by sequencing from both ends with T7 and T3 primers producing pure CTG repeats. The CTG914 fragment was cut out from pcDNA3.1 recombinant plasmid with MscI, cloned into EcoRV site of pTRE-Tight-BI-AcGFP vector and resequenced. The resulting plasmid DNA expresses GFP and CUG914 RNA from the CMV promoters regulated by doxycyclin (Dox) (see Fig. 2A). As the result, each transfected cell is green and expresses CUG914 repeats.
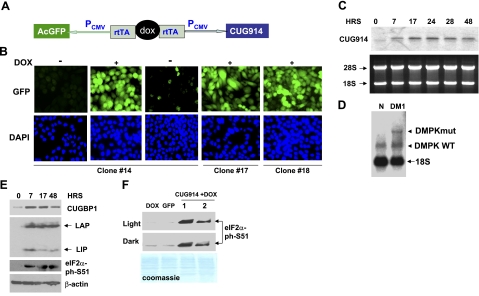
Figure 2.
Expression of the mutant RNA CUG914 in CHO double-stable cells causes DM1-like molecular abnormalities. A) Structure of the inducible TRE-GFP-CUG914 construct that was integrated into CHO cells. B) Fluorescence images of inducible CHO TRE-GFP-CUG914 clones expressing GFP and CUG914 are shown. Three clones were induced with Dox, and the expression of GFP was monitored by IF analysis. Images of the uninduced clone 14 following Dox addition and withdrawal are shown. Images of cells from clones 17 and 18 after induction with Dox are also shown. Nuclei were stained with DAPI (blue). C) Northern blot analysis of RNA CUG914 in the inducible CHO cells. RNA was extracted from cells at different time points (0, 7, 17, 24, 28, and 48 h) after Dox addition. RNA (5 μg) was separated by gel-electrophoresis, transferred on membrane, and hybridized with CAG10 probe. Gel was stained with ethidium bromide before transfer to verify RNA loading. Positions of 28S and 18S rRNAs are shown on the left. D) Northern blot analysis of the expression of endogenous DMPK mRNA in myoblasts from control and DM1 patient. Total RNA (5 μg) was used for hybridization with the CAG10 probe under conditions described above. Positions WT (5 CUG repeats) and mutant (320 CUG repeats) DMPK mRNAs are shown. Probe interacts with 18S rRNA, which serves as an internal control. E) Expression of RNA CUG914 causes alterations in protein expression that are identical to those observed in DM1 patients. Cytoplasmic and nuclear extracts were prepared from inducible CUG914-expressing cells at different time points and examined by Western blotting with antibodies to CUGBP1 (cytoplasm), C/EBPβ (nucleus), ph-S51-eIF2α (cytoplasm), and β-actin (cytoplasm). F) Phosphorylation of eIF2α at S51 is caused by CUG repeats. Western blotting was performed with cytoplasmic extracts from unmodified, regular CHO cells treated with Dox as control, from a stable clone expressing GFP only and from CUG914 stable clone treated with Dox (2 protein preparations). Dark and light exposures are shown. Membrane was stained with Coomassie blue to verify protein loading.
Transient transfections
Transient transfections and nucleofections of human myoblasts and HeLa cells were performed with FuGene (Roche Diagnostics, Mannheim, Germany) or Amaxa Biosynthesis Cell Line nucleofector kit (Amaxa, Gaithersburg, MD, USA) according to the manufacturers' protocols. The efficiency of transfection with FuGene was varied from 35 to 48% in HeLa cells and from 8 to 12% in human primary myoblasts. For nucleofection, human myoblasts grown on 5- × 10-cm plates with 60% of density (∼5×105 cells) were used for a single nucleofection, resulting in 1- × 10-cm plate containing 105 transfected cells. The efficiency of nucleofection for human myoblasts varied from 60 to 75%. The efficiency of nucleofection for HeLa cells was ∼60%.
Generation of tet-on double stable clones expressing mutant CUG RNA
Stable CHO-tTA cells (Clontech Laboratories, Mountain View, CA, USA) were transfected with TRE-GFP-CTG914 plasmid using FuGene. Control cells were transfected with empty vector. Optimal conditions for cell growth, selection, and Dox-inducible transcription were developed using the luc-expressing plasmid. Transfected cells were maintained for 2 wk in media containing hygromycin B (400 μg/ml). Cells resistant to hygromycin were incubated with Dox (300–500 ng/ml) for 24–36 h until GFP was induced and then sorted at the Baylor College of Medicine Cell Culture Sorting Facility. Individual cells were grown in 96-well dishes, expanded, and retested with Dox to identify cell lines with the tight regulation of expression by Dox assessed by the fluorescence analysis (intensity of GFP) and expression of CUG914 RNA (Northern blotting).
Separation of cytoplasmic SGs by gel-filtration chromatography
The double-stable CHO cells, expressing CUG914, were grown in DMEM containing 10% tet-free FBS (Clontech Laboratories, Mountain View, CA, USA) supplemented with geneticin and hygromycin B to 80% density. Cytoplasm fractions were collected from cells in 100-mm plates either without addition of Dox or maintained for 24 h in medium containing Dox (500 ng/ml). Expression of GFP and CUG914 RNA was verified by fluorescence analysis and by Northern blotting assay, respectively. Cytoplasmic extracts were subjected to size-exclusion chromatography on a SEC400 column precalibrated with molecular weight markers under conditions allowing separation of large aggregates from free RNAs and RNA-protein complexes. These conditions are described in our previous publications (16, 39, 40). The chromatography fractions (300 μl) were divided equally for protein and RNA analyses and kept frozen prior to study.
Western blotting
Human control primary myoblast cultures were established from muscle biopsies derived from patients with normal histological and biochemical findings. DM1 myoblasts were derived from a patient heterozygous for DM1 mutation containing 320 CTG repeats on the mutant allele of the DMPK gene. Control and DM1 biopsies were derived from biceps brachii. Human primary myoblasts were grown no more than 12 passages.
Western blot analyses were performed with cytoplasmic and nuclear proteins as described previously (9, 10, 16). Fifty to 100 μg of proteins were separated by gel-electrophoresis, transferred on membrane, and incubated with different antibodies. Mouse monoclonal anti-CUGBP1 (3B1), anti-TIA-1 (sc-48371), anti-PKR (B-10), polyclonal anti-eIF2α (FL-315), ph-eIF2α (S51, sc12412-R), and anti-MRG15 (F-19) were from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Monoclonal anti-β-actin antibodies (AC-15) were from Sigma-Aldrich (St. Louis, MO, USA). Note that the TIA-1 was detected as doublet or even triplet band in the gel filtration fractions suggesting modifications of this protein.
Immunofluorescence (IF) analysis
Human control and DM1 myoblasts were grown to 60% density as described previously (17). Cells were fixed with 3.7% formaldehyde, prepared by the dilution of 37% formaldehyde (Sigma) with 1× PBS. Cells were blocked in 1× PBS containing 0.2% normal goat serum and then incubated with polyclonal antibodies to TIA-1 (1:100) (H-120; Santa Cruz) following incubation with secondary goat anti-rabbit antibodies labeled with Texas Red (TR). For double IF analysis, fixed cells were incubated with a mix of monoclonal anti-CUGBP1 and polyclonal anti-TIA-1 for 1 h. Secondary antibodies were goat anti-rabbit IgGs labeled with TR and goat anti-mouse IgGs labeled with FITC. Nuclei were stained with DAPI. For a combination of FISH and IF, fixed cells were incubated with the mouse anti-CUGBP1 (1:10), followed by incubation with goat anti-mouse antibody labeled with FITC. FISH hybridization was performed with the probe, complementary to the CTG repeats and containing LNA modification to increase sensitivity of the assay (LNA-TYE 563–5′-CAG7-3′) (Exiqon).
UV-cross-link assay
For the UV-cross-link assay, the purified full-length CUGBP1-MBP and CUGBP1 truncated proteins fused with MBP containing different RNA-binding domains (RBDs) of CUGBP1 (RBD1, RBD2, RBD3, RBD1+2) (44) were incubated with a MRG15-specific probe labeled with 32P-γATP. The sequence of the MRG15 RNA probe is shown in Fig. 6B. Where indicated, specific (unlabeled MRG15 RNA) and nonspecific (AU-rich RNA with the sequence 5′- AUAUAUAUAUAUAUAU-3′) competitors were added at concentration 100 ng/μl. Mixtures were incubated for 30 min at room temperature, treated with UV light (125 mJ for 3 min), and then subjected to separation by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane, and the membrane was exposed to X-ray film. After exposure, the membrane was stained with Coomassie blue to verify protein loading.
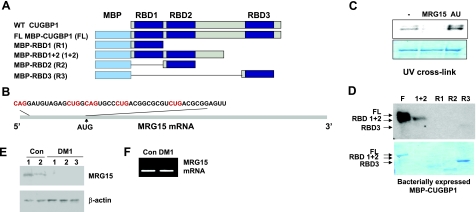
Figure 6.
CUGBP1 interacts with the 5′ region of MRG15 mRNA. A) Structure of the fusion MBP-CUGBP1 proteins used in these studies. B) Nucleotide sequence of the 5′ region of MRG15 mRNA. CUG/CAG repeats are in red. C) Purified MBP-CUGBP1 binds to the 5′ region of MRG15 mRNA. UV cross-link was performed with MRG15 RNA probe and full-length MBP-CUGBP1. Competition with specific RNA (unlabeled MRG15) and nonspecific (AU-rich) is shown. D) CUGBP1 binds to MRG15 RNA mainly through RBD1 + 2 domains. Full-length (FL) and truncated MBP-CUGBP1 proteins (see diagram in A) were used in UV cross-link with MRG15 RNA. E) MRG15 protein, but not mRNA, is reduced in DM1 myoblasts. Western blotting analysis of the protein isolated from control and DM1 myoblasts with MRG15 antibodies. Membrane was reprobed with antibodies to β-actin to verify protein loading. F) Real time RT-PCR of MRG15 mRNA shows the same levels of MRG15 mRNA in control and DM1 myoblasts using GAPDH as a standard.
Northern blot hybridization
Total RNA was isolated from primary myoblasts derived from control and DM1 patients using Trizol. CHO double-stable cells expressing GFP and CUG914 were maintained with and without Dox addition. Total RNA was extracted from the inducible CHO cells at different time points after Dox addition. All RNA samples were examined by gel electrophoresis to assess the quality of RNA. All samples contained undegraded RNA as shown by detection of 18S and 28S RNAs with ethidium bromide staining. Five micrograms of RNA was separated by the agarose gel, transferred onto membrane, and hybridized with a CAG10 probe labeled with γ-32P-ATP.
Real time RT-PCR
RNA was extracted from the gel filtration fractions and examined by real time RT-PCR with primers specific for human MRG15. The sequence of forward primer is 5′-TTGCCTAGTTGACAAAGCTGC-3′; and the sequence of the reverse primer is 5′-ATGACTTGCAAAGGATGGGC-3′. The MRG15 production was normalized by the analysis of GAPDH expression with the following primers: forward, 5′-AACTTTGGCATTGTGGAAGGGCTC-3′, and reverse, 5′-TGGAAGAGTGGGAGTTGCTGTTGA-3′.
RESULTS
DM1 myoblasts contain cytoplasmic TIA-1/CUGBP1-positive SGs
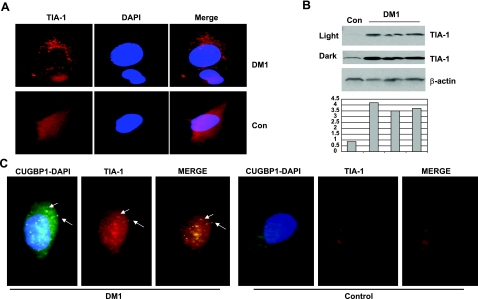
Several publications have shown that the markers of stress are increased in DM1 cells (25–27). A recent study of protein partners of TIA-1 in stressed cells has demonstrated that CUGBP1 directly interacts with TIA-1 protein, a main marker of stress (34). Here we have analyzed cytoplasmic SGs in DM1 myoblasts and asked whether CUGBP1 might be recruited to SGs. We have examined TIA-1 expression in control and DM1 myoblasts by IF analysis under quantitative conditions. We found that, in the majority of normal myoblasts (96% of 147 examined cells), TIA-1 is detected in the diffused form or in the form of small, low-intensity complexes (Fig. 1A). In DM1 myoblasts, however, in addition to the diffused form, TIA-1 is detected in large, high-intensity, cytoplasmic aggregates (Fig. 1A). The majority of DM1 myoblasts (91% of 132 examined cells) contained large TIA-1-positive cytoplasmic aggregates or SGs. To determine whether TIA-1 is elevated in DM1 myoblasts, we have performed Western blotting analysis with TIA-1 antibodies. Examination of cytoplasm of 3 DM1 myoblast lines showed that expression of TIA-1 is 3- to 4-fold increased in DM1 compared to the levels of TIA-1 in control cells (Fig. 1B). Thus, IF and Western blotting assays showed that TIA-1 is increased in DM1 myoblasts and that this increase leads to formation of cytoplasmic aggregates. Because TIA-1-positive SGs are formed in cells in response to environmental stresses, such as heat shock and oxidative stress (31–33), accumulation of TIA-1-containing SGs indicates that stress in DM1 myoblasts is increased.
Figure 1.
The increase of TIA-1-containing SGs in DM1 myoblasts. A) IF images with antibodies to TIA-1 of control and DM1 myoblasts were taken under identical conditions of brightness and time of exposure to allow comparative quantification of the signal. View is ×100. Nuclei were stained with DAPI. B) Examination of TIA-1 levels in DM1 myoblasts by Western blotting assay. Cytoplasmic protein extracts from control and from 3 DM1 myoblast lines were examined by Western blotting with antibodies to TIA-1. The membrane was reprobed with β-actin. Light and dark exposures are shown for TIA-1. Bar graphs show levels of TIA-1 as ratios to β-actin (calculated from the membrane with dark exposure). C) CUGBP1 is a component of the TIA-1-containing SGs in DM1 myoblasts. CUGBP1 was detected in DM1 and control myoblasts by immunostaining with monoclonal antibodies (3B1) and with secondary antibodies labeled by FITC. The same cells were stained with rabbit polyclonal antibodies to TIA-1 and with secondary antibodies labeled with TR. Arrows indicate examples of the cytoplasmic CUGBP1-TIA-1 aggregates in DM1 myoblasts.
To test whether the cytoplasmic TIA-1-positive SGs contain CUGBP1, we performed double IF analyses of myoblasts from control patients and myoblasts from patients with DM1 using antibodies to CUGBP1 and to TIA-1. The analysis of 122 normal myoblasts showed that both CUGBP1 and TIA-1 are present in a dispersed pattern as well as in small complexes (Fig. 1C). However, in DM1 myoblasts (based on the analysis of 131 cells) TIA-1 is also detected in large aggregates that colocalize with CUGBP1 (Fig. 1C). In summary, these data indicate that stress is increased in DM1 myoblasts and that a portion of CUGBP1 is located within cytoplasmic SGs of DM1 myoblasts.
Ectopic expression of the mutant CUG RNA causes formation of cytoplasmic SGs containing TIA-1 and CUGBP1
To test the hypothesis that the mutant CUG RNA causes stress, we have generated double-stable CHO monoclonal cell lines expressing the mutant CUG RNA (CUG914) regulated by Dox and examined whether accumulation of the mutant CUG RNA causes formation of cytoplasmic SGs. In this double-stable monoclonal cell line, noncoding pure CUG repeats are cloned under the CMV promoter activated by tet-responsive element through binding to Dox (Fig. 2A). These cells also express inducible GFP driven by another CMV promoter that is, similar to CUG repeats, activated by the tet-responsive element bound to Dox. Therefore, Dox-inducible transcription of GFP and CUG repeats could be easily monitored by fluorescence analysis of GFP. Forty individual clones expressing GFP/CUG914 were selected by cell sorting, and 10 of them were analyzed in details. Three GFP/CUG914-Dox-inducible cell lines were selected for further studies based on 1) the moderate efficiency of GFP/CUG914 transcription after Dox addition to avoid toxic effects of CUG repeats on cell viability and 2) tight control of GFP/CUG914 transcription by Dox. We have found that the addition of Dox (300–500 ng/ml) in these monoclonal lines leads to expression of GFP/CUG914 RNA in ∼97% of cells (based on the analysis of 500 cells) in 7–24 h, whereas Dox withdrawal silences GFP/CUG914 in 28–48 h (Fig. 2B).
Transcription of CUG914 RNA after Dox addition was determined by Northern blot hybridization with a CAG10 probe. CUG914 repeats are detectable at 7 h after Dox addition, reaching maximal levels at 24 h (Fig. 2C). The expression of CUG914 RNA was reduced 2-fold in 48 h, perhaps as a result of CUG914 decay, because there was no additional stimulation of CUG914 transcription by Dox during the 48-h time period (Fig. 2C). To ensure that the selected clone has the levels of CUG elevation comparable to DM1 myoblasts, we have performed Northern blotting with RNA from control and DM1 myoblasts under conditions identical to those applied for the analysis of CUG expression in the inducible clone. This analysis showed that the intensity of the signals of CUG repeats in stable clone is comparable to the CUG signals in myoblasts from DM1 patient (Fig. 2D).
It has been well documented that the accumulation of the mutant CUG RNA repeats in DM1 myoblasts causes an increase in CUGBP1 levels and disruption of its translational and splicing targets (11, 16, 18, 20–22, 24, 39, 40). Therefore, we have examined whether the accumulation of CUG914 in monoclonal CHO cells causes the same effect and found increases in CUGBP1 levels and that this increase is an early event in the toxicity of CUG914 RNA. Western blotting analysis showed that the increase of CUGBP1 is detected by 7 h after Dox addition, and CUGBP1 is slightly reduced at 48 h, correlating with the reduced expression of CUG914 RNA because of Dox degradation (Fig. 2C, E). Similar to DM1 myoblasts (16), this increase of CUGBP1 by CUG914 RNA causes an increase in translation of the CUGBP1 target, C/EBPβ, leading to the elevation of the full-length and truncated C/EBPβ proteins, LAP and LIP (Fig. 2E). We found that the levels of LAP remained at high levels during the interval from 7 to 48 h after Dox addition despite reduced levels of CUGBP1 at 48 h. It is interesting that LIP was highly induced by the increase of CUGBP1 at 7 h after Dox addition, but then was reduced to low levels. This variability could be due to differences in stability of LAP/LIP proteins and CUG914. These data indicate that the double-stable CHO monoclonal cell line expresses CUG914 under tight control of Dox and that the accumulation of CUG914 RNA causes an early elevation in levels of CUGBP1 and resulting misregulation of translation of CUGBP1 targets.
Data in the literature show that the initiation of stress by heat shock or oxidation leads to accumulation of inactive form of eIF2α, ph-S51-eIF2α, which reduces translation in stressed cells (28). It has been shown that the ectopic expression of eIF2α mutant, Ser-51A-eIF2α, also promotes stress (33). Therefore, we have examined phosphorylation status of eIF2α in the stable clone after initiation of transcription of CUG repeats. Western blotting with phospho-specific Abs showed that the addition of Dox increases phosphorylation of eIF2α (Fig. 2E). This phosphorylation is caused by CUG repeats because Dox treatment of control, uninducible cells or expression of GFP does not change the phosphorylation status of eIF2α (Fig. 2F).
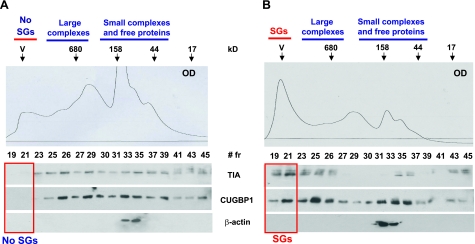
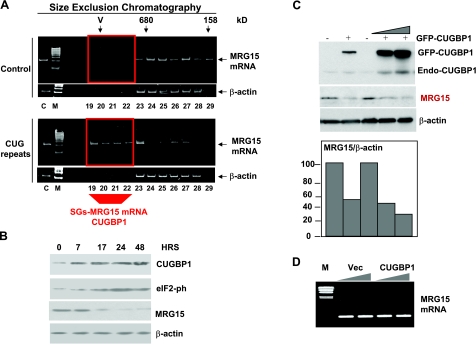
To further confirm that the accumulation of CUG914 causes stress and might increase the formation of SGs, cytoplasmic protein extracts from CHO cells grown with and without Dox were isolated and subjected to HPLC chromatography on size exclusion chromatography. We have used SEC400 size exclusion column (Bio-Rad, Hercules, CA, USA), which separates high-MW complexes and large aggregates. The upper images of Fig. 3 show the principle of separation on this column. Because of large size, SGs are expected to be located in the void volume (V, fractions 19–22), whereas high MW complexes and free RNAs should be located between the void volume and a region corresponding to complexes of 680 kDa. Optical density of the elution fractions was monitored. We found that cells expressing CUG914 RNA contain a strong peak of optical density in the fractions 19–21 (Fig. 3B), and in contrast, uninduced cells show low optical density in this region (Fig. 3A). It has been shown that SGs in stressed cells are detected after centrifugation in a sucrose gradient, as large heavy peaks (45). Thus, we determined whether the accumulation of OD in the fractions 19–21 represents SGs and analyzed the marker of stress (TIA-1 protein) in the chromatography fractions by Western blotting. TIA-1 is almost evenly distributed throughout gel filtration fractions in uninduced cells, and fractions 19–21 do not contain TIA-1 (Fig. 3A). In Dox-induced cells, however, ∼29% of TIA-1 (calculated as the percentage of TIA-1 in all fractions) is shifted to fractions 19–21 (Fig. 3B). Because CUGBP1 is a component of TIA-1-containing SGs (Fig. 1 and ref. 34), we have measured CUGBP1 in these gel filtration fractions. Fractions 19–21 of gel filtration of control cells do not contain CUGBP1 (Fig. 3A); however, after CUG914 expression these fractions accumulate ∼27% of CUGBP1 (calculated as the percentage of total CUGBP1 detected in all fractions; Fig. 3B). The translocation of TIA-1 and CUGBP1 to fractions 19–21 was specific because the reprobing of the membranes with β-actin antibodies showed no change in the distribution of β-actin in cells with or without CUG RNA (Fig. 3, bottom panels). These data reveal that the mutant CUG914 RNA causes stress and formation of TIA-1/CUGBP1 containing SGs.
Figure 3.
Ectopic expression of the mutant CUG914 RNA causes formation of SGs containing CUGBP1 and TIA-1. Cytoplasm from uninduced (A) and induced (B) CHO TRE-GFP-CUG914 cells was subjected to size exclusion chromatography (SEC400 column, HPLC, Bio-Rad) to separate free TIA-1 protein from TIA-1 bound to SGs. Top panels: optical density (OD, A280) profiles of chromatography fractions. Arrows indicate positions of molecular weight markers used for calibration of column. Blue lines indicate chromatography fractions, typically containing small and large complexes and free RNA-binding proteins. Fractions 19–21 contain SGs (marked with red boxes in bottom panels). Note that the OD profile shows that the fractions 19–21 in inducible cells (B) contain large peak in the position of SGs. Bottom panels: expression of CUG914 RNA shifts TIA-1 and CUGBP1 to the region containing SGs (indicated by red box). Chromatography fractions were sequentially analyzed by Western blotting with Abs to TIA-1, CUGBP1, and β-actin. Reprobe of the filters with Abs to β-actin shows that expanded CUG repeats do not change localization of β-actin.
Dephosphorylated isoforms of CUGBP1 are increased in cytoplasmic aggregates
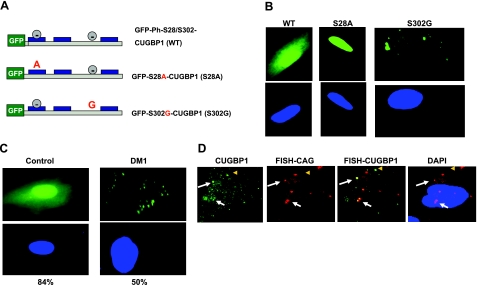
We have shown that CUGBP1, phosphorylated at Ser-302 by cyclin D3/cdk4/6, forms active translational complexes with eukaryotic translation initiation factor eIF2; however, in DM1 myotubes, the levels of cytoplasmic cyclin D3 are reduced leading to accumulation of unphosphorylated isoforms of CUGBP1 (24). We suggested that the unphosphorylated at Ser-302 isoform of CUGBP1 might be a critical regulator of SGs formation. To examine this suggestion, we generated a GFP-CUGBP1-S302G mutant that cannot be phosphorylated at Ser-302 and determined its distribution within control myoblasts. CUGBP1 is also phosphorylated by Akt kinase at Ser-28 (24), and we therefore generated a GFP-S28A mutant that cannot be phosphorylated at Ser-28 (Fig. 4A). Wild-type and CUGBP1 mutants were fused to GFP to monitor their distribution in cells. These GFP-constructs were transfected in control human myoblasts, and their intracellular distribution was examined by fluorescence analysis. We found that wild-type GFP-CUGBP1 is randomly distributed in the cytoplasm and the nucleus (Fig. 4B, C), and this distribution was observed in 97% (of 102 cells) of the control cycling myoblasts. We have found that only a small number of transfected control myoblasts (3%) contained GFP-CUGBP1 exclusively in the nucleus. In the majority of transfected cells (84% of 121 cells), the wild-type GFP-CUGBP1 was distributed in a diffused pattern or present in small complexes (Fig. 4B, C). A quite different picture was observed with the S302G mutant as the majority of transfected cells (∼80% of 108 cells) accumulated GFP-S302G-CUGBP1 in large cytoplasmic aggregates (Fig. 4B), and the location was specific for this mutant. The S28A mutant protein was mainly located in the nucleus and was not associated with cytoplasmic aggregates (Fig. 4B). A very small number of cells transfected with GFP-S28A-CUGBP1 (2% of 135 cells) contained this mutant in nucleus and cytoplasm. These data indicate that the CUGBP1 isoform unphosphorylated at Ser-302 is accumulating in cytoplasmic aggregates, whereas the S28A mutant is located in the nucleus, suggesting that specific phosphorylation of CUGBP1 by Akt at Ser-28 is required for cytoplasmic localization of CUGBP1. This result agrees with observations obtained with DM1 myoblasts in which 50% (of 104 cells) of transfected DM1 myoblasts accumulated GFP-CUGBP1 in large aggregates (Fig. 4C), whereas only 16% (of 121 cells) of myoblasts from control patients showed GFP-CUGBP1-positive aggregates. We suggest that the accumulation of GFP-CUGBP1 in cytoplasmic aggregates or SGs in DM1 myoblasts is associated with the increase of unphosphorylated CUGBP1 at Ser-302 (24).
Figure 4.
CUGBP1-S302G mutant accumulates in cytoplasmic aggregates. A) Structure of wild-type and GFP-CUGBP1 mutants Ser-28A and Ser-302G. Green box indicates GFP fusion part; gray circles indicate sites of phosphorylation; blue boxes indicate RNA binding domains within CUGBP1. B) Intracellular localization of the CUGBP1 molecule with mutant phosphorylation sites. Wild-type, S28A, and S302G mutants of GFP-CUGBP1 were transfected into control human myoblasts, and their intracellular localization was determined by fluorescence analysis. Typical pictures are shown. Green, GFP-CUGBP1; blue, nuclei stained with DAPI. View is ×100. C) Transiently transfected GFP-CUGBP1 localizes in cytoplasmic aggregates of DM1 myoblasts. Control and DM1 myoblasts were transfected with GFP-CUGBP1. Transfected cells were examined by fluorescence analysis. Average percentages of cells containing GFP-CUGBP1-positive aggregates were calculated based on 3 experiments, counting transfected cells as 100%. D) A portion of CUGBP1-positive aggregates in DM1 myoblasts contains DMPK mRNA. Left to right: IF with antibodies to CUGBP1 (green), FISH with CAG probe (red), merge of CUG and CUGBP1 signals, merge of CUG signal and DAPI. White arrows indicate examples of CUGBP1 aggregates containing DMPK mRNA; yellow arrowheads indicate CUGBP1 signals that are not colocalized with DMPK mRNA.
To determine whether endogenous CUGBP1 is associated with SGs in DM1 under physiologically relevant concentrations, we examined CUGBP1 distribution in DM1 myoblasts by IF analysis. We found that the endogenous CUGBP1 forms large complexes in DM1 myoblasts (Fig. 4D). FISH analysis with the CAG probe showed that certain CUGBP1-positive complexes contain DMPK mRNA, whereas significant amounts of CUGBP1 complexes are DMPK mRNA-free (Fig. 4D). Based on data in Figs. 1, 3, and 4B, C, we suggest that these complexes are SGs containing a translation inactive form of CUGBP1 (unph-S302-CUGBP1).
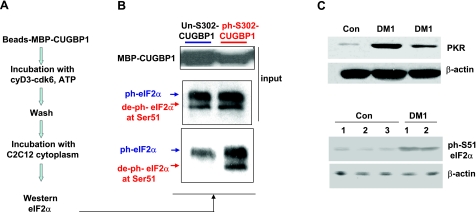
Dephosphorylated CUGBP1 binds to inactive ph-Ser-51-eIF2α
It has been previously shown that cyclin D3-cdk4/6 complexes are key regulators of the interactions of CUGBP1 with eIF2α (39, 40). To determine whether phosphorylation of CUGBP1 by cyclin D3-cdk4/6 regulates the interaction of CUGBP1 with the ph-S51-eIF2α isoform, we performed pulldown experiments with purified CUGBP1, unphosphorylated and phosphorylated at S302 by cyclin D3-cdk6. The strategy for this experiment is outlined in Fig. 5A. Bacterially expressed, purified MBP-CUGBP1 was bound to amylose beads and was subjected to phosphorylation by the purified cyclin D3-cdk6 in the presence of ATP, while control MBP-CUGBP1 was incubated with ATP in the absence of cyclin D3/cdk6. After phosphorylation, amylose beads were extensively washed to remove kinase and ATP, and unphosphorylated and phosphorylated MBP-CUGBP1 were incubated with cytoplasmic proteins from C2C12 myoblasts as source of unphosphorylated and phosphorylated eIF2α. The C2C12 myoblasts were selected for these studies because these cells contain both phosphorylated and unphosphorylated eIF2α (see Fig. 5B, input). After wash, MBP-CUGBP1 interacting proteins were analyzed by Western blotting with antibodies to eIF2α recognizing both active and inactive isoforms. CUGBP1, unphosphorylated by cyclin D3/cdk6, binds to the inactive form of eIF2α (ph-S51-eIF2α) and does not interact with active, dephosphorylated eIF2α (Fig. 5B). However, CUGBP1 incubated with cyclin D3/cdk6 binds to both active and inactive forms of eIF2α. These data suggest that cyclin D3/cdk4-dependent phosphorylation of CUGBP1 at Ser-302 might positively regulate CUGBP1 translational activity by directing CUGBP1 to the active form of eIF2α. Thus, the complex of CUGBP1 with dephosphorylated eIF2α is a positive regulator of translation on polyribosomes, whereas CUGBP1-ph-S51-eIF2 complex may inhibit translation of some mRNAs, perhaps within SGs.
Figure 5.
CUGBP1 unphosphorylated at S302 interacts preferentially with inactive form of eIF2α. A) Diagram of experimental procedure. Purified CUGBP1-MBP bound to amylose beads was phosphorylated with the cyclin D3/cdk6, and its interaction with active and inactive forms of eIF2α was tested by Western blotting assay. B) Top panel: input of CUGBPI. Middle panel: input of eIF2α. Bottom panel: Western blotting of the pulldown samples of unphosphorylated CUGBP1 and CUGBP1 phosphorylated by cyclin D3/cdk6 with antibodies to total eIF2α. Arrows indicate positions of ph-S51-eIF2α (inactive protein) and deph-S51-eIF2α. C) Protein levels of PKR are increased in DM1 myoblasts. Top panel: cytoplasmic extracts from control myoblasts and myoblasts from 2 DM1 patients were analyzed by Western blotting with Abs to PKR. Membrane was reprobed with antibodies to β-actin to verify protein loading. Bottom panel: levels of ph-S51-eIF2α are increased in DM1 myoblasts. Cytoplasmic protein extracts from control and DM1 myoblasts were examined by Western blotting with antibodies to ph-S51-eIF2α. Membrane was reprobed with antibodies to β-actin.
Elevation of stress and formation of SGs in DM1 myoblasts suggested that the levels of the inactive form of eIF2α-Ser-51-ph might be elevated in DM1. Phosphorylation of eIF2α at Ser-51 is mediated by several kinases, including PKR (reviewed in ref. 28). It has been shown previously that PKR activity is increased by CUG repeats because of accumulation of double-stranded CUG RNA (29). We have examined PKR protein levels in control and DM1 myoblasts and found that the levels of PKR are increased in DM1 myoblasts as well (Fig. 5C). The increase of PKR levels in DM1 myoblasts and the previous report showing the elevation of PKR activity by CUG repeats (29) suggest that the phosphorylation of eIF2α at Ser-51 might also be elevated in DM1 myoblasts. To examine this suggestion, we have measured the levels of ph-S51-eIF2α in myoblasts from control patients and in myoblasts from patients with DM1. These studies showed that levels of ph-eIF2α are increased in DM1 myoblasts (Fig. 5C, bottom panel).
CUGBP1 inhibits translation of a chromatin remodeling protein MRG15 (MORF4L1)
Previous data from our laboratory and from other groups showed that CUGBP1 is a positive regulator of translation (16, 17, 20, 24, 39, 40). However, the presence of CUGBP1 in SGs suggests that CUGBP1 might also inhibit translation of mRNAs within SGs. To test whether CUGBP1 has a dual role in the regulation of translation, we have searched for additional, translational targets of CUGBP1. In a parallel studies, we have pulled down a number of mRNAs that preferentially interact with CUGBP1 in DM1 cells (data not shown). Among these mRNAs, we found mRNA encoding MRG15, a protein involved in DNA repair and chromatin remodeling. It has been shown that CUGBP1 binds to the 5′ regions of mRNAs containing GC-rich sequences such as p21, cyclin D1, and C/EBPβ mRNAs (17, 24, 39, 40). We have examined the sequences of the 5′ region of MRG15 mRNA and found that this mRNA contains GC-rich motifs (Fig. 6B). Because reduction of MRG15 plays a significant role in the development of the aging phenotype (42) and because DM1 is a progressive disease in which severity of symptoms is increased with age, we suggested that MRG15 might be an appropriate candidate to be regulated by CUGBP1.
To test whether CUGBP1 binds to the 5′ region of MRG15 mRNA, the RNA probe covering the 5′ region of the MRG15 RNA (Fig. 6B) was synthesized, labeled with 32P-γATP, and used for a UV-cross-link assay with purified full-length MBP-CUGBP1 and with truncated molecules of CUGBP1 (Fig. 6A). We found that purified, full-length CUGBP1 binds to the MRG15 RNA probe (Fig. 6C). This binding is specific because the addition of a specific competitor (unlabeled MRG15 RNA) inhibits CUGBP1 binding to the MRG15 riboprobe, while this binding is not affected by a nonspecific AU-rich RNA competitor. We previously found that the first 2 RNA RBDs of CUGBP1 linked together specifically bind to RNA containing CUG repeats, whereas RBD3 binds to many GC-rich RNAs (44). Full-length and truncated CUGBP1 proteins, containing RBD1 + 2, bind to MRG15 RNA (Fig. 6D). Note that the difference in the binding between the full-length and RBD1 + 2 proteins is due to lower amounts of RBD1 + 2 protein, but not due to differences in their RNA affinities (Fig. 6D). We found that truncated proteins containing single RBDs, RBD1, and RBD2 do not bind to MRG15 RNA or bind with very low affinity. Thus, CUGBP1 specifically binds to the 5′ region of MRG15 RNA, and this binding occurs through motifs located in RBD1 + 2.
To test the effect of the increase of CUGBP1 in DM1 myoblasts on MRG15 levels, we have performed Western blot analysis of protein extracts from control and DM1 myoblasts with antibodies to MRG15. The levels of MRG15 protein are reduced in DM1 myoblasts (Fig. 6E), whereas levels of MRG15 mRNA are not changed (Fig. 6F). These data suggest that CUGBP1 might inhibit translation of MRG15 mRNA.
CUG-repeat mediated elevation of CUGBP1 inhibits translation of MRG15 mRNA by recruitment of MRG15 mRNA into SGs
The reduction of MRG15 protein, but not mRNA, in DM1 myoblasts suggests that the inactive form of CUGBP1 may trap MRG15 mRNA in SGs and inhibit its translation. To test this suggestion, we examined whether the elevation of CUGBP1 in double-stable CHO monoclonal cell lines might inhibit expression of MRG15 and whether CUGBP1-containing SGs might contain MRG15 mRNA. Mutant CUG RNA repeats were induced in the double-stable CHO cells, and SGs were separated by HPLC chromatography as described previously (Fig. 3); RNA was extracted from the chromatography fractions (19–29) and analyzed by real time RT-PCR with the primers specific for MRG15 mRNA. RT-PCR with primers to β-actin was used as the control for nonspecific shift. We found that chromatography fractions 19–22 of the extracts from CUG914 RNA-expressing cells contain MRG15 mRNA, whereas similar fractions of the extracts from cells without CUG RNA do not contain MRG15 mRNA (Fig. 7A). The shift of MRG15 mRNA to high-MW fractions in CUG-expressing cells is specific because the location of β-actin mRNA is not affected by CUG repeats (Fig. 7A). Because fractions 19–21 contain SGs (Fig. 3B), we conclude that a portion of MRG15 mRNA is trapped in SGs because of expression of CUG914 RNA. The calculation of the amount of MRG15 mRNA within fractions 19–22, containing SGs, shows that ∼44% of MRG15 mRNA translocates to SG-containing fractions in CUG-expressing cells. Thus, these data show that the mutant CUG RNA causes formation of TIA-1/CUGBP1 cytoplasmic SGs and traps MRG15 mRNA in SGs.
Figure 7.
CUGBP1 recruits MRG15 mRNA into SGs and inhibits its translation. MRG15 RNA is observed in SGs of CHO cells expressing the mutant CUG914 RNA. A) Cytoplasmic samples were fractionated as shown in Fig. 3. Total RNA was isolated from the region of the chromatographic profile (fractions 19–29), including fractions containing SGs from uninduced and induced cells, and RNA was examined by real time RT-PCR with primers specific to MRG15 mRNA and to β-actin mRNA. Fractions 19–22 (indicated by red box) in CUG-expressing cells contain MRG15, while the same fractions in cells without CUG repeats do not have MRG15 mRNA. B) Induction of CUG914 increases CUGBP1 and ph-S51-eIF2α isoform and reduces MRG15 protein. Western blotting was performed with protein extracts from induced cells using antibodies to MRG15, CUGBP1, ph-eIF2α, and β-actin. C) Transiently expressed GFP-CUGBP1 inhibits translation of MRG15. GFP-CUGBP1 was transfected into HeLa cells, and the levels of CUGBP1 and MRG15 were determined by Western blot with antibodies to CUGBP1 and MRG15, respectively. Note that expression of GFP-CUGBP1 also increases levels of endogenous CUGBP1 migrating in the position of 51 kDa. Bar graphs show levels of MRG15 as ratios to β-actin that are 2.3- to 3.0-fold reduced by GFP-CUGBP1. D) Ectopic expression of CUGBP1 does not change MRG15 mRNA levels. Total RNA extracted from cells transfected with an empty vector or with CUGBP1 was examined by real time RT-PCR with primers specific for MRG15. GAPDH levels were used as control.
Accumulation of MRG15 mRNA in SGs of CUG-expressing cells suggested that translation of MRG15 mRNA might be inhibited in these cells. To examine this possibility, we have analyzed protein extracts from uninduced and induced cells by Western blotting with antibodies to MRG15. The levels of CUGBP1 are increased at 17–48 h after Dox addition while MRG15 protein levels are significantly reduced (Fig. 7B). MRG15 levels were reversibly correlated with CUGBP1 on CUG914 induction (Fig. 7B), suggesting that CUGBP1 is a negative regulator of MRG15 expression. Because CUGBP1-eIF2-S51-ph complex is a negative regulator of translation, we examined phosphorylation status of eIF2α in these stable cells. These studies showed that the inhibition of MRG15 by CUG914 correlated with the increase of the inactive form of eIF2α during the same period (Fig. 7B). Taken together, these data show that MRG15 translation is inhibited by CUG914 through increase of inactive CUGBP1 and the inactive form of eIF2α.
To obtain additional evidence for the inhibition of translation of MRG15 mRNA by CUGBP1, we have performed transient transfections of GFP-CUGBP1 into HeLa cells. In these studies, we have used Amaxa Transfector protocol, which provides up to 60% efficiency of transfections. The results of these studies are shown in Fig. 7C, D. The transient transfection of GFP-CUGBP1 in HeLa cells reduces MRG15 levels to 40–50%. Since the efficiency of transfections was around 60%, this suggests that the inhibition of MRG15 in individual transfected cells was much higher. This down-regulation of protein levels does not involve alterations in MRG15 mRNA (Fig. 7D).
DISCUSSION
A number of studies have shown that the pathological alterations in DM1 are mediated by untranslated RNA CUG repeats. Despite this progress, little is known about the direct targets of the mutant CUGn RNA. The best characterized targets of CUG expansion are RNA-binding proteins, MBNL1 and CUGBP1. The mutant RNA may also target dsRNA-activated kinase, PKR (29). In this study, we have shown that the mutant RNA CUG repeats initiate stress and increase formation of SGs. The CUG-associated stress in DM1 myoblasts and in an inducible DM1 cell culture model is mediated by an increase in PKR levels and by the accumulation of one of the PKR substrates, inactive ph-S51-eIF2α (Fig. 8C). These results agree with the previous studies showing that DM1 atrophic fibers accumulate the inactive form of eIF2α (25). As an adaptation to the stress conditions, cells activate translation of sets of mRNAs that are needed to cope with stress, whereas translation of some mRNAs is inhibited (reviewed in ref. 28). It has been suggested that such inhibition occurs by the increase of inactive eIF2α, which forms inactive translational complexes (31, 32). As a result, mRNAs bound to inactive complexes are removed from translation on polysomes. Our data show that similar stress-related inhibition of translation occurs in DM1 myoblasts. We have identified MRG15 mRNA as a target of stress-mediated aggregates in DM1 myoblasts as it is translocated into cytoplasmic SGs after transcription of the mutant CUG914. Although we have identified one mRNA that is inhibited in cytoplasmic SGs, there is a high likelihood that the translation of other mRNAs may be blocked in SGs in DM1 cells. Our data are obtained in cultured myoblasts from DM1 patients. We suggest that the stress-mediated inhibition of translation might occur not only in DM1 muscle, but also in heart, brain, and other tissues affected in DM1. The isolation and cloning of mRNAs, associated with SGs, in different DM1 tissues is necessary to determine the portion of genes that have reduced translation in DM1 due to stress-induced SGs.
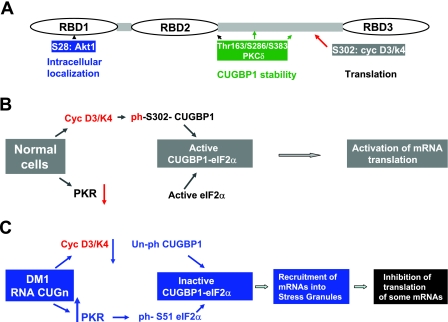
Figure 8.
Phosphorylation controls biological functions of CUGBP1. A) Positions of phosphorylation sites and their role in the CUGBP1 functions. B) Hypothetical model for phosphorylation-dependent regulation of CUGBP1 translational activity in normal cells. C) Hypothetical mechanism by which RNA CUG repeats inhibit translation of certain mRNAs via increase of stress in DM1 cells. CUG repeats increase levels of inactive eIF2α and translational inactive CUGBP1, which are recruited into cytoplasmic SGs and form inactive CUGBP1-eIF2α-S51-ph complexes. As a result, translation of mRNAs associated with these inactive complexes within SGs is inhibited.
We found that CUGBP1 binds to the 5′ region of MRG15 mRNA and inhibits its translation in double-stable clones and in transient transfection experiments. This inhibition seems to occur in SGs of DM1 myoblasts and in CHO cells expressing noncoding mutant CUG914 RNA. Note that a recent report has also identified that another CUG-binding protein, MBNL1, is a component of SGs in stressed cells (46). This finding suggests that the translation of a number of mRNAs is blocked in SGs in DM1 patients.
Data in our report emphasized a critical role of phosphorylation of CUGBP1 in the regulation of biological functions of CUGBP1 and in development of DM1 pathology. Figure 8A summarizes our data and observations from other labs showing the effects of phosphorylation on CUGBP1 functions. The Akt-mediated phosphorylation of CUGBP1 at S28 is involved in the regulation of intracellular localization of CUGBP1. Data from Cooper's group showed that PKC-mediated phosphorylation of CUGBP1 stabilizes CUGBP1 in DM1 cells (47). Although the PKC-phosphorylated residues of CUGBP1 have not been identified in this report, it is likely that Thr163/S286/S383 might be involved because these residues show high scores for PKC-mediated phosphorylation. Our recent data demonstrated that cyc-D3/cdc4-mediated phosphorylation of CUGBP1 regulates translational functions of CUGBP1. In normal cells, the cdk4-mediated phosphorylation of CUGBP1 leads to the formation of active CUGBP1-eIF2 complexes, which increases translation of mRNAs (Fig. 8B). However in DM1 cells, the activity of cyclin D3-cdk4 is reduced, and a portion of unph-S302-CUGBP1 forms an inactive complex with the ph-S51-eIF2. This inactive complex is located in SGs and inhibits translation of certain mRNAs such as MRG15. What are the biological consequences of stress caused by the accumulation of noncoding RNA in DM1 patients? It has been shown that when stress ends, SGs are disassembled, suggesting that the trapped mRNAs move to polysomes for translation (32). However, under lethal stresses, mRNAs trapped in SGs undergo degradation by RNA-binding proteins regulating RNA decay. Therefore, it remains to elucidate whether SGs in DM1 cells are reversible. We expect that SGs might be reversed by different approaches, such as reduction of CUGn RNA expression, activation of eIF2α, or normalization of RNA-binding proteins (such as phosphorylation of CUGBP1 at Ser-302) or by correction of other RNA-binding proteins associated with the formation of SGs. It is possible that SGs are reversible in young DM1 patients by unknown protective pathways that might release stress. With age, however, these protective mechanisms may be exhausted because of continuous expression of CUGn RNA. Therefore, even mild CUG expansions may cause irreversible stress and inhibition of translation of mRNAs, trapped within SGs. This possible mechanism could explain, at least in part, the progressive nature of DM1 pathology worsening with age. CUGBP1 is a good candidate to degrade mRNAs in SGs because CUGBP1 possesses multiple activities, including regulation of RNA decay and stability (36–38).
It would be of interest to study whether the accumulation of the noncoding RNA CCUGn in patients with type 2 myotonic dystrophy (DM2) causes similar stress and inhibition of translation in SGs. Our recent publication has shown that CCUGn RNA forms large cytoplasmic aggregates containing the 20S proteasome and CUGBP1-eIF2 complexes (48). The expansion of CCUG repeats in DM2 myoblasts increases levels of Hsp70 and BiP, which are components of the mega-complex binding to CCUG RNA (48). This increase of BiP suggests that cytoplasm of DM2 myoblasts may incur ER-related stress.
By analogy to DM, translation in other neuromuscular diseases caused by unstable mutations might be changed by SGs. For example, FMRP protein reduced in the patients with Fragile X syndrome is associated with SGs in the brain (45), where it may attenuate translation. Data described here suggest that the accumulation of CUGn RNA in myoblasts from patients with DM1 causes formation of SGs, which inhibit translation of certain mRNAs. The repression of protein translation by stress might lead to a muscle loss. At this stage, however, it is difficult to assess the extent to which the distinct clinical symptoms and the severity of the disease course are related to the consequences of stress. We propose that cell stress might be an important disease-modifying component in DM1. Therefore, further studies are required to understand disease pathology and to identify therapeutic approaches to reduce cell stress and its consequences.
Acknowledgments
This work was supported by National Institutes of Health grants AR052791, AR044387, AR044387-ARRA, NS063298 (to L.T.T.), GM55188, CA100070, AG025477 (to N.A.T.), and AG032134 (to O.M.P.-S.). B.S. has received research support from the Deutsche Gesellschaft für Muskelkranke, Freiburg, Germany; he is a member of the German Muscular Dystrophy Network (MD-NET/01GM0601) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany). MD-NET is a partner of TREAT-NMD (EC, 6th FP, proposal 036825). The authors thank the Muscle Tissue Culture Collection (MTCC) for providing the samples. MTCC is a partner of the EuroBioBank Network, established in 2001 thanks to EC funding (01/2003-03/2006).
REFERENCES
- 1.Harper P. S. (2001) Myotonic Dystrophy, W. B. Saunders, London [Google Scholar]
- 2.Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Clen C., Alleman J., Wormskamp N. G., Vooijs M., Buxton J., Johnson K., Sweets H. J. M., Lennon G. G., Carrano A. V. R. G., Korneluk R. G., Wieringa B., deJong P. J. (1992) Cloning of essential myotonic dystrophy region and mapping of the putative defect. Nature 355, 548–551 [DOI] [PubMed] [Google Scholar]
- 3.Brook J. D., McCurrah M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J.-P., Hudson T., Sohn R., Zemelman B., Snell R. G., Rundle S. A., Crow S., Davies J., Shelbourne P., Buxton J., Jones C., Juvonen V., Johnson K., Harper P. S., Shaw D. J., Housman D. E. (1992) Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeats at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 [DOI] [PubMed] [Google Scholar]
- 4.Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Avret M., Riley B., Williamson R., Johnson K. (1992) Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 355, 547–548 [DOI] [PubMed] [Google Scholar]
- 5.Fu Y. H., Pizzuti A., Fenwick R. G., Jr., King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P., Wieringa B., Korneluk R., Perryman M. B., Epstein H. F., Caskey C. T. (1992) An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 [DOI] [PubMed] [Google Scholar]
- 6.Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. (1992) Expansion of an unstable DMA region and phenotype variation in myotonic dystrophy. Nature 355, 545–546 [DOI] [PubMed] [Google Scholar]
- 7.Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barcelo J., O'Hoy K., LeBlond S., Earle-McDonald J., De Jong P. J., Wieringa B., Korneluk R. G. (1992) Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Pegoraro E., Menegazzo E., Gennarelli M., Hoop R. C., Angelini C., Hoffman E. P. (1995) Myotonic dystrophy: evidence for a possible dominant-negative RNA mutation. Hum. Mol. Genet. 4, 599–606 [DOI] [PubMed] [Google Scholar]
- 9.Timchenko L. T., Timchenko N. A., Caskey C. T., Roberts R. (1996) Novel proteins with binding specificity to DNA CTG and RNA CUG repeats: implications for myotonic dystrophy. Hum. Mol. Genet. 5, 115–121 [DOI] [PubMed] [Google Scholar]
- 10.Timchenko L. T., Miller J. W., Timchenko N. A., DeVore D. R., Datar K. V., Lin L., Roberts R., Caskey C. T., Swanson M. S. (1996) Identification of a (CUG)n triplet repeat binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 24, 4407–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philips A. V. L. T., Timchenko L. T., Cooper T. A. (1998) Disruption of splicing of regulated by CUG binding protein in myotonic dystrophy. Science 280, 737–741 [DOI] [PubMed] [Google Scholar]
- 12.Timchenko L. T. (1999) Myotonic dystrophy: the role of RNA CUG repeats. Am. J. Hum. Genet. 64, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mankodi M., Logigian E., Callahan L., McClain C., White R., Henderson D., Krym M., Thornton C. A. (2000) Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1772 [DOI] [PubMed] [Google Scholar]
- 14.Miller J. W., Urbinati C. R., Teng-Umnuay P., Stenberg M. G., Byrne B. J., Thornton C. A., Swanson M. S. (2000) Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 19, 4439–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seznec H., Agbulut O., Sergeant N., Savouret C., Ghestem A., Tabti N., Willer J. C., Ourth L., Duros E., Brisson E., Fouquet C., Butler-Browne G., Delacourte A., Junien C., Gourdon G. (2001) Mice transgenic for the human myotonic dystrophy with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet. 10, 2717–2726 [DOI] [PubMed] [Google Scholar]
- 16.Timchenko N. A., Cai Z.-J., Welm A. L., Reddy S., Ashizawa T., Timchenko L. T. (2001) RNA CUG repeats sequester and alter protein levels and activity of CUGBP1. J. Biol. Chem. 276, 7820–7826 [DOI] [PubMed] [Google Scholar]
- 17.Timchenko N. A., Iakova P., Cai Z.-J., Smith J. R., Timchenko L. T. (2001) Molecular basis for impaired muscle differentiation in myotonic dystrophy. Mol. Cell. Biol. 21, 6927–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savcur R. S. A. V., Philips A. V., Cooper T. A. (2001) Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 29, 40–47 [DOI] [PubMed] [Google Scholar]
- 19.Kanadia R. N., Johnstone K. A., Mankodi A., Lungu C., Thornton C. A., Esson D., Timmers A. M., Hauswirth W. W., Swanson M. S. (2003) A muscleblind knockout model for myotonic dystrophy. Science 302, 1978–1980 [DOI] [PubMed] [Google Scholar]
- 20.Timchenko N. A., Patel R., Iakova P., Cai Z.-J., Quan L., Timchenko L. T. (2004) Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J. Biol. Chem. 279, 13129–13139 [DOI] [PubMed] [Google Scholar]
- 21.Ho T. H., Bundman D., Amstrong D. L., Cooper T. A. (2005) Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 14, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 22.Mahadevan M. S., Yadava R.S., Yu Q., Balijepalli S., Frenzel-McCardell C.D., Bourne T. D., Phillips L. H. (2006) Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat. Genet. 38, 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orengo J., Chambon P., Metzger D., Mosier D. R., Snipes G. L., Copper T. A. (2008) Expanded CTG repeats within the DMPK 3′ UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc. Natl. Acad. Sci. U. S. A. 105, 2646–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salisbury E., Sakai K., Schoser B., Huichalaf C., Schneider-Gold C., Nguen H., Wang G.-L., Albrecht J. H., Timchenko L. T. (2008) Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp. Cell Res. 314, 2266–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikezoe K., Nakamori M., Furuya H., Arahata H., Kanemoto S., Kimura T., Imaizumi K., Takahashi M. P., Sakoda S., Fujii N., Kira J. (2007) Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathologica 114, 527–535 [DOI] [PubMed] [Google Scholar]
- 26.Toscano A., Messina S., Campo G. M., Di Leo R., Musumeci O., Rodolico C., Aguennouz M., Annesi G., Messina C., Vita G. (2005) Oxidative stress in myotonic dystrophy type 1. Free Radic. Res. 39, 771–776 [DOI] [PubMed] [Google Scholar]
- 27.Usuki F., Takahashi N., Sasagawa N., Ishiura S. (2000) Differential signaling pathways following oxidative stress in mutant myotonin protein kinase cDNA-transfected C2C12 cell lines. Biochem. Biophys. Res. Commun. 267, 739–743 [DOI] [PubMed] [Google Scholar]
- 28.Holcik M., Sonenberg N. N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 6, 318–327 [DOI] [PubMed] [Google Scholar]
- 29.Tian B., White R. J., Xia T., Welle S., Turner D. H., Mathews B., Thornton C. A. (2000) Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA 6, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedersha N., Anderson P. (2002) Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30, 963–969 [DOI] [PubMed] [Google Scholar]
- 31.Anderson P., Kedersha N. (2002) Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson P., Kedersha N. (2006) RNA granules. J. Cell Biol. 172, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedersha N. L., Gupta M., Li W., Miller I., Anderson P. (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimura K., Kano F., Murata M. (2008) Dual localization of the RNA binding proteins CUGBP-1 to stress granules and perinuclear compartment. Exp. Cell. Res. 314, 543–553 [DOI] [PubMed] [Google Scholar]
- 35.Woeller C. F., Fox J. T., Perry C., Stover P. J. (2007) A ferritin-responsive ribosome entry site regulates folate metabolism. J. Biol. Chem. 282, 29927–29935 [DOI] [PubMed] [Google Scholar]
- 36.Paillard L., Omilli F., Legagneux V., Bassez T., Maniey D., Osborne H. B. (1998) EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus laevis. EMBO J. 17, 278–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster P. J., Liang L., Berg C. A., Lasko P., Macdonald P. M. (2007) Translational repressor bruno plays multiple roles in development and is widely conserved. Genes Dev. 11, 2510–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moraes K. C. M., Wilusz C. J., Wilusz J. (2006) CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA 12, 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timchenko N. A., Wang G.-L., Timchenko L. T. (2005) RNA CUG-binding protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein beta by interacting with the alpha and beta subunits of eukaryotic initiation translation factor 2. J. Biol. Chem. 280, 20549–20557 [DOI] [PubMed] [Google Scholar]
- 40.Timchenko L. T., Salisbury E., Wang G.-L., Nguyen H., Albrecht J. N., Hershey J. W. B., Timchenko N. A. (2006) Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. J. Biol. Chem. 281, 32806–32819 [DOI] [PubMed] [Google Scholar]
- 41.Pardo P. S., Leung J. K., Lucchesi J. C., Pereira-Smith O. M. (2002) MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation. J. Biol. Chem. 277, 50860–50866 [DOI] [PubMed] [Google Scholar]
- 42.Pena A. N., Pereira-Smith O. M. (2007) The role of MORF/MRG family of genes in cell growth, differentiation, DNA repair, and thereby aging. Ann. N. Y. Acad. Sci. 1100, 299–305 [DOI] [PubMed] [Google Scholar]
- 43.Ordway J. M., Detloff P. J. (1996) In vitro synthesis and cloning of long CAG repeats. BioTechniques 21, 609–610 [DOI] [PubMed] [Google Scholar]
- 44.Timchenko N. A., Welm A. L., Lu X., Timchenko L. T. (1999) CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBP beta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 27, 4517–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Archrafi A., Cunningham B. A., Edelman G. M., Vanderklish P. W. (2005) The fragile X mental retardation protein and group 1 metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc. Natl. Acad. Sci. U. S. A. 102, 2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onishi H., Kino T., Futai E., Sasagawa N., Ishiura S. (2008) MBNL1 associates with YB-1 in cytoplasmic stress granules. J. Neurol. Res. 86, 1994–2002 [DOI] [PubMed] [Google Scholar]
- 47.Kuyumcu-Martinez N. M., Wang G-S., Cooper T. A. (2007) Increased steady-state levels of CUGBP1 in myotonic dystrophy are due to PKC-mediated hyperphosphorylation. Mol. Cell. 28, 68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salisbury E., Schoser B., Schneider-Gold C., Wang G.-L., Huichalaf C., Jin B., Sirito M., Sarkar P., Krahe R., Timchenko N. A., Timchenko L. T. (2009) Expression of RNA CCUG repeats dysregulates translation and degradation of proteins in DM2 patients. Am. J. Pathol. 175, 748–762 [DOI] [PMC free article] [PubMed] [Google Scholar]